Proteomic Studies of the Biofilm Matrix including Outer Membrane Vesicles of Burkholderia multivorans C1576, a Strain of Clinical Importance for Cystic Fibrosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strain and Culture Conditions
2.2. OMV Isolation and Proteins Preparations
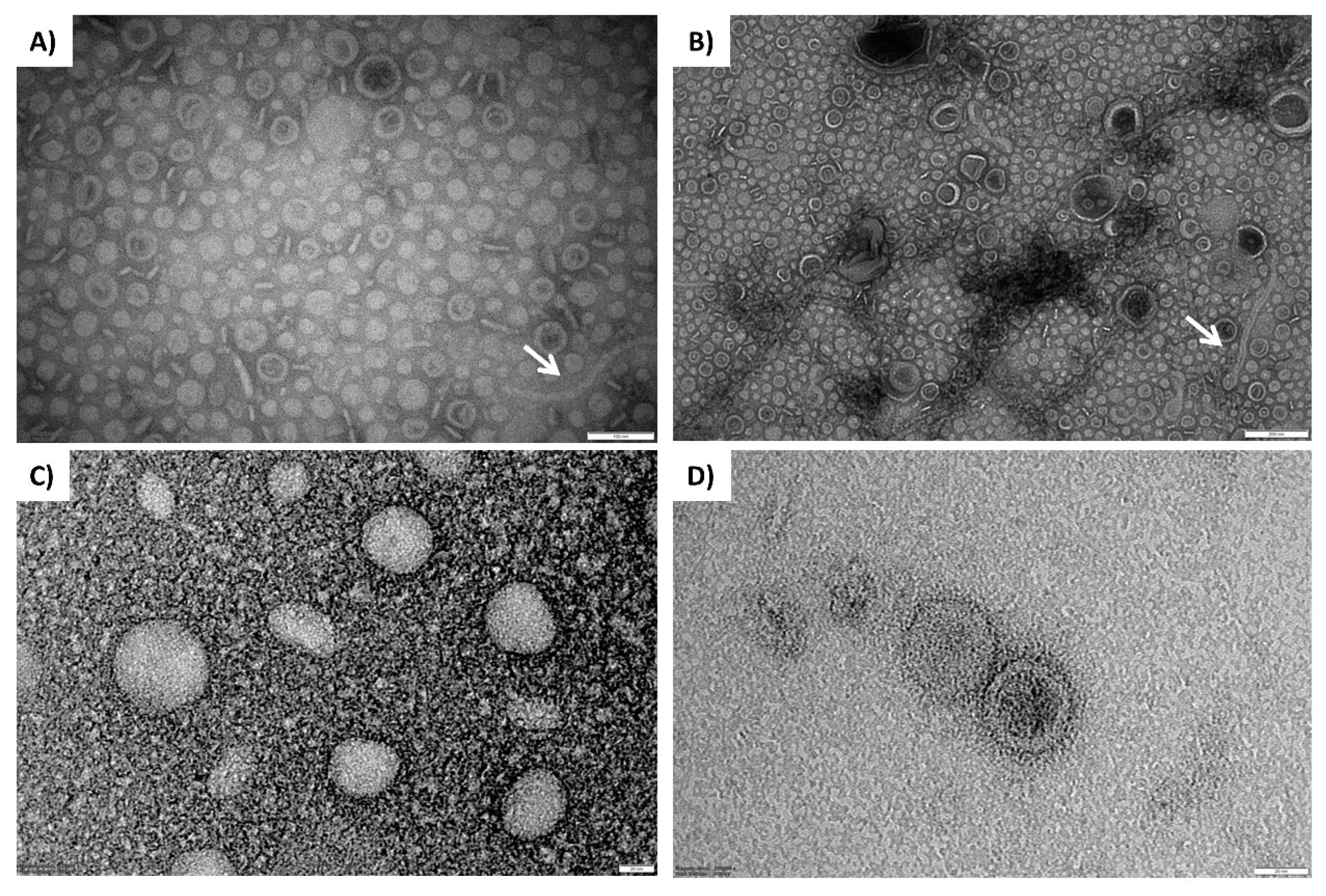
2.3. Transmission Electron Microscopy (TEM) Imaging of OMVs
2.4. Protein Preparations for Liquid Chromatography coupled to Tandem Mass Spectrometry (LC-MS/MS)
2.5. Protein Identification
2.6. Bioinformatic Analysis
3. Results
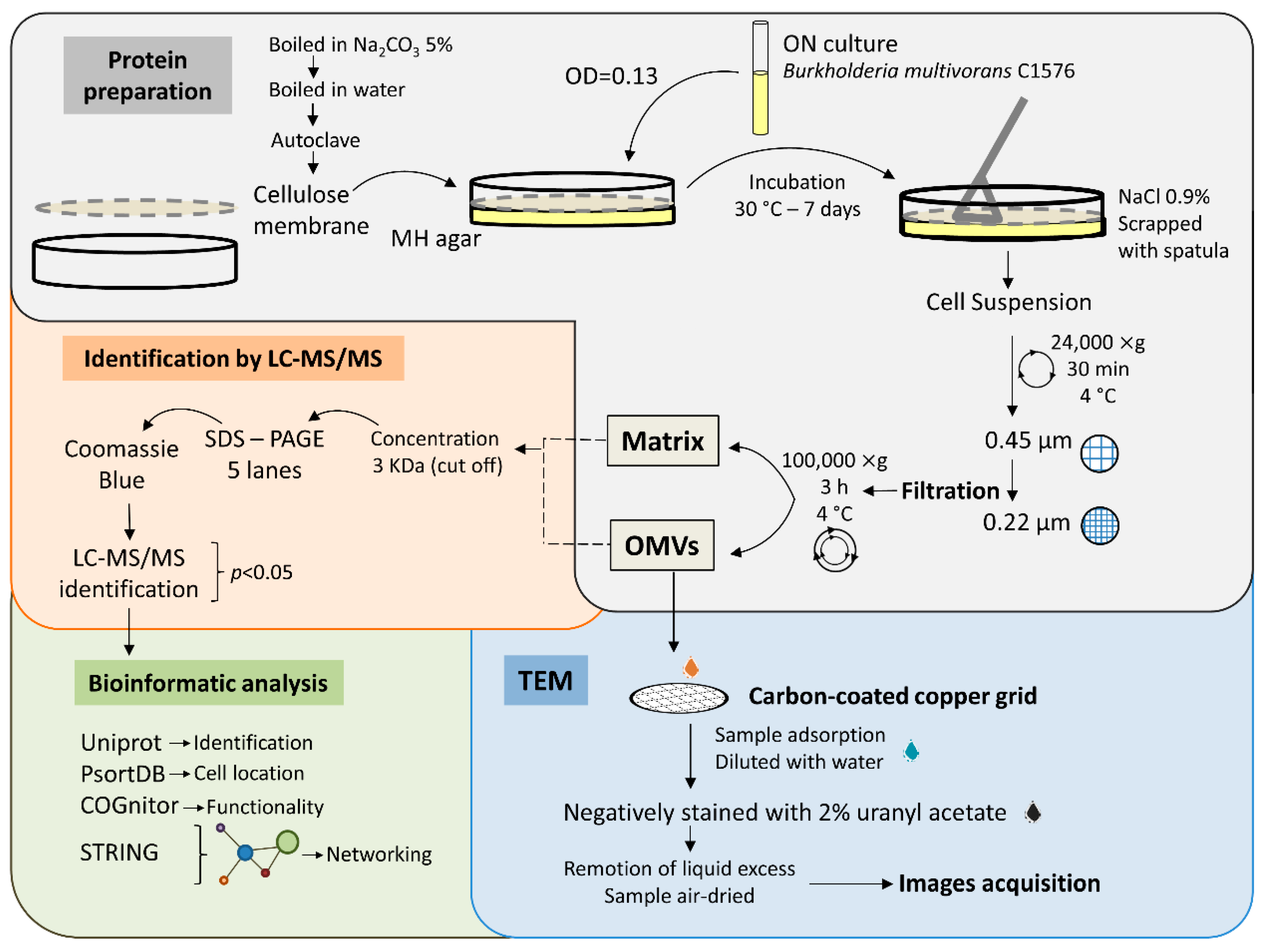
3.1. Strategy for Biofilm Production and Protein Separation
3.2. Visualization of OMVs Produced by Biofilm-Growing B. multivorans C1576
3.3. Identification of B. multivorans C1576 Proteins Associated with the Matrix and OMVs
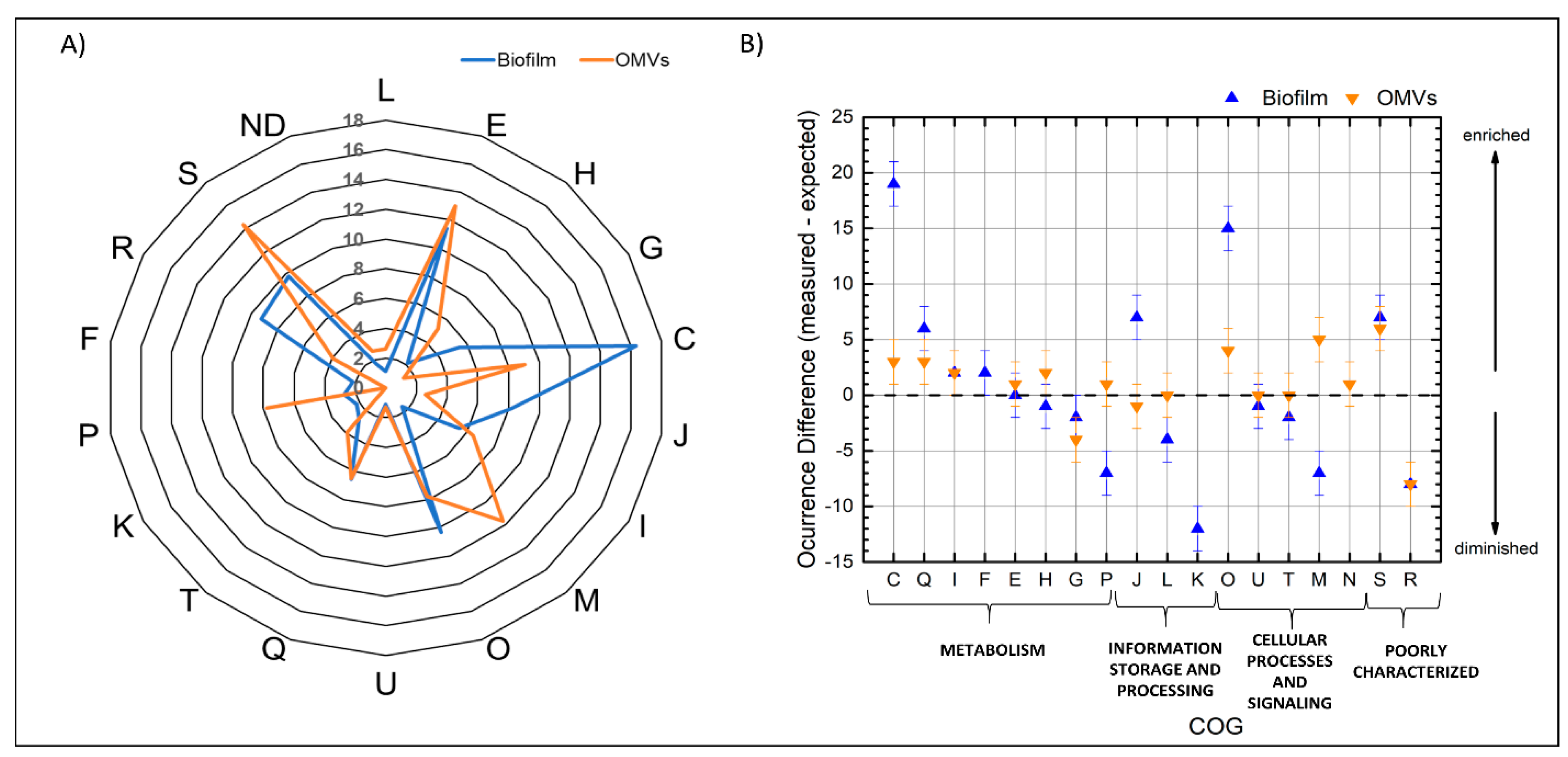
3.4. Functional Classification of the Identified Proteins from the Biofilm Matrix and OMVs
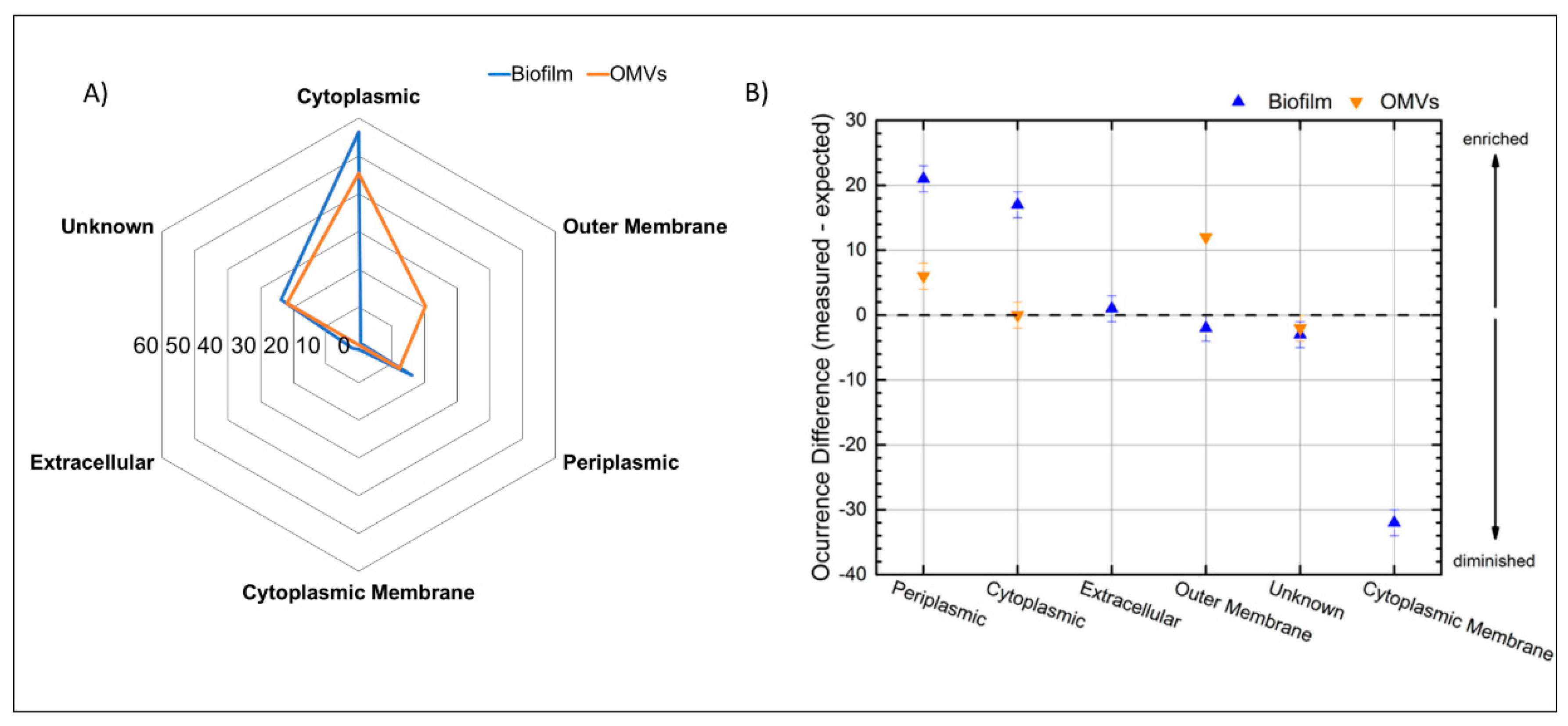
3.5. Subcellular Localization of the Identified Proteins from the Biofilm Matrix and OMVs
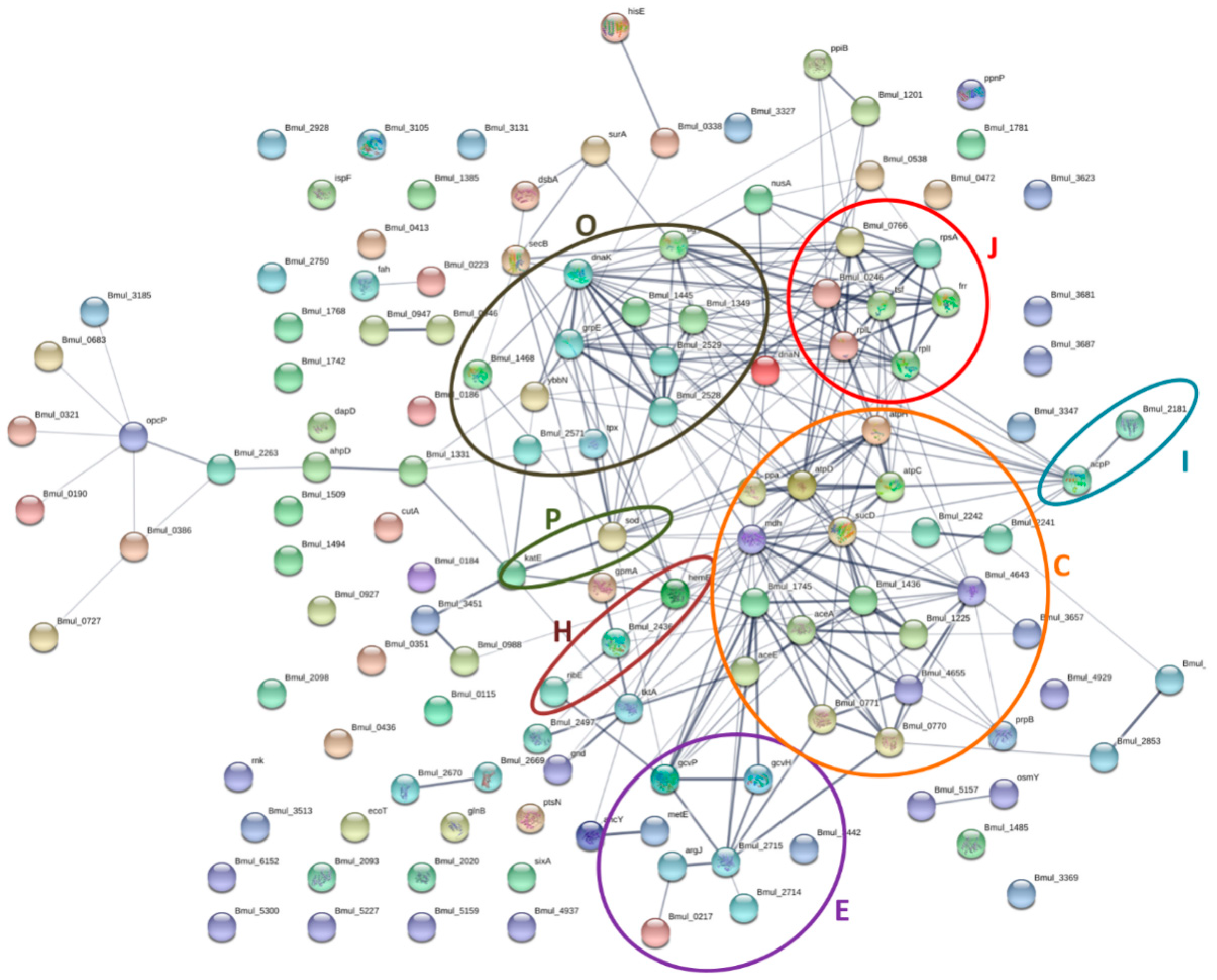
3.6. Protein–Protein Interaction Networks of the Identified Proteins from the Biofilm Matrix and OMVs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Costerton, J.W.; Lewandowski, Z.; Caldwell, D.E.; Korber, D.R.; Lappin-Scott, H.M. Microbial biofilms. Annu. Rev. Microbiol. 1995, 49, 711–745. [Google Scholar] [CrossRef] [PubMed]
- O’Toole, G.; Kaplan, H.B.; Kolter, R. Biofilm formation as microbial development. Annu. Rev. Microbiol. 2000, 54, 49–79. [Google Scholar] [CrossRef] [PubMed]
- Allison, D.G. The biofilm matrix. Biofouling 2003, 19, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Branda, S.S.; Vik, S.; Friedman, L.; Kolter, R. Biofilms: The matrix revisited. Trends Microbiol. 2005, 13, 20–26. [Google Scholar] [CrossRef]
- Fong, J.N.C.; Yildiz, F.H. Biofilm matrix proteins. Microbiol. Spectr. 2015, 3, 1–27. [Google Scholar] [CrossRef]
- Flemming, H.C.; Wingender, J. The biofilm matrix. Nat. Rev. Microbiol. 2010, 8, 623–633. [Google Scholar] [CrossRef]
- Urban, V.; Block, J.C.; Manem, J. Bioflocculation in Activated- Sludge: An Analytic Approach. Water Res. 1993, 27, 829–838. [Google Scholar] [CrossRef]
- Etterer, T.; Wilderer, P.A. Generation and properties of aerobic granular sludge. Water Sci. Technol. 2001, 43, 19–26. [Google Scholar] [CrossRef]
- Frølund, P.H.N.B.; Palmgren, R.; Keiding, K. Extraction of extracellular polymers from activated sludge using a cation exchange resin. Water Res. 1996, 30, 1749–1758. [Google Scholar] [CrossRef]
- Schooling, S.R.; Beveridge, T.J. Membrane vesicles: An overlooked component of the matrices of biofilms. J. Bacteriol. 2006, 188, 5945–5957. [Google Scholar] [CrossRef]
