Physiological Response of Corynebacterium glutamicum to Indole
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Molecular Genetic Techniques
2.2. Culture Conditions
2.3. Sequencing of Transcriptomis and Genomic Data
2.3.1. RNA Isolation, qRT-PCR, Preparation of cDNA Libraries for Sequencing and DeSeq Analysis
2.3.2. gDNA Isolation, Library Preparation and Sequencing
2.4. Quantification of Amino Acids and Organic Acids by HPLC or GC–MS
2.5. Protein Purification and Electrophoretic Mobility Shift Assay
2.6. Iron Reduction Assay
2.7. Pseudo-Dipeptid Synthesis
3. Results
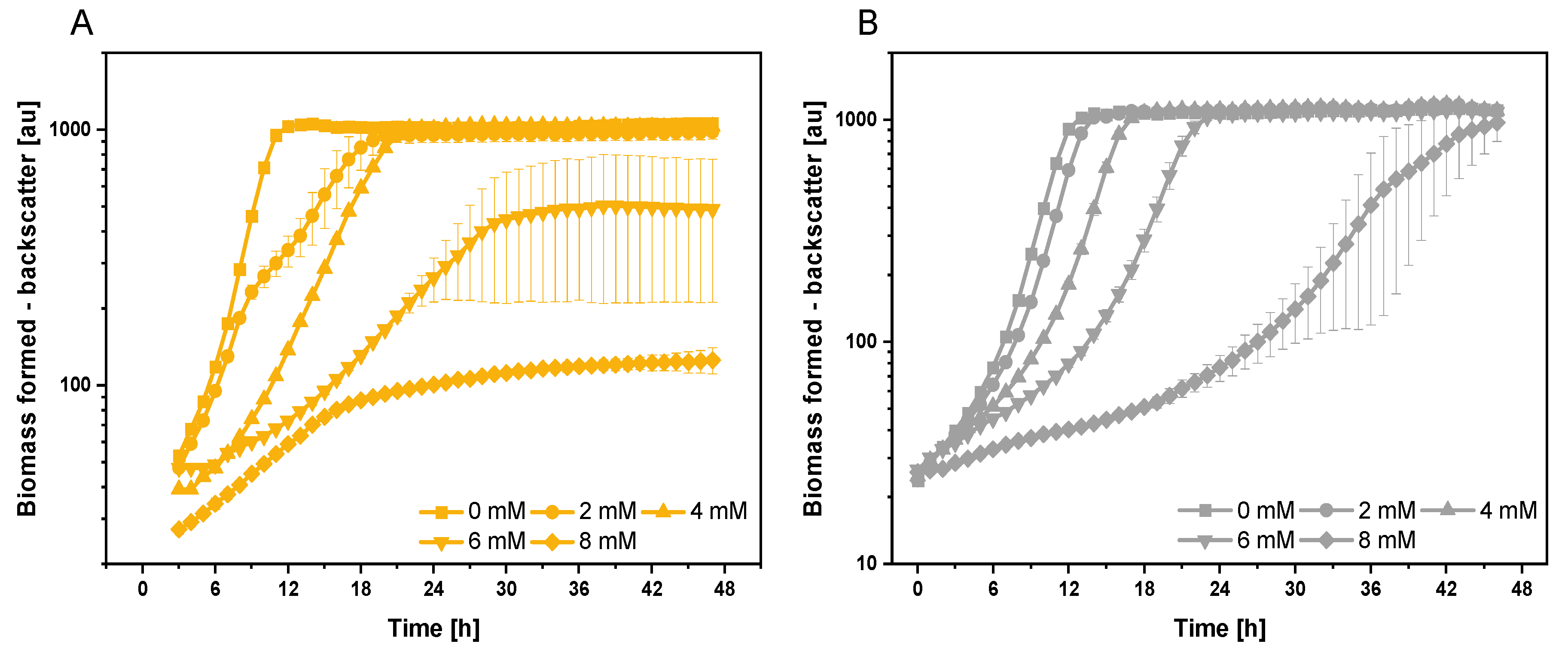
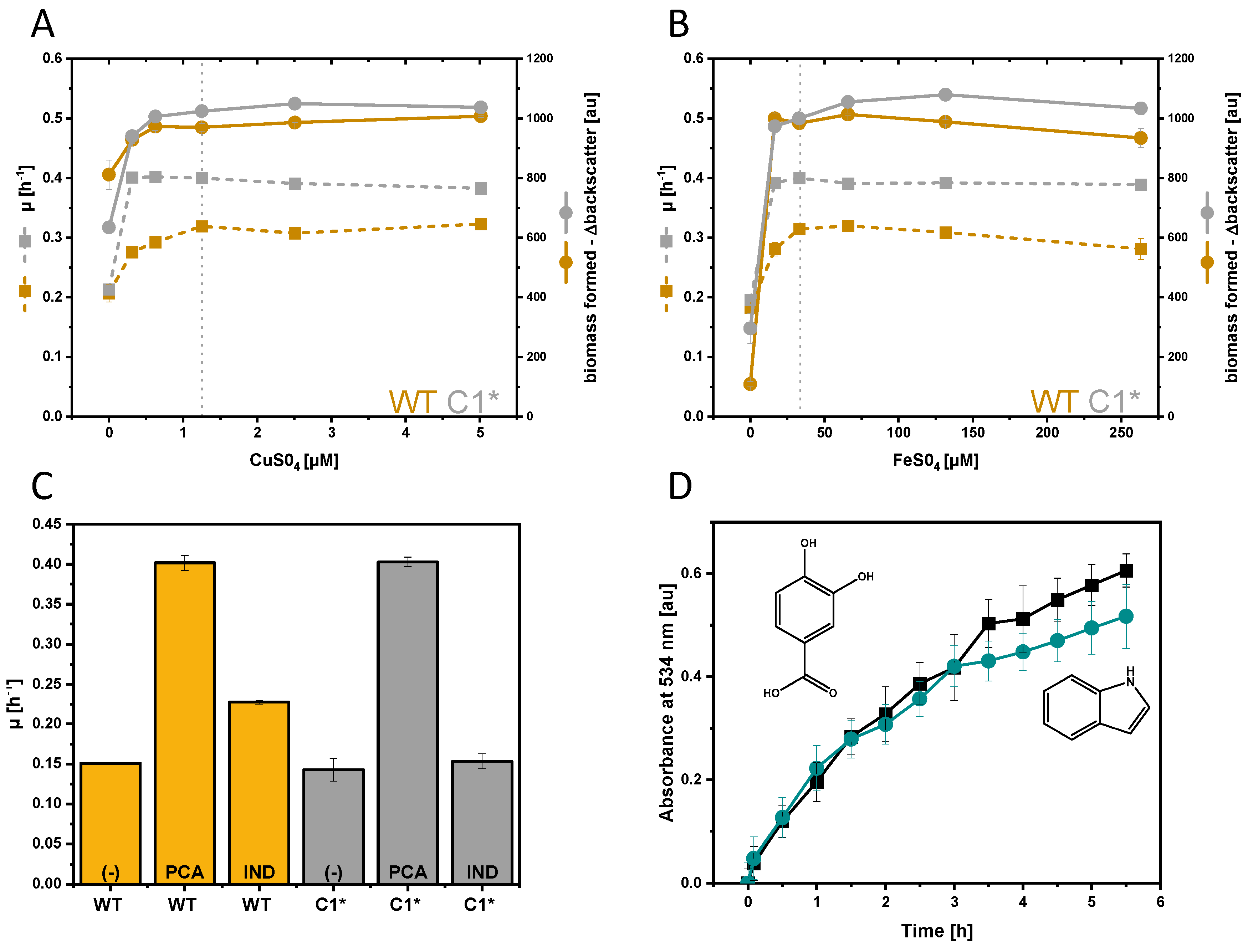
3.1. Growth and Global Gene Expression Response of C. glutamicum to Extracellularly Added Indole
3.2. Transtriptome Analysis of C. glutamicum C1* in Response to Indole-Alanine Dipeptide
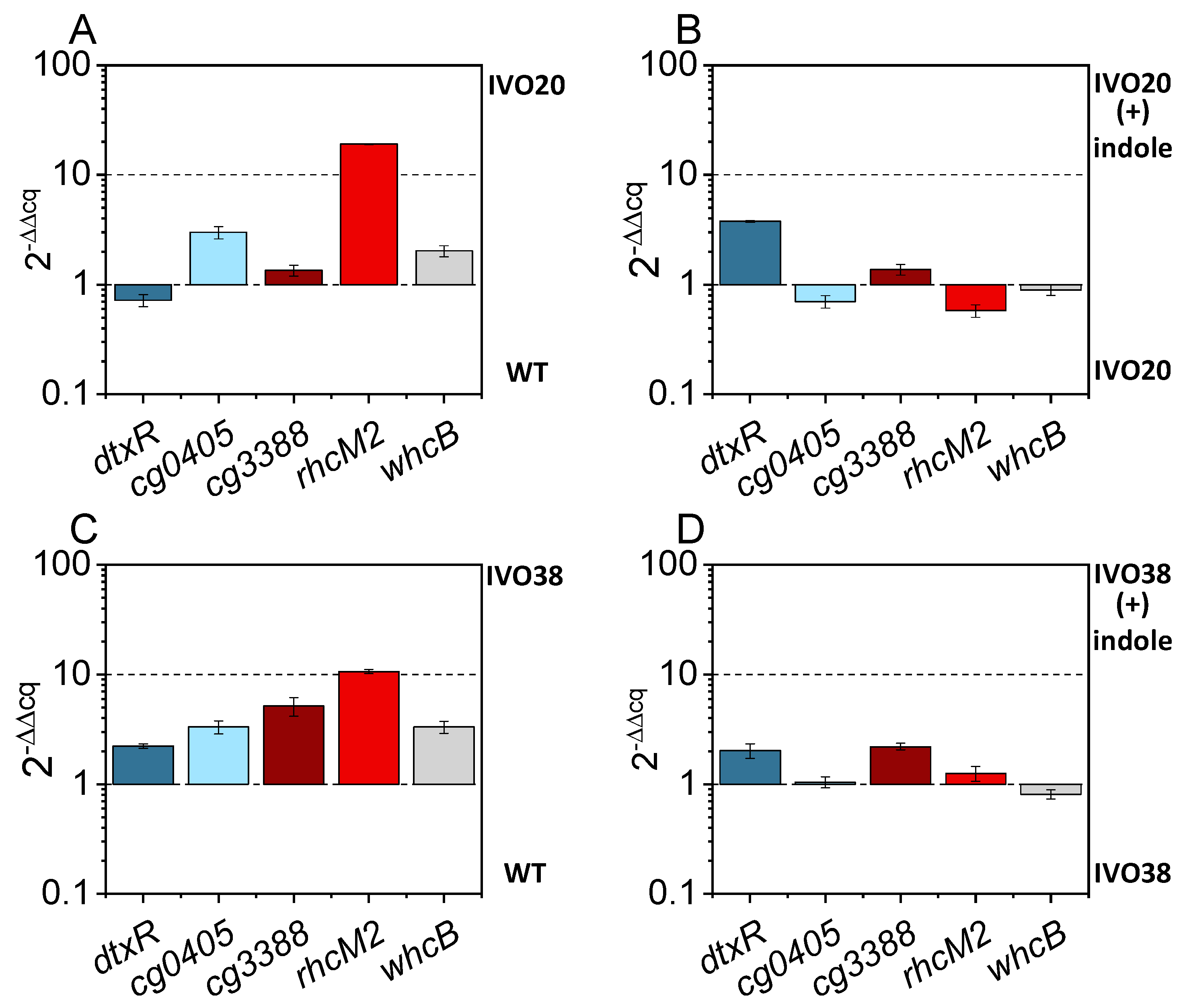
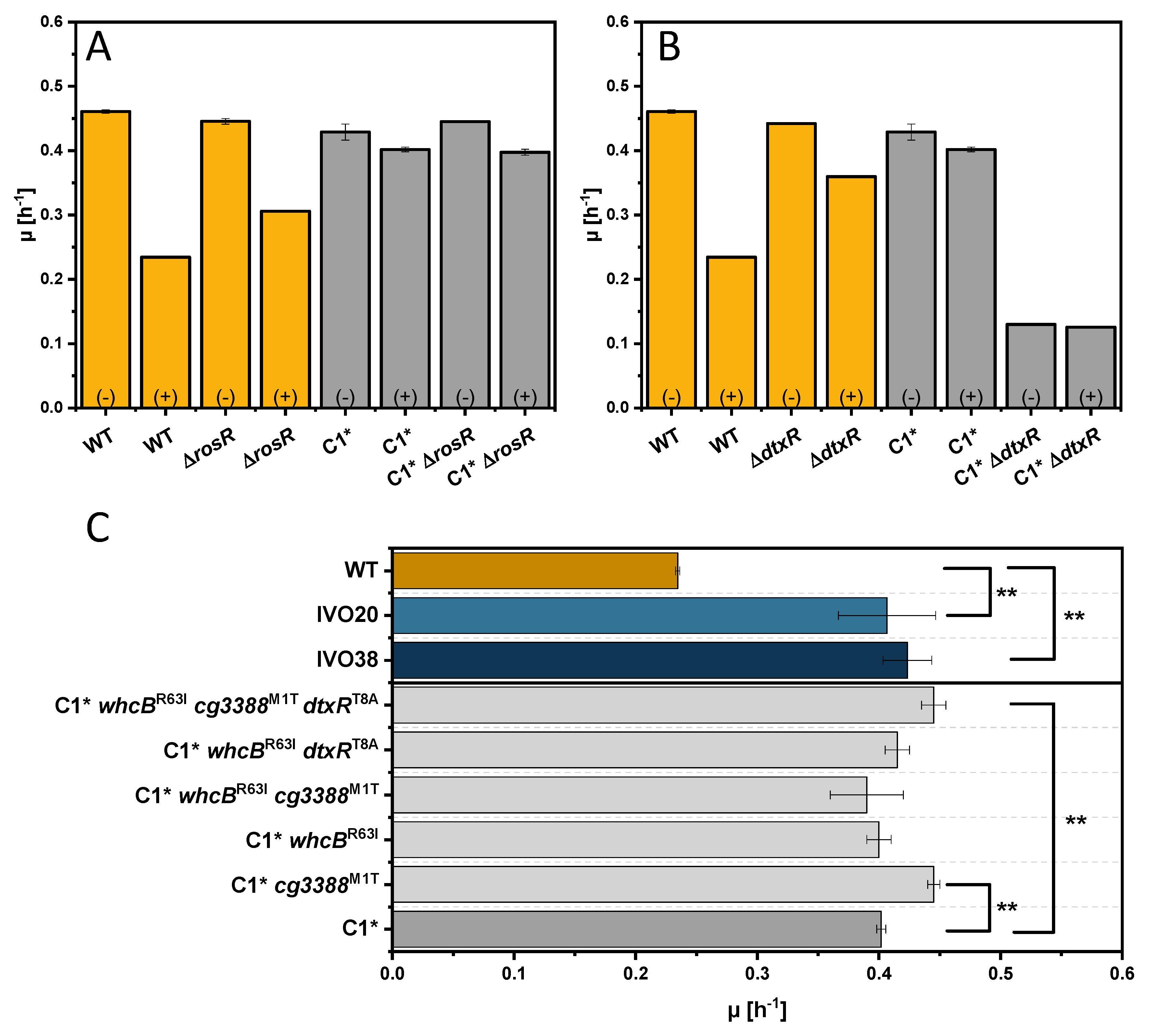
3.3. Adaptive Laboratory Evolution for Increasing Indole Tolerance
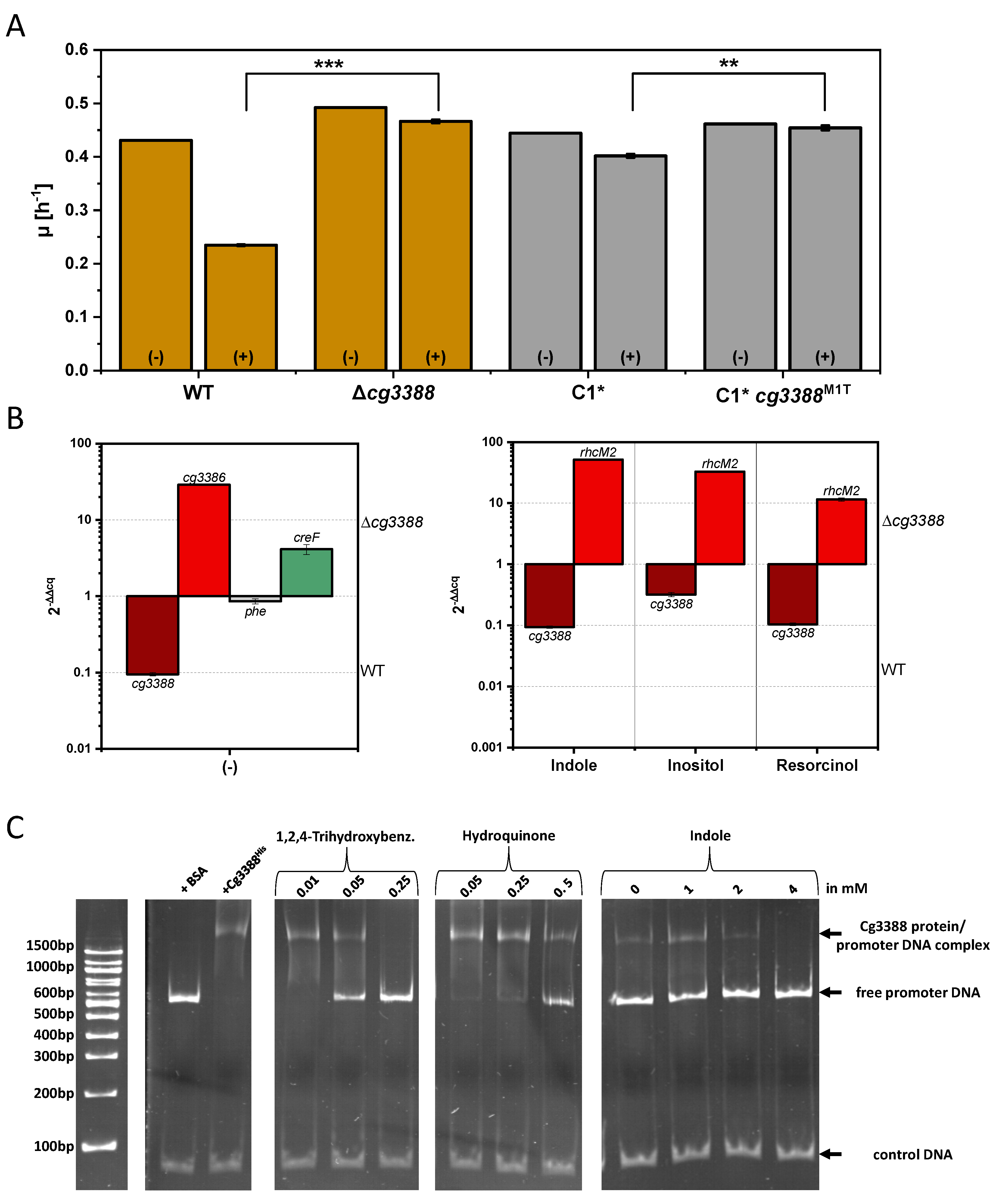
3.4. Investigation of the Transcriptional Regulator Cg3388
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Data Availability
References
- Berger, R.G. (Ed.) Flavours and Fragrances: Chemistry, Bioprocessing and Sustainability; Springer: Berlin/Heidelberg, Germany, 2007; ISBN 978-3-540-49338-9. [Google Scholar] [CrossRef]
- Chandra, S.; Askari, K.; Kumari, M. Optimization of indole acetic acid production by isolated bacteria from Stevia rebaudiana rhizosphere and its effects on plant growth. J. Genet. Eng. Biotechnol. 2018, 16, 581–586. [Google Scholar] [CrossRef]
- Fabara, A.N.; Fraaije, M.W. An overview of microbial indigo-forming enzymes. Appl. Microbiol. Biotechnol. 2020, 104, 925–933. [Google Scholar] [CrossRef]
- Lee, J.-H.; Wood, T.K.; Lee, J. Roles of indole as an interspecies and interkingdom signaling molecule. Trends Microbiol. 2015, 23, 707–718. [Google Scholar] [CrossRef]
- Zarkan, A.; Liu, J.; Matuszewska, M.; Gaimster, H.; Summers, D.K. Local and universal action: The paradoxes of indole signalling in bacteria. Trends Microbiol. 2020, 28, 566–577. [Google Scholar] [CrossRef] [PubMed]
- Frey, M.; Schullehner, K.; Dick, R.; Fiesselmann, A.; Gierl, A. Benzoxazinoid biosynthesis, a model for evolution of secondary metabolic pathways in plants. Phytochemistry 2009, 70, 1645–1651. [Google Scholar] [CrossRef]
- Snell, E.E. Tryptophanase: Structure, catalytic activities, and mechanism of action. In Advances in Enzymology and Related Areas of Molecular Biology; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2006; pp. 287–333. ISBN 978-0-470-12287-7. [Google Scholar] [CrossRef]
- Yanofsky, C.; Horn, V.; Gollnick, P. Physiological studies of tryptophan transport and tryptophanase operon induction in Escherichia coli. J. Bacteriol. 1991, 173, 6009–6017. [Google Scholar] [CrossRef]
- Vega, N.M.; Allison, K.R.; Khalil, A.S.; Collins, J.J. Signaling-mediated bacterial persister formation. Nat. Chem. Biol. 2012, 8, 431–433. [Google Scholar] [CrossRef] [PubMed]
- Piñero-Fernandez, S.; Chimerel, C.; Keyser, U.F.; Summers, D.K. Indole transport across Escherichia coli membranes. J. Bacteriol. 2011, 193, 1793–1798. [Google Scholar] [CrossRef] [PubMed]
- Kawamura-Sato, K.; Shibayama, K.; Horii, T.; Iimuma, Y.; Arakawa, Y.; Ohta, M. Role of multiple efflux pumps in Escherichia coli in indole expulsion. FEMS Microbiol. Lett. 1999, 179, 345–352. [Google Scholar] [CrossRef]
- Ma, Q.; Zhang, X.; Qu, Y. Biodegradation and biotransformation of indole: Advances and perspectives. Front. Microbiol. 2018, 9, 2625. [Google Scholar] [CrossRef]
- Arora, P.K.; Sharma, A.; Bae, H. Microbial degradation of indole and its derivatives. J. Chem. 2015, 2015, 129159. [Google Scholar] [CrossRef]
