Transcriptional Landscape of Waddlia chondrophila Aberrant Bodies Induced by Iron Starvation
Abstract
1. Introduction
2. Material and Methods
2.1. Cell Culture and Bacterial Strains
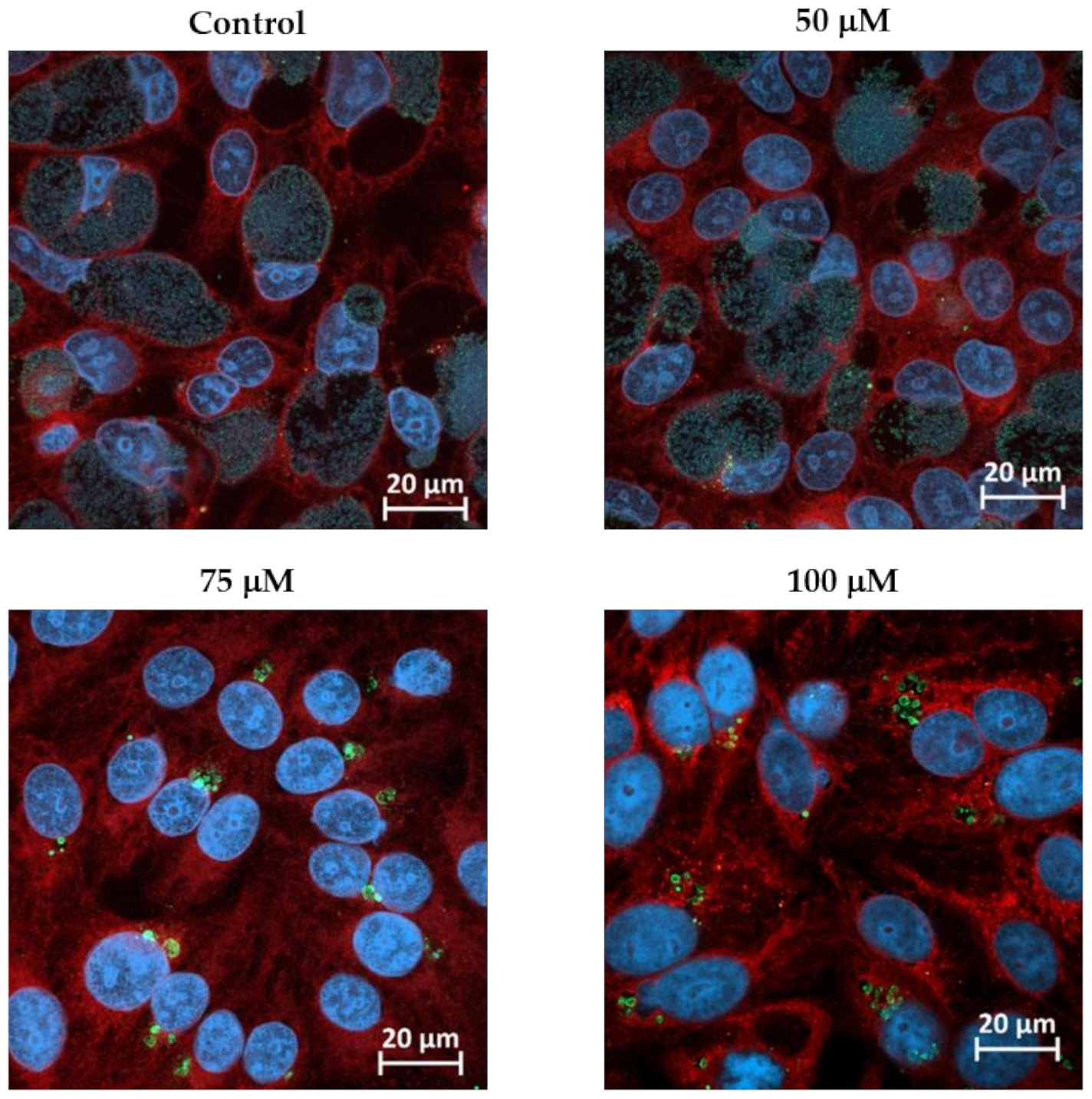
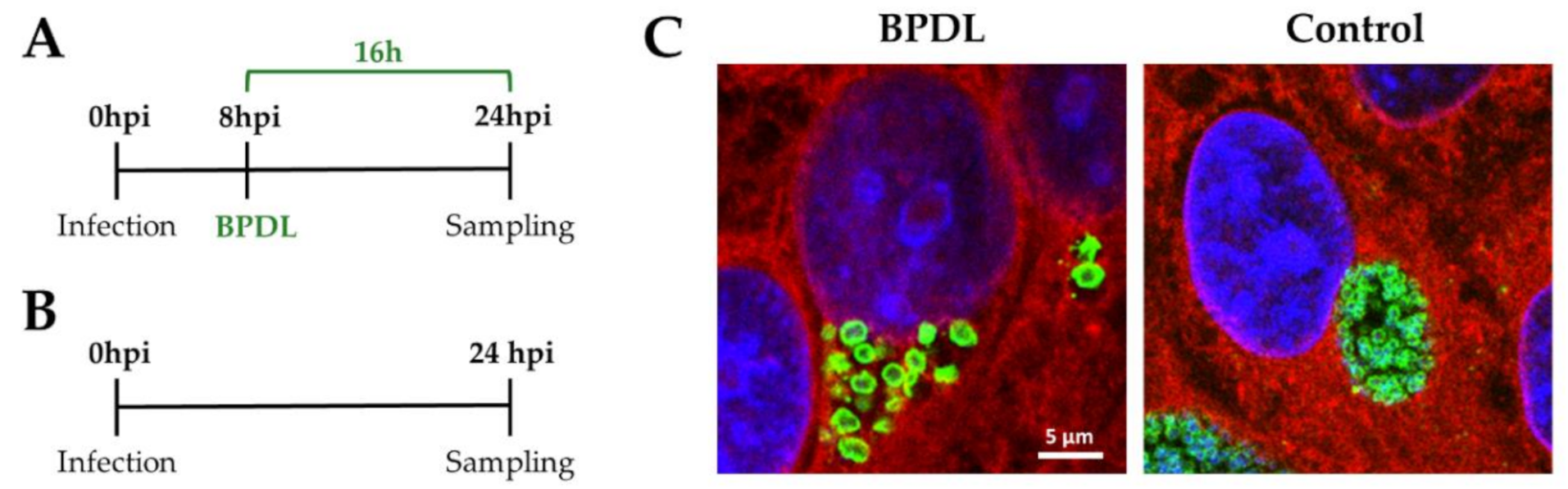
2.2. Infection Procedure and 2,2′-bipyridyl Treatment
2.3. Immunofluorescence Staining
2.4. Cell Viability Assay
2.5. RNA Extraction and Sequencing
2.6. Sequence Read Processing and Gene Expression Analysis
3. Results
3.1. BPDL Treatment Induces the Formation of Aberrant Bodies in W. chondrophila
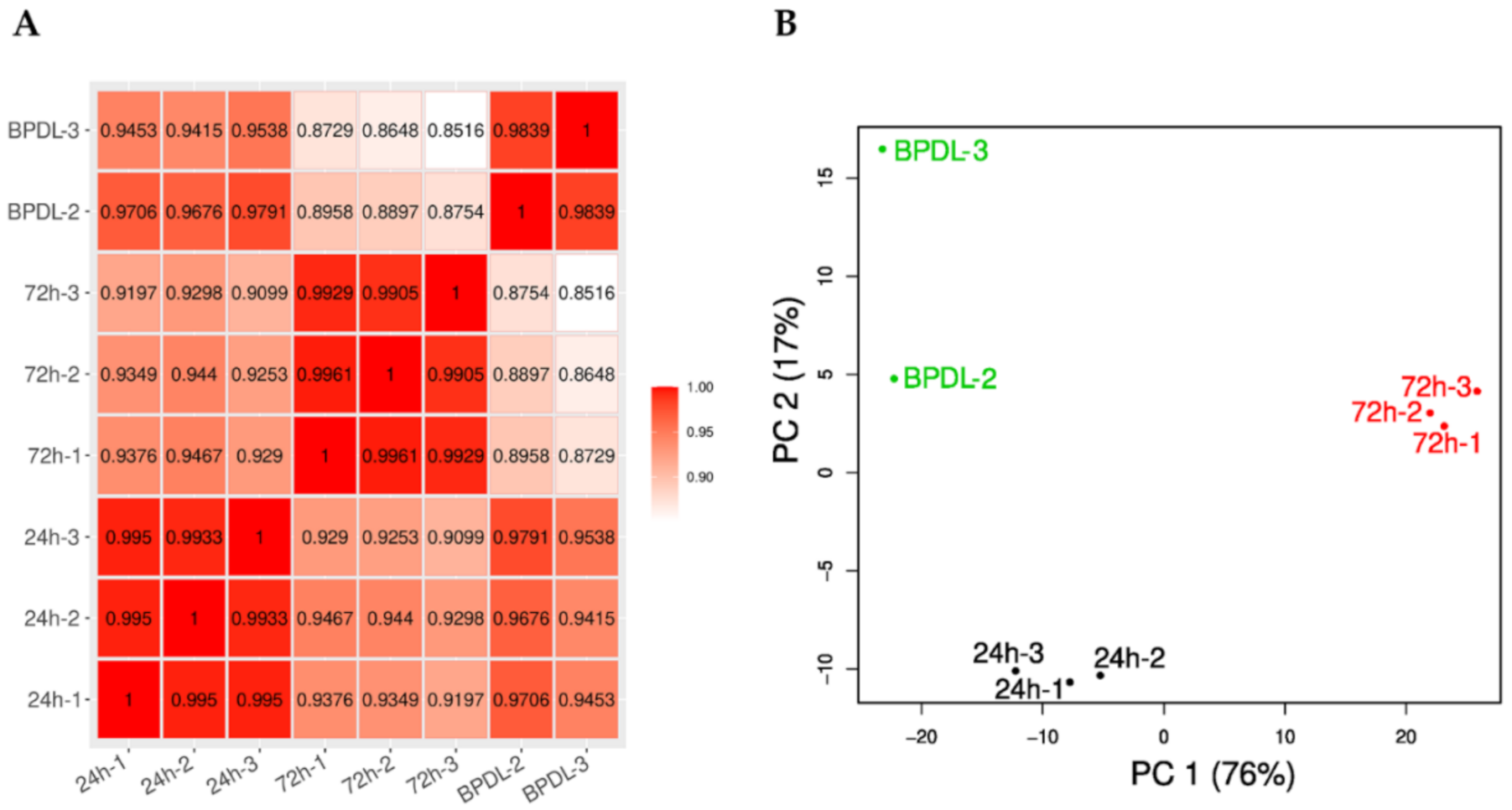
3.2. Optimization of the Conditions for the RNA Sequencing Upon BPDL Treatment
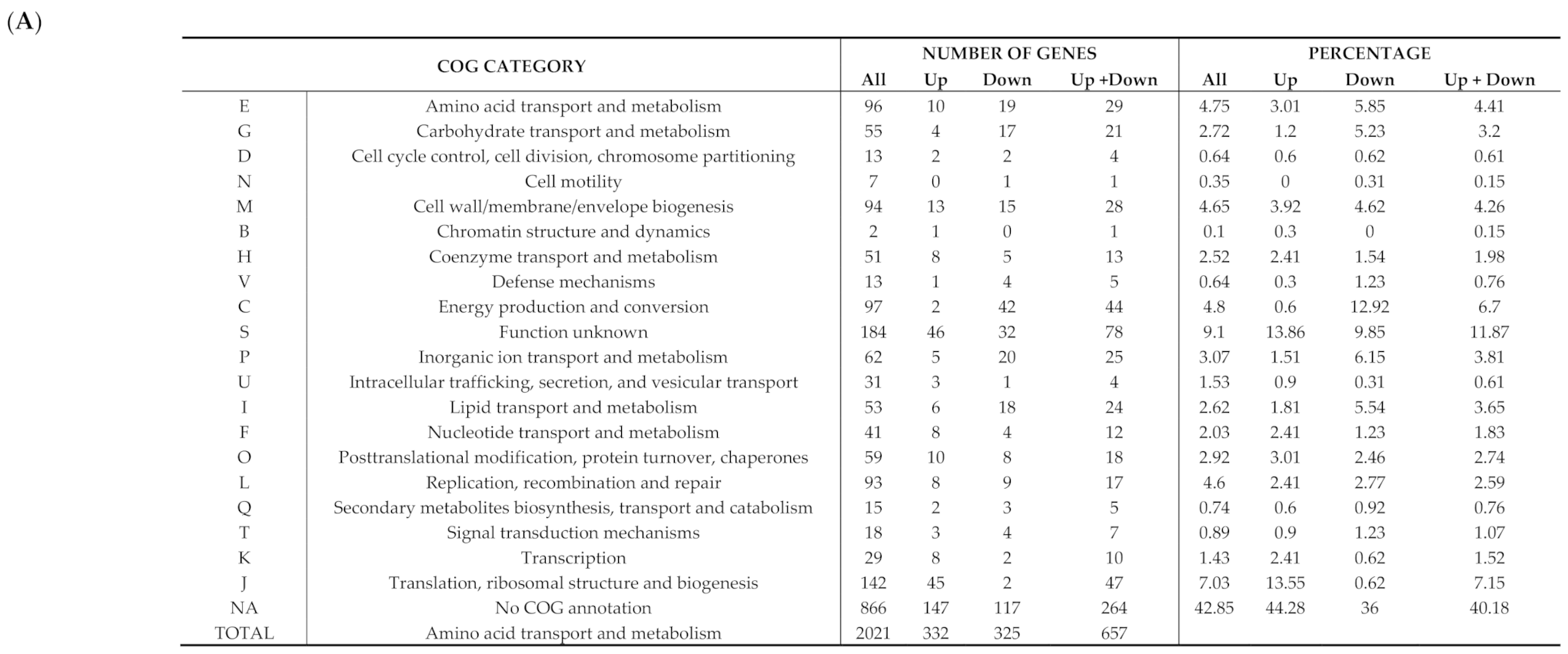
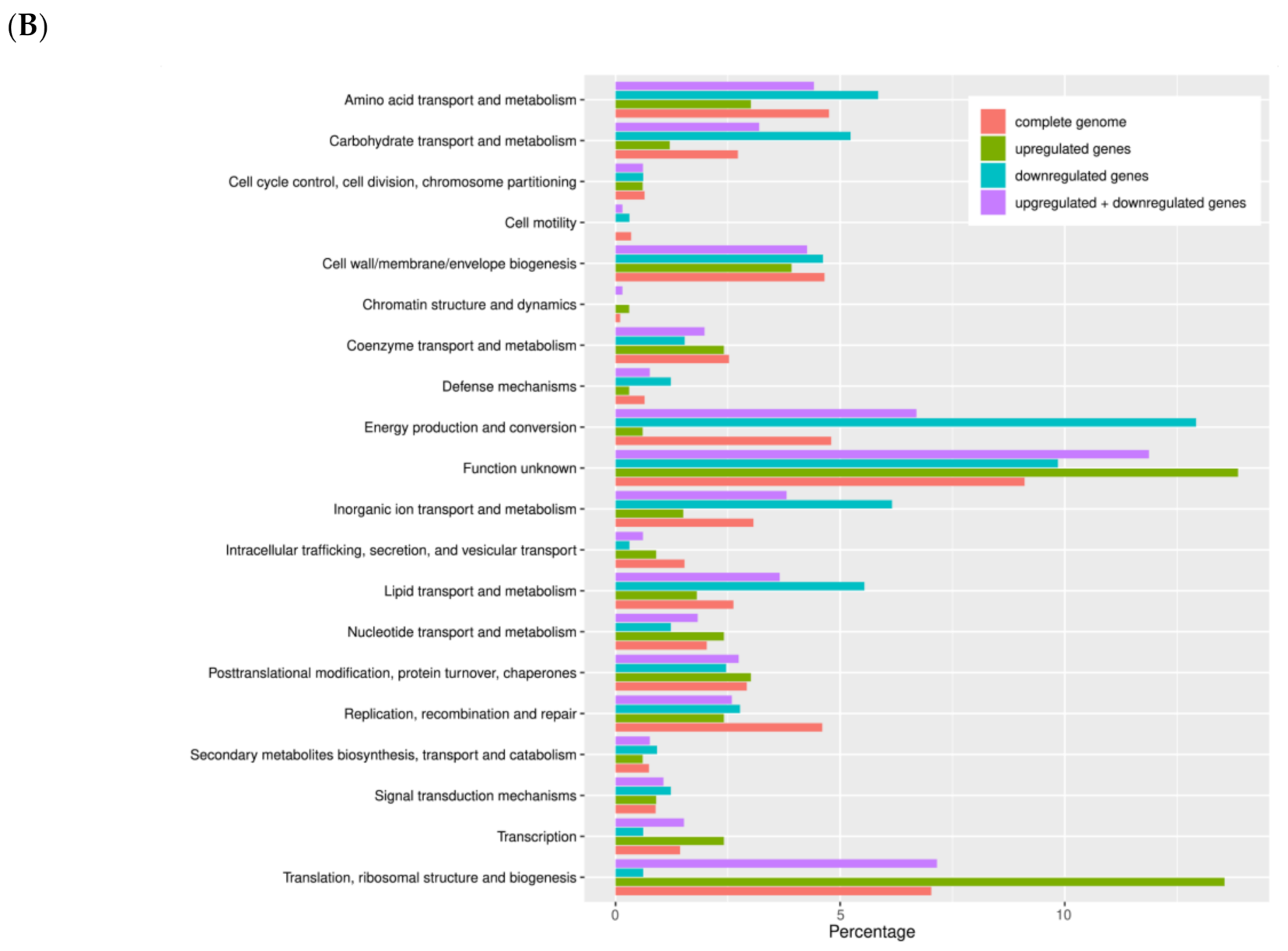
3.3. Comparative Transcriptomic Analysis of Persistent W. chondrophila Induced by Iron Starvation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pillonel, T.; Bertelli, C.; Greub, G. Environmental Metagenomic Assemblies Reveal Seven New Highly Divergent Chlamydial Lineages and Hallmarks of a Conserved Intracellular Lifestyle. Front. Microbiol. 2018, 9, 79. [Google Scholar] [CrossRef] [PubMed]
- Rowley, J.; Vander Hoorn, S.; Korenromp, E.; Low, N.; Unemo, M.; Abu-Raddad, L.J.; Chico, R.M.; Smolak, A.; Newman, L.; Gottlieb, S.; et al. Chlamydia, gonorrhoea, trichomoniasis and syphilis: Global prevalence and incidence estimates, 2016. Bull. World Health Organ. 2019, 97, 548P–562P. [Google Scholar] [CrossRef]
- Hahn, D.L.; Azenabor, A.A.; Beatty, W.L.; Byrne, G.I. Chlamydia pneumoniae as a respiratory pathogen. Front. Biosci. 2002, 7, e66–e76. [Google Scholar] [CrossRef] [PubMed]
- Corsaro, D.; Venditti, D. Emerging chlamydial infections. Crit. Rev. Microbiol. 2004, 30, 75–106. [Google Scholar] [CrossRef] [PubMed]
- Dilbeck, P.M.; Evermann, J.F.; Crawford, T.B.; Ward, A.C.; Leathers, C.W.; Holland, C.J.; Mebus, C.A.; Logan, L.L.; Rurangirwa, F.R.; McGuire, T.C. Isolation of a previously undescribed rickettsia from an aborted bovine fetus. J. Clin. Microbiol. 1990, 28, 814–816. [Google Scholar] [CrossRef] [PubMed]
- Henning, K.; Schares, G.; Granzow, H.; Polster, U.; Hartmann, M.; Hotzel, H.; Sachse, K.; Peters, M.; Rauser, M. Neospora caninum and Waddlia chondrophila strain 2032/99 in a septic stillborn calf. Vet. Microbiol. 2002, 85, 285–292. [Google Scholar] [CrossRef]
- Barkallah, M.; Gharbi, Y.; Slima, A.B.; Elleuch, F.; Mallek, Z.; Saad, R.B.; Gautier, M.; Gdoura, R.; Fendri, I. Simultaneous detection of Waddlia chondrophila and Listeria monocytogenes in aborted ruminant samples by real-time quantitative PCR. J. Microbiol. Methods 2016, 125, 64–69. [Google Scholar] [CrossRef]
- Dilbeck-Robertson, P.; McAllister, M.M.; Bradway, D.; Evermann, J.F. Results of a new serologic test suggest an association of Waddlia chondrophila with bovine abortion. J. Vet. Diagn. Investig. 2003, 15, 568–569. [Google Scholar] [CrossRef]