- Wang, W.; Chanda, W.; Zhong, M. The relationship between biofilm and outer membrane vesicles: A novel therapy overview. FEMS Microbiol. Lett. 2015, 362, fnv117. [Google Scholar] [CrossRef] [PubMed]
- Kulp, A.; Kuehn, M.J. Biological functions and biogenesis of secreted bacterial outer membrane vesicles. Annu. Rev. Microbiol. 2010, 64, 163–184. [Google Scholar] [CrossRef] [PubMed]
- Jan, A.T. Outer membrane vesicles (OMVs) of gram-negative bacteria: A perspective update. Front. Microbiol. 2017, 8, 1053. [Google Scholar] [CrossRef]
- Mashburn-Warren, L.M.; Whiteley, M. Special delivery: Vesicle trafficking in prokaryotes. Mol. Microbiol. 2006, 61, 839–846. [Google Scholar] [CrossRef] [PubMed]
- Tashiro, Y.; Uchiyama, H.; Nomura, N. Multifunctional membrane vesicles in Pseudomonas aeruginosa. Environ. Microbiol. 2012, 14, 1349–1362. [Google Scholar] [CrossRef]
- Toyofuku, M.; Roschitzki, B.; Riedel, K.; Eberl, L. Identification of proteins associated with the Pseudomonas aeruginosa biofilm extracellular matrix. J. Proteome Res. 2012, 11, 4906–4915. [Google Scholar] [CrossRef]
- Jones, A.; Dodd, M.; Govan, J.; Barcus, V.; Doherty, C.; Morris, J.; Webb, A.K. Burkholderia cenocepacia and Burkholderia multivorans: Influence on survival in cystic fibrosis. Thorax 2004, 59, 948–951. [Google Scholar] [CrossRef]
- Vanlaere, E.; Baldwin, A.; Gevers, D.; Henry, D.; De Brandt, E.; LiPuma, J.J.; Mahenthiralingam, E.; Speert, D.E.; Dowson, C.; Vandammeet, P. Taxon K, a complex within the Burkholderia cepacia complex, comprises at least two novel species, Burkholderia contaminans sp. nov. and Burkholderia lata sp. nov. Int. J. Syst. Evol. Microbiol. 2009, 59, 102–111. [Google Scholar] [CrossRef]
- De Smet, B.; Mayo, M.; Peeters, C.; Zlosnik, J.E.; Spilker, T.; Hird, T.J.; LiPuma, J.J.; Kidd, T.J.; Kaestli, M.; Ginther, J.L.; et al. Burkholderia stagnalis sp. nov. and Burkholderia territorii sp. nov.; two novel Burkholderia cepacia complex species from environmental and human sources. Int. J. Syst. Evol. Microbiol. 2015, 65, 2265–2271. [Google Scholar] [CrossRef]
- Depoorter, E.; Bull, M.J.; Peeters, C.; Coenye, T.; Vandamme, P.; Mahenthiralingam, E. Burkholderia: An update on taxonomy and biotechnological potential as antibiotic producers. Appl. Microbiol. Biotechnol. 2016, 100, 5215–5229. [Google Scholar] [CrossRef]
- Bach, E.; Sant’Anna, F.H.; Magrich dos Passos, J.F.; Balsanelli, E.; de Baura, V.A.; Pedrosa, F.d.O.; Maltempi de Souza, E.; Pereira Passaglia, L.M. Detection of misidentifications of species from the Burkholderia cepacia complex and description of a new member, the soil bacterium Burkholderia catarinensis sp. nov. Pathog. Dis. 2017, 75. [Google Scholar] [CrossRef] [PubMed]
