Neutrophil Gelatinase-Associated Lipocalin (NGAL) Is Related with the Proteinuria Degree and the Microscopic Kidney Findings in Leishmania-Infected Dogs
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals, Samples, and Ethical Statements
2.2. Clinical Signs and Serological Study
2.3. Biochemical and Hematological Analyses
2.4. Proteinuria and Azotemia Determinations and Group Allocation
2.5. Serum NGAL, Urinary NGAL, and uNGAL/C Determinations
2.6. Histopathological Study and Group Allocation
2.7. Statistical Analysis
3. Results
3.1. Clinical Signs and Laboratory Studies
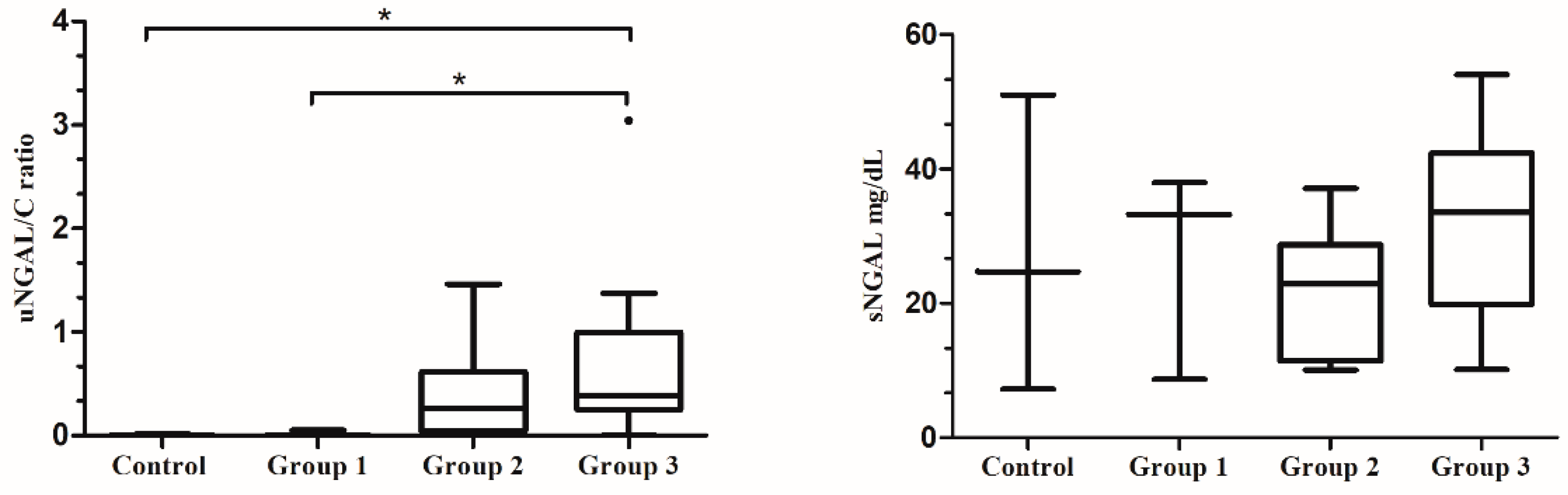
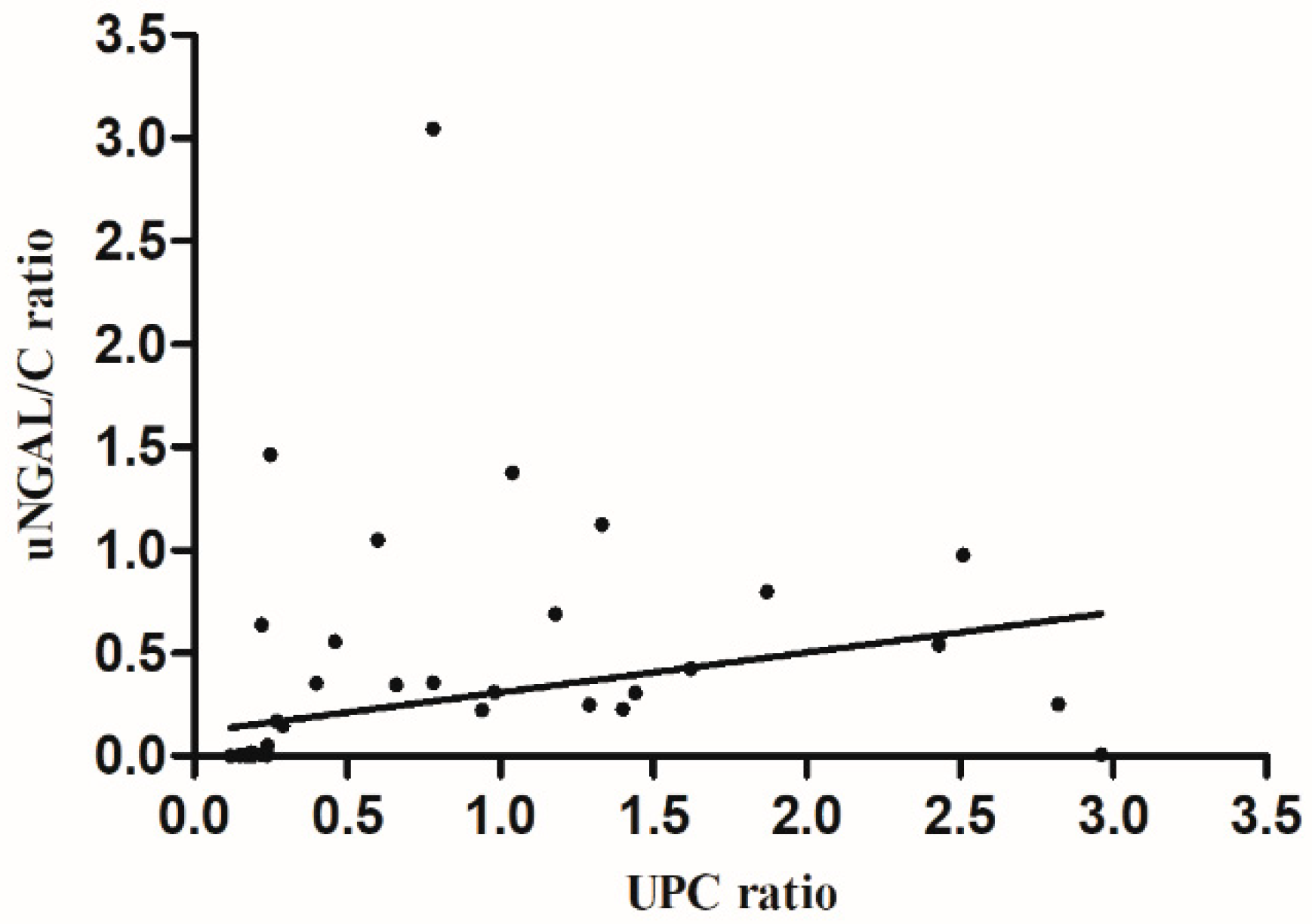