- Baud, D.; Thomas, V.; Arafa, A.; Regan, L.; Greub, G. Waddlia chondrophila, a potential agent of human fetal death. Emerg. Infect. Dis. 2007, 13, 1239–1243. [Google Scholar] [CrossRef]
- Baud, D.; Goy, G.; Osterheld, M.C.; Borel, N.; Vial, Y.; Pospischil, A.; Greub, G. Waddlia chondrophila: From bovine abortion to human miscarriage. Clin. Infect. Dis. 2011, 52, 1469–1471. [Google Scholar] [CrossRef]
- Baud, D.; Goy, G.; Osterheld, M.C.; Croxatto, A.; Borel, N.; Vial, Y.; Pospischil, A.; Greub, G. Role of Waddlia chondrophila placental infection in miscarriage. Emerg. Infect. Dis. 2014, 20, 460–464. [Google Scholar] [CrossRef] [PubMed]
- Hornung, S.; Thuong, B.C.; Gyger, J.; Kebbi-Beghdadi, C.; Vasilevsky, S.; Greub, G.; Baud, D. Role of Chlamydia trachomatis and emerging Chlamydia-related bacteria in ectopic pregnancy in Vietnam. Epidemiol. Infect. 2015, 143, 2635–2638. [Google Scholar] [CrossRef]
- Verweij, S.P.; Kebbi-Beghdadi, C.; Land, J.A.; Ouburg, S.; Morre, S.A.; Greub, G. Waddlia chondrophila and Chlamydia trachomatis antibodies in screening infertile women for tubal pathology. Microbes Infect. 2015, 17, 745–748. [Google Scholar] [CrossRef] [PubMed]
- Haider, S.; Collingro, A.; Walochnik, J.; Wagner, M.; Horn, M. Chlamydia-like bacteria in respiratory samples of community-acquired pneumonia patients. FEMS Microbiol. Lett. 2008, 281, 198–202. [Google Scholar] [CrossRef] [PubMed]
- Goy, G.; Croxatto, A.; Posfay-Barbe, K.M.; Gervaix, A.; Greub, G. Development of a real-time PCR for the specific detection of Waddlia chondrophila in clinical samples. Eur. J. Clin. Microbiol. Infect. Dis. 2009, 28, 1483–1486. [Google Scholar] [CrossRef] [PubMed]
- Greub, G.; Raoult, D. Crescent bodies of Parachlamydia acanthamoeba and its life cycle within Acanthamoeba polyphaga: An electron micrograph study. Appl. Environ. Microbiol. 2002, 68, 3076–3084. [Google Scholar] [CrossRef]
- Moulder, J.W. Interaction of chlamydiae and host cells in vitro. Microbiol. Rev. 1991, 55, 143–190. [Google Scholar] [CrossRef]
- Albrecht, M.; Sharma, C.M.; Dittrich, M.T.; Muller, T.; Reinhardt, R.; Vogel, J.; Rudel, T. The transcriptional landscape of Chlamydia pneumoniae. Genome Biol. 2011, 12, R98. [Google Scholar] [CrossRef]
- Albrecht, M.; Sharma, C.M.; Reinhardt, R.; Vogel, J.; Rudel, T. Deep sequencing-based discovery of the Chlamydia trachomatis transcriptome. Nucleic Acids Res. 2010, 38, 868–877. [Google Scholar] [CrossRef]
- Beder, T.; Saluz, H.P. Virulence-related comparative transcriptomics of infectious and non-infectious chlamydial particles. BMC Genom. 2018, 19, 575. [Google Scholar] [CrossRef]