- Martina, P.; Leguizamon, M.; Prieto, C.I.; Sousa, S.A.; Montanaro, P.; Draghi, W.O.; Stämmler, M.; Bettiol, M.; de Carvalho, C.C.; Palau, J.; et al. Burkholderia puraquae sp. nov., a novel species of the Burkholderia cepacia complex isolated from hospital settings and agricultural soils. Int. J. Syst. Evol. Microbiol. 2018, 68, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Cuzzi, B.; Herasimenka, Y.; Silipo, A.; Lanzetta, R.; Liut, G.; Rizzo, R.; Cescutti, P. Versatility of the Burkholderia cepacia Complex for the biosynthesis of exopolysaccharides: A comparative structural investigation. PLoS ONE 2014, 9, e94372. [Google Scholar] [CrossRef] [PubMed]
- Pellizzoni, E.; Ravalico, F.; Scaini, D.; Delneri, A.; Rizzo, R.; Cescutti, P. Biofilms produced by Burkholderia cenocepacia: Influence of media and solid supports on composition of matrix exopolysaccharides. Microbiology 2016, 162, 283–294. [Google Scholar] [CrossRef]
- Dolfi, S.; Sveronis, A.; Silipo, A.; Rizzo, R.; Cescutti, P. A novel rhamno-mannan exopolysaccharide isolated from biofilms of Burkholderia multivorans C1576. Carbohydr. Res. 2015, 411, 42–48. [Google Scholar] [CrossRef]
- Kuttel, M.M.; Cescutti, P.; Distefano, M.; Rizzo, R. Fluorescence and NMR spectroscopy together with molecular simulations reveal amphiphilic characteristics of a Burkholderia biofilm exopolysaccharide. J. Biol. Chem. 2017, 292, 11034–11042. [Google Scholar] [CrossRef]
- Rani, A.; Babu, S. Environmental proteomic studies: Closer step to understand bacterial biofilms. World J. Microbiol. Biotechnol. 2018, 34, 120. [Google Scholar] [CrossRef]
- Van Acker, H.; Crabbé, A.; Jurėnas, D.; Ostyn, L.; Sass, A.; Daled, S.; Dhaenens, M.; Deforce, D.; Teirlinck, E.; De Keersmaecker, H.; et al. The role of small proteins in Burkholderia cenocepacia J2315 biofilm formation, persistence and intracellular growth. Biofilm 2019, 1, 100001. [Google Scholar] [CrossRef]
- Khan, M.M.; Chattagul, S.; Tran, B.Q.; Freiberg, J.A.; Nita-Lazar, A.; Shirtliff, M.E.; Sermswan, R.W.; Ernst, R.; Goodlett, D.R. Temporal proteomic profiling reveals changes that support Burkholderia biofilms. Pathog. Dis. 2019, 77, ftz005. [Google Scholar] [CrossRef]
- Reamtong, O.; Indrawattana, N.; Rungruengkitkun, A.; Thiangtrongjit, T.; Duangurai, T.; Chongsa-nguan, M.; Pumirat, P. Altered proteome of a Burkholderia pseudomallei mutant defective in short-chain dehydrogenase affects cell adhesion, biofilm formation and heat stress tolerance. PeerJ 2020, 8, e8659. [Google Scholar] [CrossRef]
- Baker, S.M.; Davitt, C.J.H.; Motyka, N.; Kikendall, N.L.; Russell-Lodrigue, K.; Roy, C.J.; Morici, L.A. A Burkholderia pseudomallei Outer Membrane Vesicle Vaccine Provides Cross Protection against Inhalational Glanders in Mice and Non-Human Primates. Vaccines 2017, 9, 49. [Google Scholar] [CrossRef] [PubMed]