- Sadauskas, M.; Vaitekūnas, J.; Gasparavičiūtė, R.; Meškys, R. Indole biodegradation in Acinetobacter sp. strain O153: Genetic and biochemical characterization. Appl. Environ. Microbiol. 2017, 83. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.; Ma, Q.; Liu, Z.; Wang, W.; Tang, H.; Zhou, J.; Xu, P. Unveiling the biotransformation mechanism of indole in a Cupriavidus sp. strain. Mol. Microbiol. 2017, 106, 905–918. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Hong, H.; Heo, A.; Park, W. Indole toxicity involves the inhibition of adenosine triphosphate production and protein folding in Pseudomonas putida. FEMS Microbiol. Lett. 2013, 343, 89–99. [Google Scholar] [CrossRef]
- Lee, J.; Zhang, X.-S.; Hegde, M.; Bentley, W.E.; Jayaraman, A.; Wood, T.K. Indole cell signaling occurs primarily at low temperatures in Escherichia coli. ISME J. 2008, 2, 1007–1023. [Google Scholar] [CrossRef]
- Shen, X.-H.; Zhou, N.-Y.; Liu, S.-J. Degradation and assimilation of aromatic compounds by Corynebacterium glutamicum: Another potential for applications for this bacterium? Appl. Microbiol. Biotechnol. 2012, 95, 77–89. [Google Scholar] [CrossRef]
- Lee, S.Y.; Le, T.-H.; Chang, S.-T.; Park, J.-S.; Kim, Y.-H.; Min, J. Utilization of phenol and naphthalene affects synthesis of various amino acids in Corynebacterium glutamicum. Curr. Microbiol. 2010, 61, 596–600. [Google Scholar] [CrossRef]
- Chao, H.; Zhou, N.-Y. GenR, an IclR-type regulator, activates and represses the transcription of gen genes involved in 3-hydroxybenzoate and gentisate catabolism in Corynebacterium glutamicum. J. Bacteriol. 2013, 195, 1598–1609. [Google Scholar] [CrossRef]
- Kallscheuer, N.; Vogt, M.; Kappelmann, J.; Krumbach, K.; Noack, S.; Bott, M.; Marienhagen, J. Identification of the phd gene cluster responsible for phenylpropanoid utilization in Corynebacterium glutamicum. Appl. Microbiol. Biotechnol. 2016, 100, 1871–1881. [Google Scholar] [CrossRef]
- Merkens, H.; Beckers, G.; Wirtz, A.; Burkovski, A. Vanillate metabolism in Corynebacterium glutamicum. Curr. Microbiol. 2005, 51, 59–65. [Google Scholar] [CrossRef]
- Huang, Y.; Zhao, K.-X.; Shen, X.-H.; Chaudhry, M.T.; Jiang, C.-Y.; Liu, S.-J. Genetic characterization of the resorcinol catabolic pathway in Corynebacterium glutamicum. Appl. Environ. Microbiol. 2006, 72, 7238–7245. [Google Scholar] [CrossRef]
- Li, T.; Zhao, K.; Huang, Y.; Li, D.; Jiang, C.-Y.; Zhou, N.; Fan, Z.; Liu, S.-J. The TetR-type transcriptional repressor RolR from Corynebacterium glutamicum regulates resorcinol catabolism by binding to a unique operator, rolO. Appl. Environ. Microbiol. 2012, 78, 6009–6016. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Chen, X.; Chaudhry, M.T.; Zhang, B.; Jiang, C.-Y.; Liu, S.-J. Genetic characterization of 4-cresol catabolism in Corynebacterium glutamicum. J. Biotechnol. 2014, 192, 355–365. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Ma, L.; Qi, F.; Zheng, X.; Jiang, C.; Li, A.; Wan, X.; Liu, S.-J.; Li, S. Characterization of a unique pathway for 4-Cresol catabolism initiated by phosphorylation in Corynebacterium glutamicum. J. Biol. Chem. 2016, 291, 6583–6594. [Google Scholar] [CrossRef] [PubMed]
- Kubota, T.; Tanaka, Y.; Takemoto, N.; Hiraga, K.; Yukawa, H.; Inui, M. Identification and expression analysis of a gene encoding a shikimate transporter of Corynebacterium glutamicum. Microbiology 2015, 161, 254–263. [Google Scholar] [CrossRef]
- Lee, J.-H.; Wendisch, V.F. Biotechnological production of aromatic compounds of the extended shikimate pathway from renewable biomass. J. Biotechnol. 2017, 257, 211–221. [Google Scholar] [CrossRef]