- Belland, R.J.; Zhong, G.; Crane, D.D.; Hogan, D.; Sturdevant, D.; Sharma, J.; Beatty, W.L.; Caldwell, H.D. Genomic transcriptional profiling of the developmental cycle of Chlamydia trachomatis. Proc. Natl. Acad. Sci. USA 2003, 100, 8478–8483. [Google Scholar] [CrossRef] [PubMed]
- Humphrys, M.S.; Creasy, T.; Sun, Y.; Shetty, A.C.; Chibucos, M.C.; Drabek, E.F.; Fraser, C.M.; Farooq, U.; Sengamalay, N.; Ott, S.; et al. Simultaneous transcriptional profiling of bacteria and their host cells. PLoS ONE 2013, 8, e80597. [Google Scholar] [CrossRef] [PubMed]
- Hybiske, K.; Stephens, R.S. Mechanisms of host cell exit by the intracellular bacterium Chlamydia. Proc. Natl. Acad. Sci. USA 2007, 104, 11430–11435. [Google Scholar] [CrossRef] [PubMed]
- Kintner, J.; Lajoie, D.; Hall, J.; Whittimore, J.; Schoborg, R.V. Commonly prescribed beta-lactam antibiotics induce C. trachomatis persistence/stress in culture at physiologically relevant concentrations. Front. Cell. Infect. Microbiol. 2014, 4, 44. [Google Scholar] [CrossRef] [PubMed]
- Jacquier, N.; Frandi, A.; Pillonel, T.; Viollier, P.H.; Greub, G. Cell wall precursors are required to organize the chlamydial division septum. Nat. Commun. 2014, 5, 3578. [Google Scholar] [CrossRef]
- Slade, J.A.; Brockett, M.; Singh, R.; Liechti, G.W.; Maurelli, A.T. Fosmidomycin, an inhibitor of isoprenoid synthesis, induces persistence in Chlamydia by inhibiting peptidoglycan assembly. PLoS Pathog. 2019, 15, e1008078. [Google Scholar] [CrossRef]
- Beatty, W.L.; Byrne, G.I.; Morrison, R.P. Morphologic and antigenic characterization of interferon gamma-mediated persistent Chlamydia trachomatis infection in vitro. Proc. Natl. Acad. Sci. USA 1993, 90, 3998–4002. [Google Scholar] [CrossRef]
- Coles, A.M.; Reynolds, D.J.; Harper, A.; Devitt, A.; Pearce, J.H. Low-nutrient induction of abnormal chlamydial development: A novel component of chlamydial pathogenesis? FEMS Microbiol. Lett. 1993, 106, 193–200. [Google Scholar] [CrossRef]
- Scherler, A.; Jacquier, N.; Kebbi-Beghdadi, C.; Greub, G. Diverse Stress-Inducing Treatments cause Distinct Aberrant Body Morphologies in the Chlamydia-Related Bacterium, Waddlia chondrophila. Microorganisms 2020, 8, 89. [Google Scholar] [CrossRef]
- Al-Younes, H.M.; Rudel, T.; Brinkmann, V.; Szczepek, A.J.; Meyer, T.F. Low iron availability modulates the course of Chlamydia pneumoniae infection. Cell. Microbiol. 2001, 3, 427–437. [Google Scholar] [CrossRef]
- Freidank, H.M.; Billing, H.; Wiedmann-Al-Ahmad, M. Influence of iron restriction on Chlamydia pneumoniae and C. trachomatis. J. Med. Microbiol. 2001, 50, 223–227. [Google Scholar] [CrossRef] [PubMed]
- Raulston, J.E. Response of Chlamydia trachomatis serovar E to iron restriction in vitro and evidence for iron-regulated chlamydial proteins. Infect. Immun. 1997, 65, 4539–4547. [Google Scholar] [CrossRef] [PubMed]
- Thompson, C.C.; Carabeo, R.A. An optimal method of iron starvation of the obligate intracellular pathogen, Chlamydia trachomatis. Front. Microbiol. 2011, 2, 20. [Google Scholar] [CrossRef] [PubMed]