- Nieves, W.; Petersen, H.; Judy, B.M.; Blumentritt, C.A.; Russell-Lodrigue, K.; Roy, C.J.; Torres, A.G.; Morici, L.A. A Burkholderia pseudomallei outer membrane vesicle vaccine provides protection against lethal sepsis. Clin. Vaccine Immunol. 2014, 21, 747–754. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Kim, M.H.; Son, J.H.; Kim, S.I.; Yun, S.H.; Kim, K.; Kim, S.; Shin, M.; Lee, J.C. Outer membrane vesicles produced by Burkholderia cepacia cultured with subinhibitory concentrations of ceftazidime enhance pro-inflammatory responses. Virulence 2020, 11, 995–1005. [Google Scholar] [CrossRef]
- Wang, Y.; Hoffmann, J.P.; Chou, C.W.; Höner, Z.; Bentrup, K.; Fuselier, J.A.; Bitoun, J.P.; Wimley, W.C.; Morici, L.A. Burkholderia thailandensis outer membrane vesicles exert antimicrobial activity against drug-resistant and competitor microbial species. J. Microbiol. 2020, 58, 550–562. [Google Scholar] [CrossRef] [PubMed]
- Merritt, J.H.; Kadouri, D.E.; O’Toole, G.A. Growing and analyzing static biofilms. Curr. Protoc. Microbiol. 2005. [Google Scholar] [CrossRef]
- Mueller, J.H.; Hinton, J. A protein-free medium for primary isolation of the Gonococcus and Meningococcus. Exp. Biol. Med. 1941, 48, 330–333. [Google Scholar] [CrossRef]
- Sgarra, R.; Furlan, C.; Zammitti, S.; Lo Sardo, A.; Maurizio, E.; Di Bernardo, J.; Giancotti, V.; Manfioletti, G. Interaction proteomics of the HMGA chromatin architectural factors. Proteomics 2008, 8, 4721–4732. [Google Scholar] [CrossRef]
- Galperin, M.Y.; Makarova, K.; Wolf, Y.; Koonin, E. Expanded microbial genome coverage and improved protein family annotation in the COG database. Nucleic Acids Res. 2015, 43, D261–D269. [Google Scholar] [CrossRef]
- Taboada, B.; Estrada, K.; Ciria, R.; Merino, E. Operon-mapper: A web server for precise operon identification in bacterial and archaeal genomes. Bioinformatics 2018, 34, 4118–4120. [Google Scholar] [CrossRef]
- Peabody, M.A.; Laird, M.R.; Vlasschaert, C.; Lo, R.; Brinkman, F.S. PSORTdb: Expanding the bacteria and archaea protein subcellular localization database to better reflect diversity in cell envelope structures. Nucleic Acids Res. 2016, 44, D663–D668. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Gable, A.L.; Lyon, D.; Junge, A.; Wyder, S.; Huerta-Cepas, J.; Simonovic, M.; Doncheva, N.T.; Morris, J.H.; Bork, P.; et al. STRING v11: Protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019, 47, D607–D613. [Google Scholar] [CrossRef] [PubMed]
- Webb, J.S.; Thompson, L.S.; James, S.; Charlton, T.; Tolker-Nielsen, T.; Koch, B.; Givskov, M.; Kjelleberget, S. Cell death in Pseudomonas aeruginosa biofilm development. J. Bacteriol. 2003, 185, 4585–4592. [Google Scholar] [CrossRef] [PubMed]
