Mycobacterium microti Interferes with Bovine Tuberculosis Surveillance
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Statement
2.2. Ante-Mortem Tests
2.3. Post-Mortem Diagnosis
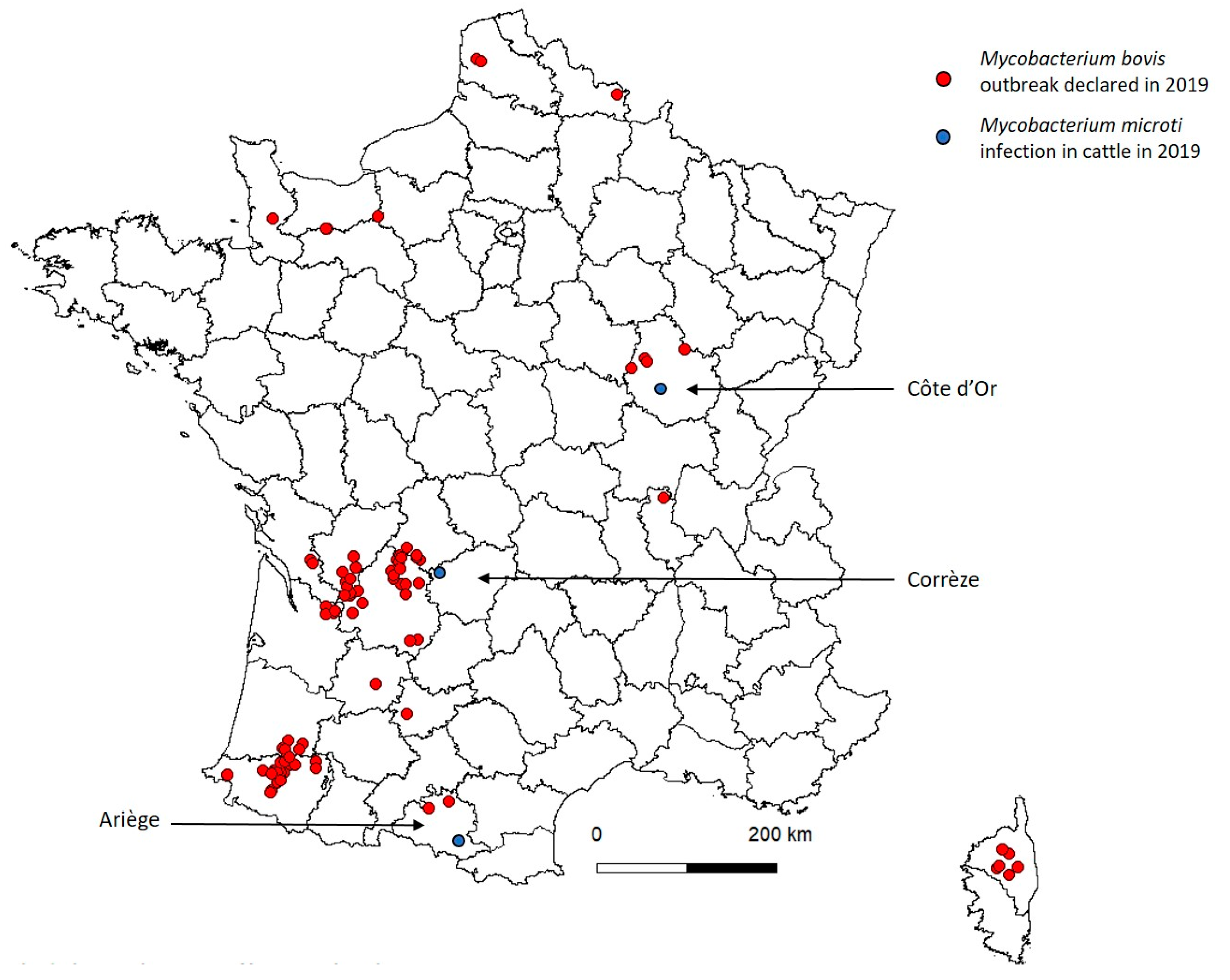
3. Results
3.1. Case 1
3.2. Case 2
3.3. Case 3
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Delavenne, C.; Pandolfi, F.; Girard, S.; Réveillaud, E.; Jabert, P.; Boschiroli, L.; Dommergues, L.; Garapin, F.; Keck, N.; Martin, F.; et al. Bovine tuberculosis: Results and analysis of the epidemiological status of metropolitan France between 2015 and 2017. Bull. Epid Santé Anim. Alim. 2020, 85. [Google Scholar]
- Reveillaud, E.; Desvaux, S.; Boschiroli, M.L.; Hars, J.; Faure, E.; Fediaevsky, A.; Cavalerie, L.; Chevalier, F.; Jabert, P.; Poliak, S.; et al. Infection of Wildlife by Mycobacterium bovis in France Assessment Through a National Surveillance System, Sylvatub. Front. Vet. Sci. 2018, 5, 262. [Google Scholar] [CrossRef] [PubMed]
- Michelet, L.; de Cruz, K.; Zanella, G.; Aaziz, R.; Bulach, T.; Karoui, C.; Henault, S.; Joncour, G.; Boschiroli, M.L. Infection with Mycobacterium microti in animals in France. J. Clin. Microbiol. 2015, 53, 981–985. [Google Scholar] [CrossRef] [PubMed]
- Michelet, L.; de Cruz, K.; Phalente, Y.; Karoui, C.; Henault, S.; Boschiroli, M.L. Mycobacterium microti detection in French wildlife. Vet. Rec. 2015, 177, 446. [Google Scholar] [CrossRef] [PubMed]
- Michelet, L.; de Cruz, K.; Phalente, Y.; Karoui, C.; Henault, S.; Beral, M.; Boschiroli, M.L. Mycobacterium microti Infection in Dairy Goats, France. Emerg. Infect. Dis. 2016, 22, 569–570. [Google Scholar] [CrossRef] [PubMed]
- Michelet, L.; de Cruz, K.; Karoui, C.; Henault, S.; Boschiroli, M.L. Mycobacterium microti infection in a cow in France. Vet. Rec. 2017, 180, 429. [Google Scholar]
- Michelet, L.; de Cruz, K.; Karoui, C.; Tambosco, J.; Moyen, J.L.; Henault, S.; Boschiroli, M.L. Second line molecular diagnosis for bovine tuberculosis to improve diagnostic schemes. PLoS ONE 2018, 13, e0207614. [Google Scholar] [CrossRef] [PubMed]
- Lesellier, S.; Boschiroli, M.L.; Barrat, J.; Wanke, C.; Salguero, F.J.; Garcia-Jimenez, W.L.; Nunez, A.; Godinho, A.; Spiropoulos, J.; Palmer, S.; et al. Detection of live M. bovis BCG in tissues and IFN-gamma responses in European badgers (Meles meles) vaccinated by oropharyngeal instillation or directly in the ileum. BMC Vet. Res. 2019, 15, 445. [Google Scholar] [CrossRef]