- Shen, X.; Liu, S. Key enzymes of the protocatechuate branch of the β-ketoadipate pathway for aromatic degradation in Corynebacterium glutamicum. Sci. China C Life Sci. 2005, 48, 241–249. [Google Scholar] [CrossRef][Green Version]
- Li, D.-F.; Zhang, N.; Hou, Y.-J.; Huang, Y.; Hu, Y.; Zhang, Y.; Liu, S.-J.; Wang, D.-C. Crystal structures of the transcriptional repressor RolR reveals a novel recognition mechanism between inducer and regulator. PLoS ONE 2011, 6, e19529. [Google Scholar] [CrossRef]
- Feng, J.; Che, Y.; Milse, J.; Yin, Y.-J.; Liu, L.; Rückert, C.; Shen, X.-H.; Qi, S.-W.; Kalinowski, J.; Liu, S.-J. The gene ncgl2918 encodes a novel maleylpyruvate isomerase that needs mycothiol as cofactor and links mycothiol biosynthesis and gentisate assimilation in Corynebacterium glutamicum. J. Biol. Chem. 2006, 281, 10778–10785. [Google Scholar] [CrossRef]
- Shang, X.; Zhang, Y.; Zhang, G.; Chai, X.; Deng, A.; Liang, Y.; Wen, T. Characterization and molecular mechanism of AroP as an aromatic amino acid and histidine transporter in Corynebacterium glutamicum. J. Bacteriol. 2013, 195, 5334–5342. [Google Scholar] [CrossRef]
- Zhao, Z.; Ding, J.-Y.; Li, T.; Zhou, N.-Y.; Liu, S.-J. The ncgl1108 (PheP (Cg)) gene encodes a new l-Phe transporter in Corynebacterium glutamicum. Appl. Microbiol. Biotechnol. 2011, 90, 2005–2013. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, S.-H.; Chao, H.-J.; Liu, S.-J.; Zhou, N.-Y. Biochemical and molecular characterization of the gentisate transporter GenK in Corynebacterium glutamicum. PLoS ONE 2012, 7, e38701. [Google Scholar] [CrossRef] [PubMed]
- Chaudhry, M.T.; Huang, Y.; Shen, X.-H.; Poetsch, A.; Jiang, C.-Y.; Liu, S.-J. Genome-wide investigation of aromatic acid transporters in Corynebacterium glutamicum. Microbiology 2007, 153, 857–865. [Google Scholar] [CrossRef] [PubMed]
- Teramoto, H.; Inui, M.; Yukawa, H. Regulation of expression of genes involved in quinate and shikimate utilization in Corynebacterium glutamicum. Appl. Environ. Microbiol. 2009, 75, 3461–3468. [Google Scholar] [CrossRef]
- Brinkrolf, K.; Brune, I.; Tauch, A. Transcriptional regulation of catabolic pathways for aromatic compounds in Corynebacterium glutamicum. Genet. Mol. Res. 2006, 5, 773–789. [Google Scholar] [PubMed]
- Chen, X.; Kohl, T.A.; Rückert, C.; Rodionov, D.A.; Li, L.-H.; Ding, J.-Y.; Kalinowski, J.; Liu, S.-J. Phenylacetic acid catabolism and its transcriptional regulation in Corynebacterium glutamicum. Appl. Environ. Microbiol. 2012, 78, 5796–5804. [Google Scholar] [CrossRef]
- Heravi, K.M.; Lange, J.; Watzlawick, H.; Kalinowski, J.; Altenbuchner, J. Transcriptional Regulation of the vanillate utilization genes (vanABK Operon) of Corynebacterium glutamicum by VanR, a PadR-like repressor. J. Bacteriol. 2015, 197, 959–972. [Google Scholar] [CrossRef]
- Zhao, K.-X.; Huang, Y.; Chen, X.; Wang, N.-X.; Liu, S.-J. PcaO positively regulates pcaHG of the beta-ketoadipate pathway in Corynebacterium glutamicum. J. Bacteriol. 2010, 192, 1565–1572. [Google Scholar] [CrossRef]
- Kubota, T.; Tanaka, Y.; Takemoto, N.; Watanabe, A.; Hiraga, K.; Inui, M.; Yukawa, H. Chorismate-dependent transcriptional regulation of quinate/shikimate utilization genes by LysR-type transcriptional regulator QsuR in Corynebacterium glutamicum: Carbon flow control at metabolic branch point. Mol. Microbiol. 2014, 92, 356–368. [Google Scholar] [CrossRef]
- Purwanto, S.H.; Kang, M.-S.; Ferrer, L.; Han, S.-S.; Lee, J.-Y.; Kim, H.-S.; Lee, J.-H. Rational engineering of the shikimate and related pathways in Corynebacterium glutamicum for 4-hydroxybenzoate production. J. Biotechnol. 2018, 282, 92–100. [Google Scholar] [CrossRef]