- Gallaher, T.K.; Wu, S.; Webster, P.; Aguilera, R. Identification of biofilm proteins in non-typeable Haemophilus influenzae. BMC Microbiol. 2006, 6, 65. [Google Scholar] [CrossRef] [PubMed]
- Curtis, P.D.; Atwood, J.; Orlando, R.; Shimkets, L.J. Proteins associated with the Myxococcus xanthus extracellular matrix. J. Bacteriol. 2007, 189, 7634–7642. [Google Scholar] [CrossRef]
- Eboigbodin, K.E.; Biggs, C.A. Characterization of the extracellular polymeric substances produced by Escherichia coli using infrared spectroscopic, proteomic; aggregation studies. Biomacromolecules 2008, 9, 686–695. [Google Scholar] [CrossRef]
- Park, C.; Novak, J.T.; Helm, R.F.; Ahn, Y.O.; Esen, A. Evaluation of the extracellular proteins in full-scale activated sludges. Water Res. 2008, 42, 3879–3889. [Google Scholar] [CrossRef]
- Jiao, Y.; D’haeseleer, P.; Dill, B.D.; Shah, M.; Verberkmoes, N.C.; Hettich, R.L.; Banfield, J.F.; Thelenet, M.P. Identification of biofilm matrix-associated proteins from an acid mine drainage microbial community. Appl. Environ. Microbiol. 2011, 77, 5230–5237. [Google Scholar] [CrossRef]
- Allan, N.D.; Kooi, C.; Sokol, P.A.; Beveridge, T.J. Putative virulence factors are released in association with membrane vesicles from Burkholderia cepacia. Can. J. Microbiol. 2003, 49, 613–624. [Google Scholar] [CrossRef]
- Lee, E.Y.; Bang, Y.B.; Park, G.W.; Choi, D.S.; Kang, J.S.; Kim, H.-J.; Park, K.-S.; Lee, J.-O.; Kwon, K.-H.; Kim, K.P.; et al. Global proteomic profiling of native outer membrane vesicles derived from Escherichia coli. Proteomics 2007, 7, 3143–3153. [Google Scholar] [CrossRef]
- Choi, D.S.; Kim, D.K.; Choi, S.J.; Lee, J.; Choi, J.P.; Rho, S.; Park, S.-H.; Kim, Y.-K.; Hwang, D.; Gho, Y.S. Proteomic analysis of outer membrane vesicles derived from Pseudomonas aeruginosa. Proteomics 2011, 11, 3424–3429. [Google Scholar] [CrossRef]
- Riley, M.A.; Gordon, D.M. The ecological role of bacteriocins in bacterial competition. Trends Microbiol. 1999, 7, 129–133. [Google Scholar] [CrossRef]
- Bakkal, S.; Robinson, S.M.; Ordonez, C.L.; Waltz, D.A.; Riley, M.A. Role of bacteriocins in mediating interactions of bacterial isolates taken from cystic fibrosis patients. Microbiology 2010, 156, 2058–2067. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tseng, B.S.; Reichhardt, C.; Merrihew, G.E.; Araujo-Hernandez, S.A.; Harrison, J.J.; MacCoss, M.J.; Parsek, M.R. A biofilm matrix-associated protease inhibitor protects Pseudomonas aeruginosa from proteolytic attack. MBio 2018, 9, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Hassett, D.J.; Cohen, S. Bacterial adaptation to oxidative stress: Implications for pathogenesis and interaction with phagocytic cells. FASEB J. 1989, 3, 2574–2582. [Google Scholar] [CrossRef]
- Thomas, M.S. Iron acquisition mechanisms of the Burkholderia cepacia complex. BioMetals 2007, 431–452. [Google Scholar] [CrossRef]