- Kebbi-Beghdadi, C.; Cisse, O.; Greub, G. Permissivity of Vero cells, human pneumocytes and human endometrial cells to Waddlia chondrophila. Microbes Infect. 2011, 13, 566–574. [Google Scholar] [CrossRef]
- Lewis, M.E.; Belland, R.J.; AbdelRahman, Y.M.; Beatty, W.L.; Aiyar, A.A.; Zea, A.H.; Greene, S.J.; Marrero, L.; Buckner, L.R.; Tate, D.J.; et al. Morphologic and molecular evaluation of Chlamydia trachomatis growth in human endocervix reveals distinct growth patterns. Front. Cell. Infect. Microbiol. 2014, 4, 71. [Google Scholar] [CrossRef]
- Angeli, A.; Laine, F.; Lavenu, A.; Ropert, M.; Lacut, K.; Gissot, V.; Sacher-Huvelin, S.; Jezequel, C.; Moignet, A.; Laviolle, B.; et al. Joint Model of Iron and Hepcidin during the Menstrual Cycle in Healthy Women. AAPS J. 2016, 18, 490–504. [Google Scholar] [CrossRef]
- Kim, I.; Yetley, E.A.; Calvo, M.S. Variations in iron-status measures during the menstrual cycle. Am. J. Clin. Nutr. 1993, 58, 705–709. [Google Scholar] [CrossRef] [PubMed]
- Pokorzynski, N.D.; Thompson, C.C.; Carabeo, R.A. Ironing Out the Unconventional Mechanisms of Iron Acquisition and Gene Regulation in Chlamydia. Front. Cell. Infect. Microbiol. 2017, 7, 394. [Google Scholar] [CrossRef]
- Ouellette, S.P.; Carabeo, R.A. A Functional Slow Recycling Pathway of Transferrin is Required for Growth of Chlamydia. Front. Microbiol. 2010, 1, 112. [Google Scholar] [CrossRef]
- Luo, Z.; Neville, S.L.; Campbell, R.; Morey, J.R.; Menon, S.; Thomas, M.; Eijkelkamp, B.A.; Ween, M.P.; Huston, W.M.; Kobe, B.; et al. Structure and Metal Binding Properties of Chlamydia trachomatis YtgA. J. Bacteriol. 2019, 202. [Google Scholar] [CrossRef]
- Miller, J.D.; Sal, M.S.; Schell, M.; Whittimore, J.D.; Raulston, J.E. Chlamydia trachomatis YtgA is an iron-binding periplasmic protein induced by iron restriction. Microbiology 2009, 155, 2884–2894. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Raulston, J.E.; Miller, J.D.; Davis, C.H.; Schell, M.; Baldwin, A.; Ferguson, K.; Lane, H. Identification of an iron-responsive protein that is antigenic in patients with Chlamydia trachomatis genital infections. FEMS Immunol. Med. Microbiol. 2007, 51, 569–576. [Google Scholar] [CrossRef] [PubMed]
- Thompson, C.C.; Nicod, S.S.; Malcolm, D.S.; Grieshaber, S.S.; Carabeo, R.A. Cleavage of a putative metal permease in Chlamydia trachomatis yields an iron-dependent transcriptional repressor. Proc. Natl. Acad. Sci. USA 2012, 109, 10546–10551. [Google Scholar] [CrossRef] [PubMed]
- Panzetta, M.E.; Valdivia, R.H.; Saka, H.A. Chlamydia Persistence: A Survival Strategy to Evade Antimicrobial Effects in-vitro and in-vivo. Front. Microbiol. 2018, 9, 3101. [Google Scholar] [CrossRef] [PubMed]