3.2. NGAL Values and Proteinuria Degree
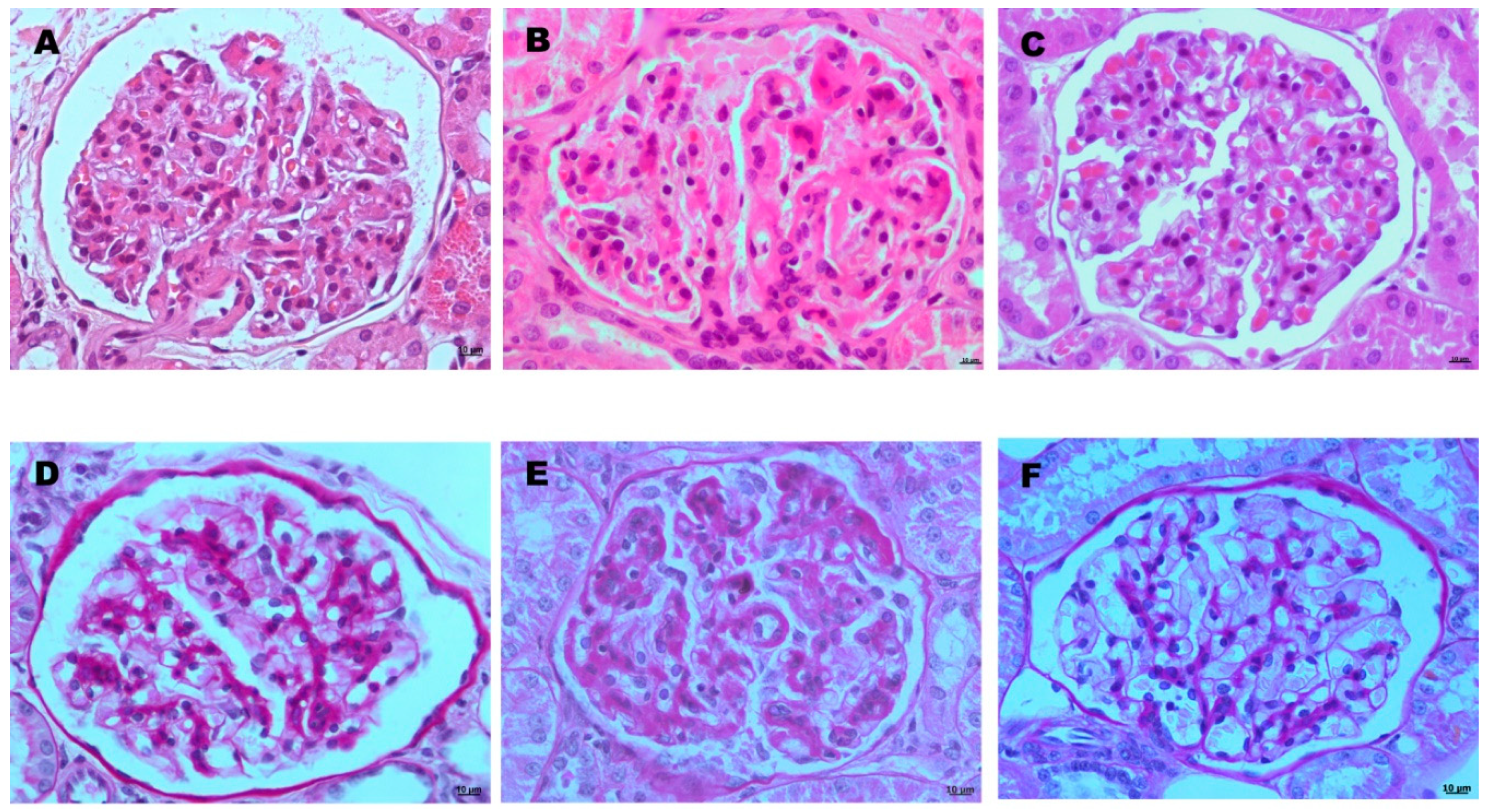
3.3. NGAL Values and Histopathological Study
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Koutinas, A.F.; Koutinas, C.K. Pathologic mechanisms underlying the clinical findings in canine leishmaniasis due to Leishmania infantum/chagasi. Vet. Pathol. 2014, 51, 527–538. [Google Scholar] [CrossRef] [PubMed]
- Cianciolo, R.; Mohr, F. Urinary system. In Jubb, Kennedy and Palmer´s, Pathology of Domestic Animals, 6th ed.; Grant Maxie, M., Ed.; Saunders: St. Louis, MO, USA, 2016; Volume 2, pp. 401–410. [Google Scholar]
- Costa, F.A.; Goto, H.; Saldanha, L.C.; Silva, S.M.; Sinhorini, I.L.; Silva, T.C.; Guerra, J.L. Histopathologic patterns of nephropathy in naturally acquired canine visceral leishmaniasis. Vet. Pathol. 2003, 40, 677–684. [Google Scholar] [CrossRef] [PubMed]
- Zatelli, A.; Borgarelli, M.; Santilli, R.; Bonfanti, U.; Nigrisoli, E.; Zanatta, R.; Tarducci, A.; Guarraci, A. Glomerular lesions in dogs infected with Leishmania organisms. Am. J. Vet. Res. 2003, 64, 558–561. [Google Scholar] [CrossRef] [PubMed]
- International Renal Interest Society Guidelines. Treatment Recommendations for CKD. 2017. Available online: http://www.iris-kidney.com/guidelines/recommendations.html (accessed on 15 July 2018).