- Cornelis, P.; Dingemans, J. Pseudomonas aeruginosa adapts its iron uptake strategies in function of the type of infections. Front. Cell. Infect. Microbiol. 2013, 3, 75. [Google Scholar] [CrossRef]
- Ganz, T. Iron and infection. Int. J. Hematol. 2018, 107, 7–15. [Google Scholar] [CrossRef]
- Whitby, P.W.; VanWagoner, T.M.; Springer, J.M.; Morton, D.J.; Seale, T.W.; Stull, T.L. Burkholderia cenocepacia utilizes ferritin as an iron source. J. Med. Microbiol. 2016, 55, 661–668. [Google Scholar] [CrossRef]
- Jeffery, C.J. Moonlighting proteins: Old proteins learning new tricks. Trends Genet. 2003, 19, 415–417. [Google Scholar] [CrossRef]
- Kunert, A.; Losse, J.; Gruszin, C.; Huhn, M.; Kaendler, K.; Mikkat, S.; Volke, D.; Hoffmann, R.; Jokiranta, T.S.; Seeberger, H.; et al. Immune evasion of the human pathogen Pseudomonas aeruginosa: Elongation factor Tuf Is a factor H and plasminogen binding protein. J. Immunol. 2007, 179, 2979–2988. [Google Scholar] [CrossRef]
- Granato, D.; Bergonzelli, G.E.; Pridmore, R.D.; Marvin, L.; Rouvet, M.; Corthésy-Theulazet, I.E. Cell surface-associated elongation factor Tu mediates the attachment of Lactobacillus johnsonii NCC533 (La1) to Human intestinal cells and mucins. Infect. Immun. 2004, 72, 2160–2169. [Google Scholar] [CrossRef] [PubMed]
- Bergonzelli, G.E.; Granato, D.; Pridmore, R.D.; Marvin-Guy, L.F.; Donnicola, D.; Corthésy-Theulaz, I.E. GroEL of Lactobacillus johnsonii La1 (NCC 533) is cell surface associated: Potential role in interactions with the host and the gastric pathogen Helicobacter pylori. Infect. Immun. 2006, 74, 425–434. [Google Scholar] [CrossRef] [PubMed]
- Kolling, G.L.; Matthews, K.R. Export of virulence genes and Shiga toxin by membrane vesicles of Escherichia coli O157:H7. Appl. Environ. Microbiol. 1999, 65, 1843–1848. [Google Scholar] [CrossRef] [PubMed]
- Yaron, S.; Kolling, G.L.; Simon, L.; Matthews, K.R. Vesicle-mediated transfer of virulence genes from Escherichia coli O157:H7 to other enteric bacteria. Appl. Environ. Microbiol. 2000, 66, 4414–4420. [Google Scholar] [CrossRef] [PubMed]
- Whitchurch, C.B.; Tolker-Nielsen, T.; Ragas, P.C.; Mattick, J.S. Extracellular DNA required for bacterial biofilm formation. Science 2002, 295, 1487. [Google Scholar] [CrossRef] [PubMed]
- Mann, E.E.; Rice, K.C.; Boles, B.R.; Endres, J.L.; Ranjit, D.; Chandramohan, L.; Tsang, L.H.; Smeltzer, M.S.; Horswill, A.R.; Bayles, K.W. Modulation of eDNA release and degradation affects Staphylococcus aureus biofilm maturation. PLoS ONE 2009, 4, e5822. [Google Scholar] [CrossRef] [PubMed]
- Harmsen, M.; Lappann, M.; Knøchel, S.; Molin, S. Role of extracellular DNA during biofilm formation by Listeria monocytogenes. Appl. Environ. Microbiol. 2010, 76, 2271–2279. [Google Scholar] [CrossRef]