- Kukurba, K.R.; Montgomery, S.B. RNA Sequencing and Analysis. Cold Spring Harb. Protoc. 2015, 2015, 951–969. [Google Scholar] [CrossRef] [PubMed]
- Brinkworth, A.J.; Wildung, M.R.; Carabeo, R.A. Genomewide Transcriptional Responses of Iron-Starved Chlamydia trachomatis Reveal Prioritization of Metabolic Precursor Synthesis over Protein Translation. mSystems 2018, 3, e00184-17. [Google Scholar] [CrossRef] [PubMed]
- König, L.; Siegl, A.; Penz, T.; Haider, S.; Wentrup, C.; Polzin, J.; Mann, E.; Schmitz-Esser, S.; Domman, D.; Horn, M. Biphasic Metabolism and Host Interaction of a Chlamydial Symbiont. mSystems 2017, 2, e00202-16. [Google Scholar] [CrossRef]
- Bertelli, C.; Collyn, F.; Croxatto, A.; Ruckert, C.; Polkinghorne, A.; Kebbi-Beghdadi, C.; Goesmann, A.; Vaughan, L.; Greub, G. The Waddlia genome: A window into chlamydial biology. PLoS ONE 2010, 5, e10890. [Google Scholar] [CrossRef]
- Omsland, A.; Sixt, B.S.; Horn, M.; Hackstadt, T. Chlamydial metabolism revisited: Interspecies metabolic variability and developmental stage-specific physiologic activities. FEMS Microbiol. Rev. 2014, 38, 779–801. [Google Scholar] [CrossRef]
- Borel, N.; Pospischil, A.; Dowling, R.D.; Dumrese, C.; Gaydos, C.A.; Bunk, S.; Hermann, C.; Ramirez, J.A.; Summersgill, J.T. Antigens of persistent Chlamydia pneumoniae within coronary atheroma from patients undergoing heart transplantation. J. Clin. Pathol. 2012, 65, 171–177. [Google Scholar] [CrossRef]
- Somboonna, N.; Ziklo, N.; Ferrin, T.E.; Hyuk Suh, J.; Dean, D. Clinical Persistence of Chlamydia trachomatis Sexually Transmitted Strains Involves Novel Mutations in the Functional alphabetabetaalpha Tetramer of the Tryptophan Synthase Operon. mBio 2019, 10, e01464-19. [Google Scholar] [CrossRef] [PubMed]
- Croxatto, A.; Greub, G. Early intracellular trafficking of Waddlia chondrophila in human macrophages. Microbiology 2010, 156, 340–355. [Google Scholar] [CrossRef] [PubMed]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [PubMed]
- Li, H. Aligning sequence reads, clone sequences and assembly contigs with BWA-MEM. arXiv 2013, arXiv:1303.3997v2. [Google Scholar]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. edgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef]
- Robinson, M.D.; Oshlack, A. A scaling normalization method for differential expression analysis of RNA-seq data. Genome Biol. 2010, 11, R25. [Google Scholar] [CrossRef]
- Kolde, R. Pheatmap: Pretty Heatmaps. R Package Version 1.0.10. 2012. Available online: https://CRAN.R-project.org/package=pheatmap (accessed on 8 January 2020).
- Kanehisa, M.; Furumichi, M.; Tanabe, M.; Sato, Y.; Morishima, K. KEGG: New perspectives on genomes, pathways, diseases and drugs. Nucleic Acids Res. 2017, 45, D353–D361. [Google Scholar] [CrossRef]