- Kitade, Y.; Hashimoto, R.; Suda, M.; Hiraga, K.; Inui, M. Production of 4-hydroxybenzoic acid by an aerobic growth-arrested bioprocess using metabolically engineered Corynebacterium glutamicum. Appl. Environ. Microbiol. 2018, 84, e02587-17. [Google Scholar] [CrossRef] [PubMed]
- Veldmann, K.H.; Dachwitz, S.; Risse, J.M.; Lee, J.-H.; Sewald, N.; Wendisch, V.F. Bromination of l-tryptophan in a fermentative process with Corynebacterium glutamicum. Front. Bioeng. Biotechnol. 2019, 7. [Google Scholar] [CrossRef]
- Veldmann, K.H.; Minges, H.; Sewald, N.; Lee, J.-H.; Wendisch, V.F. Metabolic engineering of Corynebacterium glutamicum for the fermentative production of halogenated tryptophan. J. Biotechnol. 2019, 291, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Walter, T.; Al Medani, N.; Burgardt, A.; Cankar, K.; Ferrer, L.; Kerbs, A.; Lee, J.-H.; Mindt, M.; Risse, J.M.; Wendisch, V.F. Fermentative N-Methylanthranilate production by engineered Corynebacterium glutamicum. Microorganisms 2020, 8, 866. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.W.; Cho, J.S.; Lee, S.Y. Microbial production of methyl anthranilate, a grape flavor compound. Proc. Natl. Acad. Sci. USA 2019, 116, 10749–10756. [Google Scholar] [CrossRef] [PubMed]
- Milke, L.; Mutz, M.; Marienhagen, J. Synthesis of the character impact compound raspberry ketone and additional flavoring phenylbutanoids of biotechnological interest with Corynebacterium glutamicum. Microb. Cell Fact 2020, 19, 92. [Google Scholar] [CrossRef]
- Hanahan, D. Studies on transformation of Escherichia coli with plasmids. J. Mol. Biol. 1983, 166, 557–580. [Google Scholar] [CrossRef]
- Green, M.R.; Sambrook, J.; Sambrook, J. Molecular Cloning: A Laboratory Manual; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 2012; ISBN 978-1-936113-41-5. [Google Scholar]
- Eggeling, L.; Bott, M. Handbook of Corynebacterium glutamicum; CRC Press: Boca Raton, FL, USA, 2005; ISBN 978-1-4200-3969-6. [Google Scholar]
- Baumgart, M.; Unthan, S.; Kloß, R.; Radek, A.; Polen, T.; Tenhaef, N.; Müller, M.F.; Küberl, A.; Siebert, D.; Brühl, N.; et al. Corynebacterium glutamicum Chassis C1*: Building and besting a bovel platform host for synthetic biology and industrial biotechnology. ACS Synth. Biol. 2018, 7, 132–144. [Google Scholar] [CrossRef]
- Wennerhold, J.; Bott, M. The DtxR regulon of Corynebacterium glutamicum. J. Bacteriol. 2006, 188, 2907–2918. [Google Scholar] [CrossRef]
- Bussmann, M.; Baumgart, M.; Bott, M. RosR (Cg1324), a hydrogen peroxide-sensitive MarR-type transcriptional regulator of Corynebacterium glutamicum. J. Biol. Chem. 2010, 285, 29305–29318. [Google Scholar] [CrossRef]
- Lange, J.; Münch, E.; Müller, J.; Busche, T.; Kalinowski, J.; Takors, R.; Blombach, B. Deciphering the adaptation of Corynebacterium glutamicum in transition from aerobiosis via microaerobiosis to anaerobiosis. Genes 2018, 9, 297. [Google Scholar] [CrossRef]
- Simon, R.; Priefer, U.; Pühler, A. A broad host range mobilization system for in vivo genetic engineering: Transposon mutagenesis in gram negative bacteria. Bio/Technology 1983, 1, 784–791. [Google Scholar] [CrossRef]
- Studier, F.W.; Moffatt, B.A. Use of bacteriophage T7 RNA polymerase to direct selective high-level expression of cloned genes. J. Mol. Biol. 1986, 189, 113–130. [Google Scholar] [CrossRef]
- Schäfer, A.; Tauch, A.; Jäger, W.; Kalinowski, J.; Thierbach, G.; Pühler, A. Small mobilizable multi-purpose cloning vectors derived from the Escherichia coli plasmids pK18 and pK19: Selection of defined deletions in the chromosome of Corynebacterium glutamicum. Gene 1994, 145, 69–73. [Google Scholar] [CrossRef]
- Salis, H.M.; Mirsky, E.A.; Voigt, C.A. Automated design of synthetic ribosome binding sites to control protein expression. Nat. Biotechnol. 2009, 27, 946–950. [Google Scholar] [CrossRef] [PubMed]