- Solano-Gallego, L.; Koutinas, A.; Miró, G.; Cardoso, L.; Pennisi, M.G.; Ferrer, L.; Bourdeau, P.; Oliva, G.; Baneth, G. Directions for the diagnosis, clinical staging, treatment and prevention of canine leishmaniosis. Vet. Parasitol. 2009, 165, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Palacio, J.; Liste, F.; Gascón, M. Urinary protein/creatinine ratio in the evaluation of renal failure in canine leishmaniasis. Vet. Record. 1995, 137, 567–568. [Google Scholar] [CrossRef]
- Finco, D.R.; Brown, S.A.; Vaden, S.L.; Ferguson, D.C. Relationship between plasma creatinine concentration and glomerular filtration rate in dogs. J. Vet. Pharmacol. Ther. 1995, 18, 418–421. [Google Scholar] [CrossRef]
- Cianciolo, R.; Hokamp, J.; Nabity, M. Advances in the evaluation of canine renal disease. Vet. J. 2016, 215, 21–29. [Google Scholar] [CrossRef]
- Nabity, M.B.; Lees, G.E.; Cianciolo, R.; Boggess, M.M.; Steiner, J.M.; Suchodolski, J.S. Urinary biomarkers of renal disease in dogs with X-linked hereditary nephropathy. J. Vet. Intern. Med. 2012, 26, 282–293. [Google Scholar] [CrossRef]
- D’Amico, G.; Bazzi, C. Pathophysiology of proteinuria. Kidney Int. 2003, 63, 809–825. [Google Scholar] [CrossRef]
- Martínez-Subiela, S.; García-Martínez, J.D.; Tvarijonaviciute, A.; Tecles, F.; Caldin, M.; Bernal, L.J.; Cerón, J.J. Urinary C reactive protein levels in dogs with leishmaniasis at different stages of renal damage. Res. Vet. Sci. 2013, 95, 924–929. [Google Scholar] [CrossRef]
- García-Martínez, J.D.; Martínez-Subiela, S.; Tvarijonaviciute, A.; Caldin, M.; Cerón, J.J. Urinary ferritin and cystatin C concentrations at different stages of kidney disease in leishmaniotic dogs. Res. Vet. Sci. 2015, 99, 204–207. [Google Scholar] [CrossRef] [PubMed]
- Sato, R.; Soeta, S.; Miyazaki, M.; Syuto, B.; Sato, J.; Miyake, Y.; Yasuda, J.; Okada, K.; Naito, Y. Clinical availability of urinary N-acetyl-beta-D-glucosaminidase index in dogs with urinary diseases. J. Vet. Med. Sci. 2002, 64, 361–365. [Google Scholar] [CrossRef] [PubMed]
- Ibba, F.; Mangiagalli, G.; Paltrinieri, S. Urinary gamma-glutamyl transferase (GGT) as a marker of tubular proteinuria in dogs with canine leishmaniasis, using sodium dodecylsulphate (SDS) electrophoresis as a reference method. Vet. J. 2016, 210, 89–91. [Google Scholar] [CrossRef] [PubMed]
- Paltrinieri, S.; Mangiagalli, G.; Ibba, F. Use of urinary γ-glutamyl transferase (GGT) to monitor the pattern of proteinuria in dogs with leishmaniasis treated with N-methylglucamine antimoniate. Res. Vet. Sci. 2018, 119, 52–55. [Google Scholar] [CrossRef] [PubMed]
- Pardo-Marín, L.; Martínez-Subiela, S.; Pastor, J.; Tvarijonaviciute, A.; Garcia-Martinez, J.D.; Segarra, S.; Cerón, J.J. Evaluation of various biomarkers for kidney monitoring during canine leishmaniosis treatment. BMC Vet. Res. 2016, 13, 31. [Google Scholar] [CrossRef]
- Kjeldsen, L.; Johnsen, A.H.; Sengeløv, H.; Borregaard, N. Isolation and primary structure of NGAL, a novel protein associated with human neutrophil gelatinase. J. Biol. Chem. 1993, 268, 10425–10432. [Google Scholar]
- Cowland, J.B.; Borregaard, N. Molecular characterization and pattern of tissue expression of the gene for neutrophil gelatinase-associated lipocalin from humans. Genomics 1997, 45, 17–23. [Google Scholar] [CrossRef]