- Pillonel, T.; Tagini, F.; Bertelli, C.; Greub, G. ChlamDB: A comparative genomics database of the phylum Chlamydiae and other members of the Planctomycetes-Verrucomicrobiae-Chlamydiae superphylum. Nucleic Acids Res. 2020, 48, D526–D534. [Google Scholar] [CrossRef]
- Huntley, R.P.; Sawford, T.; Mutowo-Meullenet, P.; Shypitsyna, A.; Bonilla, C.; Martin, M.J.; O’Donovan, C. The GOA database: Gene Ontology annotation updates for 2015. Nucleic Acids Res. 2015, 43, D1057–D1063. [Google Scholar] [CrossRef]
- Klopfenstein, D.V.; Zhang, L.; Pedersen, B.S.; Ramirez, F.; Warwick Vesztrocy, A.; Naldi, A.; Mungall, C.J.; Yunes, J.M.; Botvinnik, O.; Weigel, M.; et al. GOATOOLS: A Python library for Gene Ontology analyses. Sci. Rep. 2018, 8, 10872. [Google Scholar] [CrossRef]
- Hayer-Hartl, M.; Bracher, A.; Hartl, F.U. The GroEL-GroES Chaperonin Machine: A Nano-Cage for Protein Folding. Trends Biochem. Sci. 2016, 41, 62–76. [Google Scholar] [CrossRef] [PubMed]
- Belland, R.J.; Nelson, D.E.; Virok, D.; Crane, D.D.; Hogan, D.; Sturdevant, D.; Beatty, W.L.; Caldwell, H.D. Transcriptome analysis of chlamydial growth during IFN-gamma-mediated persistence and reactivation. Proc. Natl. Acad. Sci. USA 2003, 100, 15971–15976. [Google Scholar] [CrossRef] [PubMed]
- Mäurer, A.P.; Mehlitz, A.; Mollenkopf, H.J.; Meyer, T.F. Gene expression profiles of Chlamydophila pneumoniae during the developmental cycle and iron depletion-mediated persistence. PLoS Pathog. 2007, 3, e83. [Google Scholar] [CrossRef] [PubMed]
- Goellner, S.; Schubert, E.; Liebler-Tenorio, E.; Hotzel, H.; Saluz, H.P.; Sachse, K. Transcriptional response patterns of Chlamydophila psittaci in different in vitro models of persistent infection. Infect. Immun. 2006, 74, 4801–4808. [Google Scholar] [CrossRef]
- Klos, A.; Thalmann, J.; Peters, J.; Gerard, H.C.; Hudson, A.P. The transcript profile of persistent Chlamydophila (Chlamydia) pneumoniae in vitro depends on the means by which persistence is induced. FEMS Microbiol. Lett. 2009, 291, 120–126. [Google Scholar] [CrossRef]
- Kokab, A.; Jennings, R.; Eley, A.; Pacey, A.A.; Cross, N.A. Analysis of modulated gene expression in a model of Interferon-gamma-induced persistence of Chlamydia trachomatis in HEp-2 cells. Microb. Pathog. 2010, 49, 217–225. [Google Scholar] [CrossRef]
- Justino, M.C.; Vicente, J.B.; Teixeira, M.; Saraiva, L.M. New genes implicated in the protection of anaerobically grown Escherichia coli against nitric oxide. J. Biol. Chem. 2005, 280, 2636–2643. [Google Scholar] [CrossRef]
- Justino, M.C.; Almeida, C.C.; Goncalves, V.L.; Teixeira, M.; Saraiva, L.M. Escherichia coli YtfE is a di-iron protein with an important function in assembly of iron-sulphur clusters. FEMS Microbiol. Lett. 2006, 257, 278–284. [Google Scholar] [CrossRef]
- Sass, A.M.; Coenye, T. Low iron-induced small RNA BrrF regulates central metabolism and oxidative stress responses in Burkholderia cenocepacia. PLoS ONE 2020, 15, e0236405. [Google Scholar] [CrossRef]