- Langmead, B.; Trapnell, C.; Pop, M.; Salzberg, S.L. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 2009, 10, R25. [Google Scholar] [CrossRef]
- Anders, S.; Huber, W. Differential expression analysis for sequence count data. Genome Biol. 2010, 11, R106. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Hilker, R.; Stadermann, K.B.; Schwengers, O.; Anisiforov, E.; Jaenicke, S.; Weisshaar, B.; Zimmermann, T.; Goesmann, A. ReadXplorer 2—Detailed read mapping analysis and visualization from one single source. Bioinformatics 2016, 32, 3702–3708. [Google Scholar] [CrossRef]
- Hilker, R.; Stadermann, K.B.; Doppmeier, D.; Kalinowski, J.; Stoye, J.; Straube, J.; Winnebald, J.; Goesmann, A. ReadXplorer—Visualization and analysis of mapped sequences. Bioinformatics 2014, 30, 2247–2254. [Google Scholar] [CrossRef]
- Bustin, S.A.; Benes, V.; Garson, J.A.; Hellemans, J.; Huggett, J.; Kubista, M.; Mueller, R.; Nolan, T.; Pfaffl, M.W.; Shipley, G.L.; et al. The MIQE guidelines: Minimum information for publication of quantitative real-time PCR experiments. Clin. Chem. 2009, 55, 611–622. [Google Scholar] [CrossRef]
- Higuchi, R.; Dollinger, G.; Walsh, P.S.; Griffith, R. Simultaneous amplification and detection of specific DNA sequences. Bio/Technology 1992, 10, 413–417. [Google Scholar] [CrossRef]
- Seemann, T. Snippy: Fast Bacterial Variant Calling from NGS Reads. Available online: https://github.com/tseemann/snippy (accessed on 17 January 2017).
- Henke, N.A.; Heider, S.A.E.; Hannibal, S.; Wendisch, V.F.; Peters-Wendisch, P. Isoprenoid pyrophosphate-dependent transcriptional regulation of carotenogenesis in Corynebacterium glutamicum. Front. Microbiol. 2017, 8, 633. [Google Scholar] [CrossRef]
- Müller, F.; Rapp, J.; Hacker, A.-L.; Feith, A.; Takors, R.; Blombach, B. CO2/HCO3- accelerates iron reduction through phenolic compounds. mBio 2020, 11. [Google Scholar] [CrossRef]
- Erdmann, A.; Weil, B.; Krämer, R. Lysine secretion by wild-type Corynebacterium glutamicum triggered by dipeptide uptake. Microbiology 1993, 139, 3115–3122. [Google Scholar] [CrossRef]
- Lubitz, D.; Jorge, J.M.P.; Pérez-García, F.; Taniguchi, H.; Wendisch, V.F. Roles of export genes cgmA and lysE for the production of l-arginine and l-citrulline by Corynebacterium glutamicum. Appl. Microbiol. Biotechnol. 2016, 100, 8465–8474. [Google Scholar] [CrossRef]
- Ni, Y.; Shi, F.; Wang, N. Specific γ-aminobutyric acid decomposition by gabP and gabT under neutral pH in recombinant Corynebacterium glutamicum. Biotechnol. Lett. 2015, 37, 2219–2227. [Google Scholar] [CrossRef]
- Lee, J.-Y.; Park, J.-S.; Kim, H.-J.; Kim, Y.; Lee, H.-S. Corynebacterium glutamicum whcB, a stationary phase-specific regulatory gene. FEMS Microbiol. Lett. 2012, 327, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Küberl, A.; Mengus-Kaya, A.; Polen, T.; Bott, M. The iron deficiency response of Corynebacterium glutamicum and a link to thiamine biosynthesis. Appl. Environ. Microbiol. 2020, 86. [Google Scholar] [CrossRef] [PubMed]
- Frunzke, J.; Bramkamp, M.; Schweitzer, J.-E.; Bott, M. Population heterogeneity in Corynebacterium glutamicum ATCC 13032 caused by prophage CGP3. J. Bacteriol. 2008, 190, 5111–5119. [Google Scholar] [CrossRef]
- Netzer, R.; Krause, M.; Rittmann, D.; Peters-Wendisch, P.G.; Eggeling, L.; Wendisch, V.F.; Sahm, H. Roles of pyruvate kinase and malic enzyme in Corynebacterium glutamicum for growth on carbon sources requiring gluconeogenesis. Arch. Microbiol. 2004, 182, 354–363. [Google Scholar] [CrossRef] [PubMed]
- Lindner, S.N.; Seibold, G.M.; Krämer, R.; Wendisch, V.F. Impact of a new glucose utilization pathway in amino acid-producing Corynebacterium glutamicum. Bioeng. Bugs 2011, 2, 291–295. [Google Scholar] [CrossRef] [PubMed]