- Schmidt-Ott, K.M.; Mori, K.; Kalandadze, A.; Li, J.Y.; Paragas, N.; Nicholas, T.; Devarajan, P.; Barasch, J. Neutrophil gelatinase-associated lipocalin-mediated iron traffic in kidney epithelia. Curr. Opin. Nephrol. Hypertens. 2006, 15, 442–449. [Google Scholar] [CrossRef]
- Nickolas, T.L.; Forster, C.; Sise, M.E.; Nicholas, B.; David, S.V.; Viltard, M.; Buchen, C.; Kupferman, S.; Carnevali, M.L.; Bennett, M.; et al. Monomeric neutrophil gelatinase associated lipocalin is associated with tubulointerstitial damage in chronic kidney disease. Kidney Int. 2012, 82, 718–722. [Google Scholar] [CrossRef]
- Cobrin, A.R.; Blois, S.L.; Abrams-Ogg, A.C.G.; Kruth, S.A.; Dewey, C.; Holowaychuk, M.K.; Gauthier, V. Neutrophil gelatinase-associated lipocalin in dogs with chronic kidney disease, carcinoma, lymphoma and endotoxaemia. J. Small Anim. Pract. 2016, 57, 291–298. [Google Scholar] [CrossRef]
- Hokamp, J.A.; Cianciolo, R.E.; Boggess, M.; Lees, G.E.; Benali, S.L.; Kovarsky, M.; Nabity, M.B. Correlation of urine and serum biomarkers with renal damage and survival in dogs with naturally occurring proteinuric chronic kidney disease. J. Vet. Intern. Med. 2016, 30, 591–601. [Google Scholar] [CrossRef] [PubMed]
- Abella, V.; Scotece, M.; Conde, J.; Gómez, R.; Lois, A.; Pino, J.; Gómez-Reino, J.J.; Lago, F.; Mobasheri, A.; Gualillo, O. The potential of lipocalin-2/NGAL as biomarker for inflammatory and metabolic diseases. Biomarkers 2015, 20, 565–571. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Lam, K.S.; Kraegen, E.W.; Sweeney, G.; Zhang, J.; Tso, A.W.; Chow, W.S.; Wat, N.M.; Xu, J.Y.; Hoo, R.L.; et al. Lipocalin-2 is an inflammatory marker closely associated with obesity, insulin resistance, and hyperglycemia in humans. Clin. Chem. 2007, 53, 34–41. [Google Scholar] [CrossRef]
- Axelsson, L.; Bergenfeldt, M.; Ohlsson, K. Studies of the release and turnover of a human neutrophil lipocalin. Scand. J. Clin. Lab. Investig. 1995, 55, 577–588. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Avital, Y.; Segev, G. Biomarkers of Acute Kidney Injury. Isr. J. Vet. Med. 2017, 72, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Bolignano, D.; Donato, V.; Coppolino, G.; Campo, S.; Buemi, A.; Lacquaniti, A.; Buemi, M. Neutrophil gelatinase-associated lipocalin (NGAL) as a marker of kidney damage. Am. J. Kidney Dis. 2008, 52, 595–605. [Google Scholar] [CrossRef] [PubMed]
- Segev, G.; Daminet, S.; Meyer, E.; De Loor, J.; Cohen, A.; Aroch, I.; Bruchim, Y. Characterization of kidney damage using several renal biomarkers in dogs with naturally occurring heatstroke. Vet. J. 2015, 206, 231–235. [Google Scholar] [CrossRef]
- Segev, G.; Palm, C.; LeRoy, B.; Cowgill, L.D.; Westropp, J.L. Evaluation of neutrophil gelatinase-associated lipocalin as a marker of kidney injury in dogs. J. Vet. Intern. Med. 2013, 27, 1362–1367. [Google Scholar] [CrossRef]
- Guy, M.; Bailey, W.; Snowder, K. Canine leishmaniasis. Vet. Record. 1993, 132, 396. [Google Scholar] [CrossRef]