- Lappann, M.; Claus, H.; Van Alen, T.; Harmsen, M.; Elias, J.; Molin, S.; Vogel, U. A dual role of extracellular DNA during biofilm formation of Neisseria meningitides. Mol. Microbiol. 2010, 75, 1355–1371. [Google Scholar] [CrossRef]
- Seper, A.; Fengler, V.H.I.; Roier, S.; Wolinski, H.; Kohlwein, S.D.; Bishop, A.L.; Camilli, J.; Schild, S. Extracellular nucleases and extracellular DNA play important roles in Vibrio cholerae biofilm formation. Mol. Microbiol. 2011, 82, 1015–1037. [Google Scholar] [CrossRef]
- Allesen-Holm, M.; Barken, K.B.; Yang, L.; Klausen, M.; Webb, J.S.; Kjelleberg, S.; Molin, S.; Givskon, M.; Tolker-Nielsen, T. A characterization of DNA release in Pseudomonas aeruginosa cultures and biofilms. Mol. Microbiol. 2016, 59, 1114–1128. [Google Scholar] [CrossRef]
- Dillon, S.C.; Dorman, C.J. Bacterial nucleoid-associated proteins, nucleoid structure and gene expression. Nat. Rev. Microbiol. 2010, 8, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Inhülsen, S.; Aguilar, C.; Schmid, N.; Suppiger, A.; Riedel, K.; Eberl, L. Identification of functions linking quorum sensing with biofilm formation in Burkholderia cenocepacia H111. Microbiologyopen 2012, 1, 225–242. [Google Scholar] [CrossRef] [PubMed]


| Proteins | Gene Name/Identifier | Accession Number a | Number of Matches b | Subcellular Localization c |
|---|---|---|---|---|
| Acyl carrier protein | acpP | ACP_BURCA | 35 | cytoplasm |
| thiol peroxidase | tpx | WP_006400399.1 | 30 | periplasmic space |
| carboxymuconolactone decarboxylase | ahpD | EGD01850.1 | 22 | cytoplasm |
| beta-ketoacyl-ACP reductase | Bmul_2181 | AOL03371.1 | 17 | cytoplasm |
| branched-chain amino acid ABC transporter substrate-binding protein | livK/Bmul_3623 | WP_006396439.1 | 16 | periplasmic space |
| thiol: disulfide interchange protein DsbA/DsbL | dsbA | WP_006398112.1 | 15 | periplasmic space |
| Malate dehydrogenase | mdh | WP_006399543.1 | 13 | unknown |
| ABC transporter substrate-binding protein | Bmul_3347 | WP_006397294.1 | 13 | periplasmic space |
| Fe(3+) ABC transporter substrate-binding protein | Bmul_2093 | WP_035953191.1 | 11 | periplasmic space |
| glutamate/aspartate ABC transporter substrate-binding protein | Bmul_2714 | WP_006408338.1 | 10 | periplasmic space |
| RNA chaperone Hfq | hfq/Bmul_1468 | WP_063496600.1 | 10 | cytoplasm |
| thioredoxin | Bmul_1445 | ABC39250.1 | 9 | cytoplasm |
| ribosomal protein S1 | rpsA | EJO59460.1 | 9 | cytoplasm |
| Protein-export protein SecB | secB | SECB_BURCA | 8 | cytoplasm |
| hypothetical protein | Bmul_2263 | SAK23673.1 | 8 | unknown |
| 5-methyltetrahydropteroyltriglutamate-homocysteine S-methyltransferase | metE | WP_060098089.1 | 8 | cytoplasm |