- Schröder, J.; Tauch, A. Transcriptional regulation of gene expression in Corynebacterium glutamicum: The role of global, master and local regulators in the modular and hierarchical gene regulatory network. FEMS Microbiol. Rev. 2010, 34, 685–737. [Google Scholar] [CrossRef]
- Brune, I.; Werner, H.; Hüser, A.T.; Kalinowski, J.; Pühler, A.; Tauch, A. The DtxR protein acting as dual transcriptional regulator directs a global regulatory network involved in iron metabolism of Corynebacterium glutamicum. BMC Genom. 2006, 7, 21. [Google Scholar] [CrossRef]
- Morosov, X.; Davoudi, C.-F.; Baumgart, M.; Brocker, M.; Bott, M. The copper-deprivation stimulon of Corynebacterium glutamicum comprises proteins for biogenesis of the actinobacterial cytochrome bc1-aa3 supercomplex. J. Biol. Chem. 2018, 293, 15628–15640. [Google Scholar] [CrossRef]
- Chen, C.; Zhang, Y.; Xu, L.; Zhu, K.; Feng, Y.; Pan, J.; Si, M.; Zhang, L.; Shen, X. Transcriptional control of the phenol hydroxylase gene phe of Corynebacterium glutamicum by the AraC-type regulator PheR. Microbiol. Res. 2018, 209, 14–20. [Google Scholar] [CrossRef]
- Xiao, X.; Si, M.; Yang, Z.; Zhang, Y.; Guan, J.; Chaudhry, M.T.; Wang, Y.; Shen, X. Molecular characterization of a eukaryotic-like phenol hydroxylase from Corynebacterium glutamicum. J. Gen. Appl. Microbiol. 2015, 61, 99–107. [Google Scholar] [CrossRef][Green Version]
- Bäumchen, C.; Krings, E.; Bringer, S.; Eggeling, L.; Sahm, H. Myo-inositol facilitators IolT1 and IolT2 enhance d-mannitol formation from d-fructose in Corynebacterium glutamicum. FEMS Microbiol. Lett. 2009, 290, 227–235. [Google Scholar] [CrossRef]
- Brüsseler, C.; Radek, A.; Tenhaef, N.; Krumbach, K.; Noack, S.; Marienhagen, J. The myo-inositol/proton symporter IolT1 contributes to d-xylose uptake in Corynebacterium glutamicum. Bioresour. Technol. 2018, 249, 953–961. [Google Scholar] [CrossRef]
- Klaffl, S.; Brocker, M.; Kalinowski, J.; Eikmanns, B.J.; Bott, M. Complex Regulation of the phosphoenolpyruvate carboxykinase gene pck and characterization of its GntR-type regulator IolR as a repressor of myo-Inositol utilization genes in Corynebacterium glutamicum. J. Bacteriol. 2013, 195, 4283–4296. [Google Scholar] [CrossRef]

| Strains | Description | Source |
|---|---|---|
| Corynebacterium glutamicum | ||
| WT | C. glutamicum wild type ATCC13032 | ATCC |
| C1* | Genome-reduced chassis strain derived from WT | [52] |
| ΔdtxR | WT containing dtxR deletion | [53] |
| ΔrosR | WT containing rosR deletion | [54] |
| Δcg3388 | WT containing cg3388/ihtR deletion | [55] |
| C1* ΔdtxR | C1* containing dtxR deletion | This study |
| C1* ΔrosR | C1* containing rosR deletion | This study |
| IVO20 | Strain evolved from WT after 20 transfers in the presence of indole | This study |
| IVO38 | Strain evolved from WT after 38 transfers in the presence of indole | This study |
| C1* cg3388M1T | C1* with SNP in cg3388/ihtR, resulting in amino acid exchange M1T | This study |
| C1* whcBR63L | C1* with SNP in whcB, resulting in amino acid exchange R63L | This study |
| C1* cg3388M1T dtxRT8A | C1* with SNP in cg3388/ihtR and dtxR, resulting in amino acid exchange M1T and T8A | This study |
| C1* cg3388M1T whcBR63L | C1* with SNP in cg3388/ihtR and whcB, resulting in amino acid exchanges M1T and R63L, respectively | This study |
| C1* cg3388M1T dtxRT8A whcBR63L | C1* with SNPs in cg3388/ihtR, dtxR and whcB, resulting in amino acid exchanges M1T, T8A and R63L, respectively | This study |
| C1* (pVWEx1) | C1* carrying pVWEx1 | This study |
| C1* (pVWEx1-phe) | C1* carrying pVWEx1-phe | This study |
| C1* Δphe (pVWEx1) | C1* containing phe deletion, carrying pVWEx1 | This study |