- Alcolea, P.J.; Alonso, A.; Esteban, A.; Peris, P.; Cortés, A.; Castillo, J.A.; Larraga, V. IL12 p35 and p40 subunit genes administered as pPAL plasmid constructs do not improve protection of pPAL-LACK vaccine against canine leishmaniasis. PLoS ONE 2019, 14, e0212136. [Google Scholar] [CrossRef]
- Mauricio, I.L.; Stothard, J.R.; Miles, M.A. Leishmania donovani complex: Genotyping with the ribosomal internal transcribed spacer and the mini-exon. Parasitology 2004, 128, 263–267. [Google Scholar] [CrossRef] [PubMed]
- Solano-Gallego, L.; Villanueva-Saz, S.; Carbonell, M.; Trotta, M.; Furlanello, T.; Natale, A. Serological diagnosis of canine leishmaniosis: Comparison of three commercial ELISA tests (Leiscan®, ID Screen® and Leishmania 96®), a rapid test (Speed Leish K®) and an in-house IFAT. Parasites Vectors 2013, 7, 111. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Ochoa, P.; Castillo, J.A.; Lucientes, J.; Gascón, M.; Zarate, J.J.; Arbea, J.I.; Larraga, V.; Rodriguez, C. Modified direct agglutination test for simplified serologic diagnosis of leishmaniasis. Clin. Diagn. Lab. Immunol. 2003, 10, 967–968. [Google Scholar] [CrossRef] [PubMed]
- Elliott, J.; Watson, A.D.J. Chronic kidney disease: Staging and management. In Kirk´s Current Veterinary Therapy, 14th ed.; Bonagura, J.D., Twedr, D.C., Eds.; Saunders: Amsterdam, The Netherlands, 2009; pp. 883–892. [Google Scholar]
- Ahn, H.; Hyun, C. Evaluation of serum associated lipocalin (NGAL) activity in dogs with chronic kidney disease. Vet. Record. 2013, 173, 452. [Google Scholar] [CrossRef] [PubMed]
- Steinbach, S.; Weis, J.; Schweighauser, A.; Francey, T.; Neiger, R. Plasma and urine neutrophil gelatinase-associated lipocalin (NGAL) in dogs with acute kidney injury or chronic kidney disease. J. Vet. Intern. Med. 2014, 28, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Schober, P.; Boer, C.; Schwarte, L.A. Correlation coefficients: Appropriate use and interpretation. Anesth. Analg. 2018, 126, 1763–1768. [Google Scholar] [CrossRef]
- Kuwabara, T.; Mori, K.; Mukoyama, M.; Kasahara, M.; Yokoi, H.; Saito, Y.; Yoshioka, T.; Ogawa, Y.; Imamaki, H.; Kusakabe, T.; et al. Urinary neutrophil gelatinase-associated lipocalin levels reflect damage to glomeruli, proximal tubules, and distal nephrons. Kidney Int. 2009, 75, 285–294. [Google Scholar] [CrossRef]
- Hsu, W.L.; Lin, Y.S.; Hu, Y.Y.; Wong, M.L.; Lin, F.Y.; Lee, Y.J. Neutrophil gelatinase-associated lipocalin in dogs with naturally occurring renal diseases. J. Vet. Intern. Med. 2014, 28, 437–442. [Google Scholar] [CrossRef]
- Vaden, S.; Elliot, J. Management of proteinuria in dogs and cats with chronic kidney disease. Vet. Clin. N. Am. Small Anim. Pract. 2016, 46, 1115–1130. [Google Scholar] [CrossRef]
- Mori, K.; Nakao, K. Neutrophil gelatinase-associated lipocalin as the real-time indicator of active kidney damage. Kidney Int. 2007, 71, 967–970. [Google Scholar] [CrossRef]
- Soares, M.; Moraes, J.; Moraes, F. Renal involvement in canine leishmaniasis: A morphological and immunohistochemical study. Arq. Bras. Med. Vet. Zootec. 2009, 61, 785–790. [Google Scholar] [CrossRef]
- Daure, E.; Belanger, M.C.; Beauchamp, G.; Lapointe, C. Elevation of neutrophil gelatinase-associated lipocalin (NGAL) in non-azotemic dogs with urinary tract infection. Res. Vet. Sci. 2013, 95, 1181–1185. [Google Scholar] [CrossRef] [PubMed]