- Zhang, J.; Abadia, E.; Refregier, G.; Tafaj, S.; Boschiroli, M.L.; Guillard, B.; Andremont, A.; Ruimy, R.; Sola, C. Mycobacterium tuberculosis complex CRISPR genotyping: Improving efficiency, throughput and discriminative power of ‘spoligotyping’ with new spacers and a microbead-based hybridization assay. J. Med. Microbiol. 2010, 59, 285–294. [Google Scholar] [CrossRef]
- Smith, N.H.; Upton, P. Naming spoligotype patterns for the RD9-deleted lineage of the Mycobacterium tuberculosis complex; www.Mbovis.org. Infect. Genet. Evol. 2012, 12, 873–876. [Google Scholar] [CrossRef] [PubMed]
- Perez de Val, B.; Sanz, A.; Soler, M.; Allepuz, A.; Michelet, L.; Boschiroli, M.L.; Vidal, E. Mycobacterium microti Infection in Free-Ranging Wild Boar, Spain, 2017–2019. Emerg. Infect. Dis. 2019, 25, 2152–2154. [Google Scholar] [CrossRef] [PubMed]
- Jahans, K.; Palmer, S.; Inwald, J.; Brown, J.; Abayakoon, S. Isolation of Mycobacterium microti from a male Charolais-Hereford cross. Vet. Rec. 2004, 155, 373–374. [Google Scholar]
- Faye, S.; Moyen, J.L.; Gares, H.; Benet, J.J.; Garin-Bastuji, B.; Boschiroli, M.L. Determination of decisional cut-off values for the optimal diagnosis of bovine tuberculosis with a modified IFNgamma assay (Bovigam(R)) in a low prevalence area in France. Vet. Microbiol. 2011, 151, 60–67. [Google Scholar] [CrossRef][Green Version]
- Srinivasan, S.; Jones, G.; Veerasami, M.; Steinbach, S.; Holder, T.; Zewude, A.; Fromsa, A.; Ameni, G.; Easterling, L.; Bakker, D.; et al. A defined antigen skin test for the diagnosis of bovine tuberculosis. Sci. Adv. 2019, 5, eaax4899. [Google Scholar] [CrossRef] [PubMed]
- Oevermann, A.; Pfyffer, G.E.; Zanolari, P.; Meylan, M.; Robert, N. Generalized tuberculosis in llamas (Lama glama) due to Mycobacterium microti. J. Clin. Microbiol. 2004, 42, 1818–1821. [Google Scholar] [CrossRef] [PubMed]
- Palgrave, C.J.; Benato, L.; Eatwell, K.; Laurenson, I.F.; Smith, N.H. Mycobacterium microti infection in two meerkats (Suricata suricatta). J. Comp. Pathol. 2012, 146, 278–282. [Google Scholar] [CrossRef]
- Rufenacht, S.; Bogli-Stuber, K.; Bodmer, T.; Jaunin, V.F.; Jmaa, D.C.; Gunn-Moore, D.A. Mycobacterium microti infection in the cat: A case report, literature review and recent clinical experience. J. Feline Med. Surg. 2011, 13, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Hauer, A.; De Cruz, K.; Cochard, T.; Godreuil, S.; Karoui, C.; Henault, S.; Bulach, T.; Banuls, A.L.; Biet, F.; Boschiroli, M.L. Genetic evolution of Mycobacterium bovis causing tuberculosis in livestock and wildlife in France since 1978. PLoS ONE 2015, 10, e0117103. [Google Scholar] [CrossRef] [PubMed]