| C1* Δphe (pVWEx1-phe) | C1* containing phe deletion, carrying pVWEx1-phe | This study |
| C1* (pEKEx3) | C1* carrying pEKEx3 | This study |
| C1* (pEKEx3-cg2796-cg2797) | C1* carrying pEKEx3-cg2796-cg2797 | This study |
| C1* Δcg2796-cg2797 (pEKEx3) | C1* containing cg2796-cg2797 deletion, carrying pEKEx3 | This study |
| C1* Δcg2796-cg2797 (pEKEx3-cg2796-cg2797) | C1* containing cg2796-cg2797 deletion, carrying pEKEx3-cg2796-cg2797 | This study |
| Escherichia coli | ||
| S17-1 | recA pro hsdR RP4-2-Tc::Mu-Km::Tn7 | [56] |
| DH5α | F-thi-1 endA1 hsdr17(r-, m-) supE44 1lacU169 (Φ80lacZ1M15) recA1 gyrA96 | [49] |
| BL21 (DE3) | F– ompT gal dcm lon hsdSB(rB–mB–) λ(DE3[lacI lacUV5-T7p07 ind1 sam7 nin5])[malB+]K-12(λS) | [57] |
| Locus | Gene | Gene Product | Ind/- |
|---|---|---|---|
| cg0018 | uncharacterized membrane protein | 4.09 | |
| cg0405 | ABC-type Fe3+-siderophore transport systems | 2.57 | |
| cg0470 | htaB | heme binding protein | 3.12 |
| cg0471 | htaC | heme binding protein | 2.91 |
| cg0637 | creC | benzylaldehyde dehydrogenases | 2.85 |
| cg0638 | creD | phosphohydrolase | 3.11 |
| cg0639 | creE | class I P450 system subunit | 3.59 |
| cg0640 | creF | class I P450 system subunit | 3.81 |
| cg0641 | creG | NAD+-dependent 4-hydroxybenzyl alcohol dehydrogenase | 3.01 |
| cg0642 | creH | 4-methylbenzyl phosphate synthase subunit | 3.60 |
| cg0644 | creI | 4-methylbenzyl phosphate synthase subunit | 3.95 |
| cg0645 | creJ | class I P450 system subunit | 3.96 |
| cg1120 | ripA | AraC-type DNA-binding domain-containing proteins | 2.47 |
| cg1152 | seuB | acyl-CoA dehydrogenases | 3.20 |
| cg1298 | cydC | ATP-binding/permease protein | 3.69 |
| cg1299 | cydD | ATP-binding/permease protein | 3.77 |
| cg1300 | cydB | cytochrome bd-type quinol oxidase, subunit 2 | 3.84 |
| cg1301 | cydA | cytochrome bd-type quinol oxidase, subunit 1 | 3.72 |
| cg1773 | ctaB | polyprenyltransferase | 2.45 |
| cg1881 | predicted iron-dependent peroxidase | 3.15 | |
| cg1883 | uncharacterized secreted protein | 3.15 | |
| cg1884 | copC | membrane-bound copper chaperone | 3.08 |
| cg1930 | hypothetical protein | 3.46 | |
| cg1931 | hypothetical protein | 2.72 | |
| cg2678 | ABC-type transporter. periplasmic component | 2.44 | |
| cg2796 | uncharacterized protein involved in propionate catabolism | 6.87 | |
| cg2797 | uncharacterized ACR | 6.29 | |
| cg2962 | putative enzyme synthesing extracellular polysaccharides | 3.69 | |
| cg2966 | phe | putative phenol 2-monooxygenase | 6.14 |
| cg3280 | uncharacterized secreted protein | 2.70 | |
| cg3281 | copB | cation transport ATPases | 2.67 |
| cg3286 | hypothetical protein | 3.09 | |
| cg3287 | copO | multicopper oxidase | 3.11 |
| cg3289 | thioredoxin-like protein | 3.82 | |
| cg3327 | dps | starvation-inducible DNA-binding protein | 4.40 |
| Locus | Gene | Gene Product | Ind-Ala/- |
|---|---|---|---|
| cg0012 | ssuR | transcriptional activator of sulfonate(ester) utilization | 1.84 |
| cg0018 | uncharacterized membrane protein | 5.36 | |
| cg0120 | putative hydrolase | 1.66 | |
| cg0175 | putative secreted protein | 1.85 | |
| cg0192 | hypothetical protein | 1.97 | |
| cg0286 | putative membrane protein | −2.23 | |
| cg0464 | putative Cu2+ transporting P-type ATPase | 1.59 | |
| cg0569 | cation transporting ATPase | 2.05 | |
| cg0759 | prpD2 | methylcitrate dehydratase, involved in propionate catabolism | 2.38 |
| cg0760 | prpB2 | methylisocitrate lyase, involved in propionate metabolism | 2.53 |
| cg0762 | prpC2 | methylcitrate synthase, involved in propionate catabolism | 2.57 |
| cg0796 | prpD1 | putative (2-methyl) citrate dehydratase | 1.65 |
| cg0797 | prpB1 | putative (methyl)isocitrate lyase | 1.82 |