- Rosario, C.J.; Tan, M. The early gene product EUO is a transcriptional repressor that selectively regulates promoters of Chlamydia late genes. Mol. Microbiol. 2012, 84, 1097–1107. [Google Scholar] [CrossRef]
- Ouellette, S.P.; Hatch, T.P.; AbdelRahman, Y.M.; Rose, L.A.; Belland, R.J.; Byrne, G.I. Global transcriptional upregulation in the absence of increased translation in Chlamydia during IFNgamma-mediated host cell tryptophan starvation. Mol. Microbiol. 2006, 62, 1387–1401. [Google Scholar] [CrossRef] [PubMed]
- Timms, P.; Good, D.; Wan, C.; Theodoropoulos, C.; Mukhopadhyay, S.; Summersgill, J.; Mathews, S. Differential transcriptional responses between the interferon-gamma-induction and iron-limitation models of persistence for Chlamydia pneumoniae. J. Microbiol. Immunol. Infect. 2009, 42, 27–37. [Google Scholar] [PubMed]
- Xue, Y.; Zheng, H.; Mai, Z.; Qin, X.; Chen, W.; Huang, T.; Chen, D.; Zheng, L. An in vitro model of azithromycin-induced persistent Chlamydia trachomatis infection. FEMS Microbiol. Lett. 2017, 364. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, S.; Miller, R.D.; Sullivan, E.D.; Theodoropoulos, C.; Mathews, S.A.; Timms, P.; Summersgill, J.T. Protein expression profiles of Chlamydia pneumoniae in models of persistence versus those of heat shock stress response. Infect. Immun. 2006, 74, 3853–3863. [Google Scholar] [CrossRef]
- Hogan, R.J.; Mathews, S.A.; Mukhopadhyay, S.; Summersgill, J.T.; Timms, P. Chlamydial persistence: Beyond the biphasic paradigm. Infect. Immun. 2004, 72, 1843–1855. [Google Scholar] [CrossRef]
- Ramage, H.R.; Connolly, L.E.; Cox, J.S. Comprehensive functional analysis of Mycobacterium tuberculosis toxin-antitoxin systems: Implications for pathogenesis, stress responses, and evolution. PLoS Genet. 2009, 5, e1000767. [Google Scholar] [CrossRef]
- Schuessler, D.L.; Cortes, T.; Fivian-Hughes, A.S.; Lougheed, K.E.; Harvey, E.; Buxton, R.S.; Davis, E.O.; Young, D.B. Induced ectopic expression of HigB toxin in Mycobacterium tuberculosis results in growth inhibition, reduced abundance of a subset of mRNAs and cleavage of tmRNA. Mol. Microbiol. 2013, 90, 195–207. [Google Scholar] [CrossRef]
- Page, R.; Peti, W. Toxin-antitoxin systems in bacterial growth arrest and persistence. Nat. Chem. Biol. 2016, 12, 208–214. [Google Scholar] [CrossRef]
- Keren, I.; Minami, S.; Rubin, E.; Lewis, K. Characterization and transcriptome analysis of Mycobacterium tuberculosis persisters. MBio 2011, 2, e00100-11. [Google Scholar] [CrossRef]
- Wen, Y.; Behiels, E.; Devreese, B. Toxin-Antitoxin systems: Their role in persistence, biofilm formation, and pathogenicity. Pathog. Dis. 2014, 70, 240–249. [Google Scholar] [CrossRef]