- Proverbio, D.; Spada, E.; Baggiani, L.; De Giorgi, G.B.; Ferro, E.; Martino, P.A.; Perego, R. Relationship between Urinary Neutrophil Gelatinase-Associated Lipocalin and Noninfectious Pyuria in Dogs. Dis. Markers 2015, 2015, 387825. [Google Scholar]

| ALB gr/dL | ALP IU/L | Urea mg/dL | sCr mg/dL | AST IU/L | ALT IU/L | LDH IU/L | T-Bil mg/dL | T-Pro gr/dL | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 180 dpi | Normal | N | 29 | 29 | 29 | 29 | 29 | 29 | 29 | 29 | 29 |
| Value | 4 ±0.15 | 59.9 ±34.3 | 35.5 ±5.3 | 0.67 ±0.1 | 25.7 ±5.5 | 41.3 ±8.6 | 117.5 ±40.7 | 0.39 ±0.06 | 6.25 ±0.4 | ||
| Altered | N | - | - | - | - | - | - | - | - | - | |
| Value | - | - | - | - | - | - | - | - | - | ||
| 240 dpi | Normal | N | 29 | 29 | 29 | 29 | 29 | 29 | 24 | 29 | 29 |
| Value | 3.65 ±0.23 | 66.5 ±36.4 | 38.2 ±1.1 | 0.73 ±0.15 | 35.8 ±14.5 | 72.2 ±17 | 172.2 ±17.2 | 0.38 ±0.07 | 6.16 ±0.8 | ||
| Altered | N | - | - | - | - | - | - | 5 | - | - | |
| Value | - | - | - | - | - | - | 303.1 ±15.2 | - | - | ||
| 300 dpi | Normal | N | 29 | 29 | 29 | 4 | 29 | 29 | 24 | 29 | 29 |
| Value | 3.72 ±0.28 | 57.5 ±30.3 | 32.3 ±6.2 | 0.72 ±0.12 | 42.6 ±19.6 | 69.3 ±15.7 | 185.5 ±24.8 | 0.37 ±0.03 | 6.84 ±0.6 | ||
| Altered | N | - | - | - | 25 | - | - | 5 | - | - | |
| Value | - | - | - | 1.05 * ±0.09 | - | - | 358.8 ±21.7 | - | - | ||
| 360 dpi | Normal | N | 29 | 29 | 26 | 4 | 29 | 29 | 24 | 29 | 29 |
| Value | 3.6 ±0.32 | 77.3 ±50.9 | 33.1 ±5.6 | 0.73 ±0.08 | 49.1 ±7.6 | 78.4 ±22.3 | 191.4 ±12.5 | 0.36 ±0.05 | 6.73 ±1.03 | ||
| Altered | N | - | - | 3 | 25 | - | - | 5 | - | - | |
| Value | - | - | 86.6 ±38.9 | 1.02 * ±0.04 | - | - | 683.2 ±257.9 | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peris, M.P.; Morales, M.; Ares-Gómez, S.; Esteban-Gil, A.; Gómez-Ochoa, P.; Gascón, M.; Moreno, B.; Castillo, J.A. Neutrophil Gelatinase-Associated Lipocalin (NGAL) Is Related with the Proteinuria Degree and the Microscopic Kidney Findings in Leishmania-Infected Dogs. Microorganisms 2020, 8, 1966. https://doi.org/10.3390/microorganisms8121966
Peris MP, Morales M, Ares-Gómez S, Esteban-Gil A, Gómez-Ochoa P, Gascón M, Moreno B, Castillo JA. Neutrophil Gelatinase-Associated Lipocalin (NGAL) Is Related with the Proteinuria Degree and the Microscopic Kidney Findings in Leishmania-Infected Dogs. Microorganisms. 2020; 8(12):1966. https://doi.org/10.3390/microorganisms8121966
Chicago/Turabian StylePeris, María Paz, Mariano Morales, Sonia Ares-Gómez, Adriana Esteban-Gil, Pablo Gómez-Ochoa, Manuel Gascón, Bernardino Moreno, and Juan Antonio Castillo. 2020. "Neutrophil Gelatinase-Associated Lipocalin (NGAL) Is Related with the Proteinuria Degree and the Microscopic Kidney Findings in Leishmania-Infected Dogs" Microorganisms 8, no. 12: 1966. https://doi.org/10.3390/microorganisms8121966
APA StylePeris, M. P., Morales, M., Ares-Gómez, S., Esteban-Gil, A., Gómez-Ochoa, P., Gascón, M., Moreno, B., & Castillo, J. A. (2020). Neutrophil Gelatinase-Associated Lipocalin (NGAL) Is Related with the Proteinuria Degree and the Microscopic Kidney Findings in Leishmania-Infected Dogs. Microorganisms, 8(12), 1966. https://doi.org/10.3390/microorganisms8121966