| Skin-Fold Thickness at the Bovine PPD Injection (DB 1 = B3 − B0) | Difference of Skin-Fold Thickness at the Points of Bovine PPD Injection (DB1 = B3 − B0) and Avian PPD Injection (DA 2 = A3 − A0) | Result of SICCT |
|---|---|---|
| DB 1 > 2 mm | DB 1 − DA 2 > 4 mm | Positive |
| 1 mm ≤ DB 1 − DA 2 ≤ 4 mm | Doubtful | |
| DB 1 − DA 2 < 1 mm | Negative | |
| DB 1 ≤ 2 mm | Negative |
| Cattle | DA | DB | DB-DA | Interpretation | MTBC PCR | Molecular Identification |
|---|---|---|---|---|---|---|
| 1 | 1.9 | 3.7 | 1.8 | Doubtful | Positive (RP LN 1) | M. microti SB0118 |
| 2 | 0.7 | 2.8 | 2.1 | Doubtful | Negative | NA 3 |
| 3 | 0 | 2.7 | 2.7 | Doubtful | Negative | NA 3 |
| 4 | 1.9 | 4.9 | 3 | Doubtful | Negative | NA 3 |
| 5 | 0.4 | 9 | 8.6 | Positive | Negative | NA 3 |
| 6 | 0.5 | 4.8 | 4.3 | Positive | Positive (TB NL 2) | M. microti SB0118 |
| 7 | 1.5 | 3 | 1.5 | Doubtful | Negative | NA 3 |
| 8 | 1.5 | 3.3 | 1.8 | Doubtful | Negative | NA 3 |
| 9 | 0.1 | 7.3 | 7.2 | Positive | Negative | NA 3 |
| Case 1a | Case 1b | Case 2 | Case 3 | |
|---|---|---|---|---|
| Geographic location | Côte d’Or | Côte d’Or | Corrèze | Ariège |
| Breed | Salers | Charolaise | Limousine | Gasconne |
| Age | 2 years | 2 years | 4 years | 5 years |
| Skin test result | Doubtful | Positive | Doubtful | Positive |
| Presence of lesion | NVL | NVL | NVL | NVL |
| Infected lymph node | RP | TB | MD | RP/TB |
| First line PCR | Positive (CT 29) | Positive (CT 34) | Positive (CT 32) | Positive (CT 27) |
| NRL identification | M. microti SB0118 | M. microti SB0118 | M. microti SB0118 | M. microti SB0112 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Michelet, L.; de Cruz, K.; Tambosco, J.; Hénault, S.; Boschiroli, M.L. Mycobacterium microti Interferes with Bovine Tuberculosis Surveillance. Microorganisms 2020, 8, 1850. https://doi.org/10.3390/microorganisms8121850
Michelet L, de Cruz K, Tambosco J, Hénault S, Boschiroli ML. Mycobacterium microti Interferes with Bovine Tuberculosis Surveillance. Microorganisms. 2020; 8(12):1850. https://doi.org/10.3390/microorganisms8121850
Chicago/Turabian StyleMichelet, Lorraine, Krystel de Cruz, Jennifer Tambosco, Sylvie Hénault, and Maria Laura Boschiroli. 2020. "Mycobacterium microti Interferes with Bovine Tuberculosis Surveillance" Microorganisms 8, no. 12: 1850. https://doi.org/10.3390/microorganisms8121850
APA StyleMichelet, L., de Cruz, K., Tambosco, J., Hénault, S., & Boschiroli, M. L. (2020). Mycobacterium microti Interferes with Bovine Tuberculosis Surveillance. Microorganisms, 8(12), 1850. https://doi.org/10.3390/microorganisms8121850