- Leplae, R.; Geeraerts, D.; Hallez, R.; Guglielmini, J.; Dreze, P.; Van Melderen, L. Diversity of bacterial type II toxin-antitoxin systems: A comprehensive search and functional analysis of novel families. Nucleic Acids Res. 2011, 39, 5513–5525. [Google Scholar] [CrossRef] [PubMed]
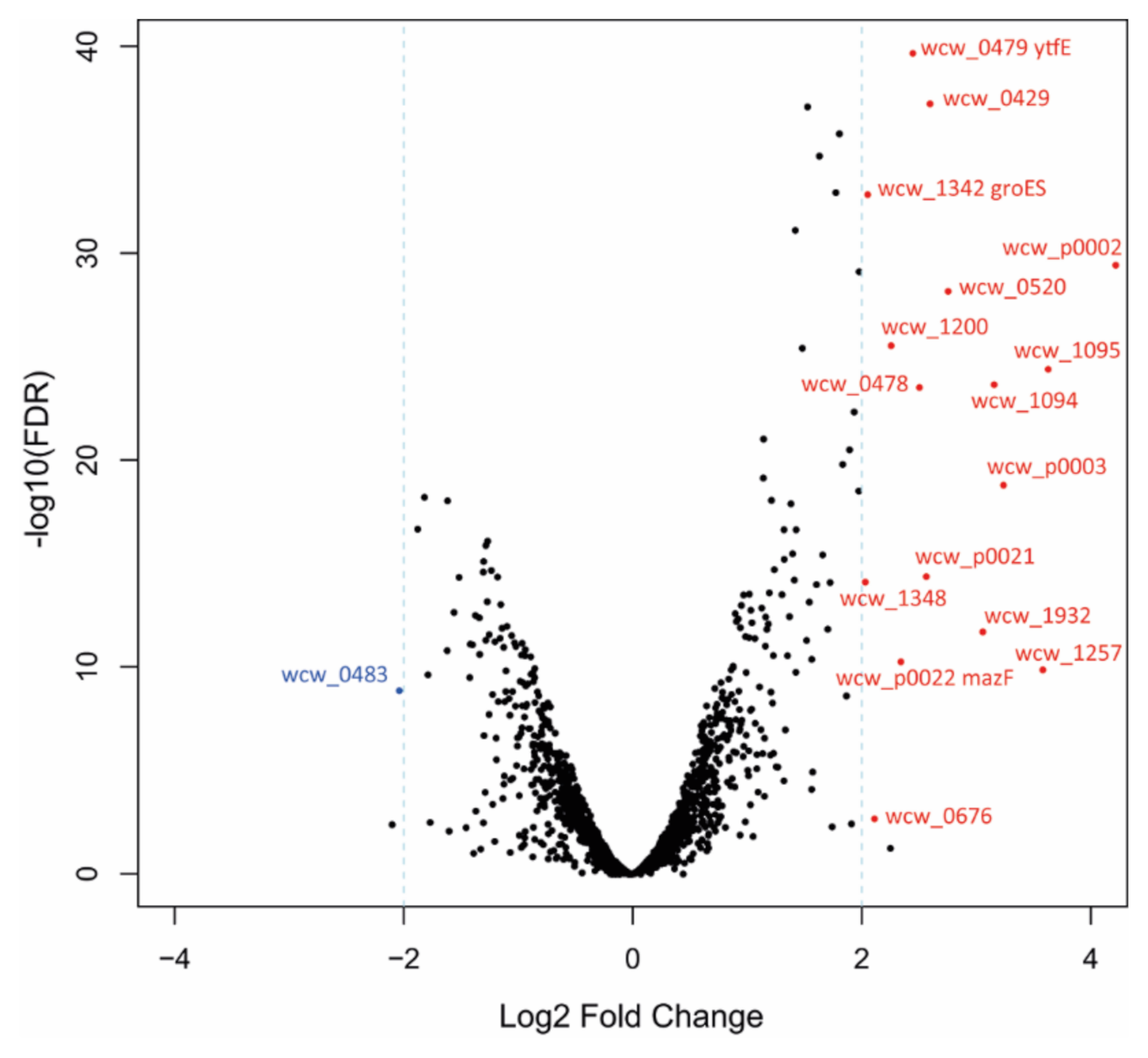
| Gene 1 | Locus tag | LogFC | FC | FDR | Product | Conservation 2 |
|---|---|---|---|---|---|---|
| wcw_0483 | wcw_0483 | −2.0 | 0.2 | 2.03 × 10−8 | Transposase | B |
| wcw_1348 | wcw_1348 | 2.0 | 4.1 | 3.15 × 10−13 | Putative addiction module killer protein | B |
| groES1 | wcw_1342 | 2.1 | 4.2 | 3.69 × 10−31 | Co-chaperonin GroES | A |
| wcw_0676 | wcw_0676 | 2.1 | 4.3 | 0.0064 | tRNA-Ser | A |
| wcw_1200 | wcw_1200 | 2.3 | 4.8 | 4.31 × 10−24 | Hypothetical protein | C |
| mazF | wcw_p0022 | 2.4 | 5.1 | 1.01 × 10−9 | Putative MazF-like toxin | B |
| wcw_0479 | wcw_0479 | 2.5 | 5.5 | 3.82 × 10−37 | Putative regulator of cell morphogenesis | B |
| wcw_0478 | wcw_0478 | 2.5 | 5.7 | 3.39 × 10−22 | Putative HTH-type transcriptional repressor | B |
| wcw_p0021 | wcw_p0021 | 2.6 | 6.0 | 1.88 × 10−13 | Hypothetical protein | B |
| wcw_0429 | wcw_0429 | 2.6 | 6.1 | 4.94 × 10−35 | Hypothetical protein | C |
| wcw_0520 | wcw_0520 | 2.8 | 6.8 | 1.11 × 10−26 | Hypothetical protein | B |
| wcw_1932 | wcw_1932 | 3.1 | 8.4 | 4.90 × 10−11 | tRNA-Gly | A |
| wcw_1094 | wcw_1094 | 3.2 | 9.0 | 2.64 × 10−22 | Hypothetical protein | B |
| wcw_p0003 | wcw_p0003 | 3.2 | 9.5 | 1.30 × 10−17 | Hypothetical protein | B |
| wcw_1257 | wcw_1257 | 3.6 | 12.0 | 2.35 × 10−9 | tRNA-Ala | A |
| wcw_1095 | wcw_1095 | 3.6 | 12.4 | 5.10 × 10−23 | Hypothetical protein | B |
| wcw_p0002 | wcw_p0002 | 4.2 | 18.7 | 7.40 × 10−28 | Putative component of the toxin-antitoxin plasmid stabilization module | B |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ardissone, S.; Scherler, A.; Pillonel, T.; Martin, V.; Kebbi-Beghdadi, C.; Greub, G. Transcriptional Landscape of Waddlia chondrophila Aberrant Bodies Induced by Iron Starvation. Microorganisms 2020, 8, 1848. https://doi.org/10.3390/microorganisms8121848
Ardissone S, Scherler A, Pillonel T, Martin V, Kebbi-Beghdadi C, Greub G. Transcriptional Landscape of Waddlia chondrophila Aberrant Bodies Induced by Iron Starvation. Microorganisms. 2020; 8(12):1848. https://doi.org/10.3390/microorganisms8121848
Chicago/Turabian StyleArdissone, Silvia, Aurélie Scherler, Trestan Pillonel, Virginie Martin, Carole Kebbi-Beghdadi, and Gilbert Greub. 2020. "Transcriptional Landscape of Waddlia chondrophila Aberrant Bodies Induced by Iron Starvation" Microorganisms 8, no. 12: 1848. https://doi.org/10.3390/microorganisms8121848
APA StyleArdissone, S., Scherler, A., Pillonel, T., Martin, V., Kebbi-Beghdadi, C., & Greub, G. (2020). Transcriptional Landscape of Waddlia chondrophila Aberrant Bodies Induced by Iron Starvation. Microorganisms, 8(12), 1848. https://doi.org/10.3390/microorganisms8121848