| cg0798 | prpC1 | putative (methyl)citrate synthase | 1.76 |
| cg1279 | putative secreted protein | 2.05 | |
| cg1327 | putative transcriptional regulator, Crp-family | 1.53 | |
| cg1328 | putative heavy-metal ion transporting P-type ATPase | 1.72 | |
| cg1393 | putative acetyltransferase, GNAT family | 1.78 | |
| cg1470 | hypothetical protein | 2.58 | |
| cg1471 | hypothetical protein | 1.51 | |
| cg1635 | putative membrane protein | 1.51 | |
| cg1710 | bacA | undecaprenol kinase | −1.77 |
| cg2096 | putative membrane protein | −1.59 | |
| cg2391 | aroG | 3-deoxy-7-phosphoheptulonate synthase | −1.54 |
| cg2500 | putative transcriptional regulator. ArsR-family | 1.59 | |
| cg2559 | aceB | malate synthase | 1.79 |
| cg2560 | aceA | isocitrate lyase | 2.34 |
| cg2719 | putative enterochelin esterase | −1.6 | |
| cg2836 | sucD | succinate-CoA ligase (ADP-forming), alpha subunit | 2.06 |
| cg2837 | sucC | succinate-CoA ligase (ADP-forming), beta subunit | 2.23 |
| cg2962 | putative enzyme synthesing extracellular polysaccharides | 2.12 | |
| cg2966 | phe | putative phenol 2-monooxygenase | 2.08 |
| cg3169 | pck | phosphoenolpyruvate carboxykinase (GTP) | 2.00 |
| cg3195 | putative flavin-containing monooxygenase | 1.60 | |
| cg3202 | farR | transcriptional regulator. GntR-family | 1.88 |
| cg3226 | putative MFS-type L-lactate permease | 2.05 | |
| cg3303 | putative PadR-family transcriptional regulator | 2.26 | |
| cg3327 | dps | starvation-inducible DNA-binding protein | 1.85 |
| cg3367 | ABC-type multidrug transport system, ATPase | 1.72 | |
| cg3402 | copper chaperone | 2.03 | |
| cg3431 | rnpA | ribonuclease P | −1.70 |
| cg4019 | hypothetical protein | 3.57 | |
| cg4028 | hypothetical protein | 2.05 |
| Locus | Gene Name | Amino Acid Exchange | Gene Product |
|---|---|---|---|
| cg0695 | whcB | R63L in IVO20 | Stationary phase repressor protein |
| cg2103 | dtxR | T8A in IVO20 | Transcriptional iron homeostasis repressor |
| cg3388 | - | M1T in IVO20 | Putative transcriptional regulator. IclR-family |
| cg0566 | gabT | insertion in IVO20 (ACCGCAT pos. 17 to 23) | 4-aminobutyrate aminotransferase |
| cg1324 | rosR | T2I in IVO38 | Transcriptional regulator of oxidative stress response |
| cg1420 | gatB | D452G in IVO38 | Glutamyl-tRNA (Gln) amidotransferase. subunit B |
| cg2103 | dtxR | R103H in IVO38 | Transcriptional iron homeostasis repressor |
| cg3132 | - | V310A in IVO38 | Putative membrane protein |
| cg3388 | - | G69D in IVO38 | Putative transcriptional regulator. IclR-family |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Walter, T.; Veldmann, K.H.; Götker, S.; Busche, T.; Rückert, C.; Kashkooli, A.B.; Paulus, J.; Cankar, K.; Wendisch, V.F. Physiological Response of Corynebacterium glutamicum to Indole. Microorganisms 2020, 8, 1945. https://doi.org/10.3390/microorganisms8121945
Walter T, Veldmann KH, Götker S, Busche T, Rückert C, Kashkooli AB, Paulus J, Cankar K, Wendisch VF. Physiological Response of Corynebacterium glutamicum to Indole. Microorganisms. 2020; 8(12):1945. https://doi.org/10.3390/microorganisms8121945
Chicago/Turabian StyleWalter, Tatjana, Kareen H. Veldmann, Susanne Götker, Tobias Busche, Christian Rückert, Arman Beyraghdar Kashkooli, Jannik Paulus, Katarina Cankar, and Volker F. Wendisch. 2020. "Physiological Response of Corynebacterium glutamicum to Indole" Microorganisms 8, no. 12: 1945. https://doi.org/10.3390/microorganisms8121945
APA StyleWalter, T., Veldmann, K. H., Götker, S., Busche, T., Rückert, C., Kashkooli, A. B., Paulus, J., Cankar, K., & Wendisch, V. F. (2020). Physiological Response of Corynebacterium glutamicum to Indole. Microorganisms, 8(12), 1945. https://doi.org/10.3390/microorganisms8121945