| NADP-dependent phosphogluconate dehydrogenase | gnd | WP_006399510.1 | 8 | cytoplasm |
| Endoribonuclease L-PSP | Bmul_0927 | WP_006398680.1 | 7 | unknown |
| NADP-dependent isocitrate dehydrogenase | Bmul_0771 | WP_006398523.1 | 7 | cytoplsm |
| superoxide dismutase | sod | WP_006398503.1 | 6 | periplasmic space |
| OMV Proteins | Gene Name/Identifier | Accession Number a | Number of Matches b | Subcellular Localization c |
|---|---|---|---|---|
| porin | opcP | WP_054315301.1 | 30 | outer membrane |
| OmpA-like protein | Bmul_2265 | WP_006400736.1 | 17 | outer membrane |
| chaperonin GroEL | groL/Bmul_2528 | WP_006400973.1 | 16 | cytoplasm |
| porin | opcP | WP_059451674.1 | 15 | outer membrane |
| iron complex outer membrane receptor protein | Bmul_1594 | BAG43570.1 | 12 | outer membrane |
| glutamate/aspartate ABC transporter, periplasmic glutamate/aspartate-binding protein | gltI | EED98255.1 | 10 | unknown |
| hypothetical OmpA-like protein | opcP | WP_059451674.1 | 9 | outer membrane |
| putative lipoprotein | Bmul_1389 | WP_006402280.1 | 7 | unknown |
| TonB-dependent hemoglobin/transferrin/lactoferrin family receptor | Bmul_3338 | WP_048804300.1 | 7 | outer membrane |
| aspartate/tyrosine/aromatic aminotransferase | aspC | WP_038714441.1 | 4 | cytoplasm |
| adenosylhomocysteinase | ahcY | EEE03553.1 | 5 | cytoplasm |
| acyl carrier protein | acpP | ACP_BURCA | 4 | cytoplasm |
| malate dehydrogenase | mdh | MDH_BURCJ | 4 | unknown |
| putative outer membrane protein | Bmul_1507 | OJD05646.1 | 4 | outer membrane |
| enolase | eno | ENO_BURM1 | 4 | cytoplasm |
| aldehyde dehydrogenase family protein | Bmul_3451 | WP_054317513.1 | 4 | cytoplasm |
| citrate synthase | Bmul_4643 | OJD04157.1 | 3 | cytoplasm |
| redoxin family protein | Bmul_1331 | KOS88719.1 | 3 | cytoplasm |
| phasin protein | Bmul_1136 | WP_006401963.1 | 3 | unknown |
| bacterioferritin | bfr/Bmul_1081 | ABO01605.1 | 2 | cytoplasm |
| Protein | Gene Name/ Identifier | Accession Number a | Subcellular localization b |
|---|---|---|---|
| Porin | opcP | WP_054315301.1 | Outer membrane |
| Chaperonin GroEL | groL | WP_006400973.1 | Cytoplasmic |
| Citrate (Si)-synthase | Bmul_4643 | OJD04157.1 | Cytoplasmic |
| Aldehyde dehydrogenase family protein | Bmul_3451 | WP_054317513.1 | Cytoplasmic |
| Malate dehydrogenase | mdh | WP_006399543.1 | Unknown |
| Leucine ABC transporter subunit substrate binding protein LivK | Bmul_3623 | OJD03740.1 | Periplasmic |
| Glycine dehydrogenase | gcvP | GCSP_BURM1 | Unknown |
| Beta-ketoacyl-ACP reductase | Bmul_2181 | AOL03371.1 | Cytoplasmic |
| 2,3,4,5- tetrahydropyridine-2,6- dicarboxylate N succinyltransferase | dapD | DAPD_BURCA | Cytoplasmic |
| ATP-dependent Clp protease, proteolytic subunit ClpP | clpP/Bmul_1349 | EED97471.1 | Cytoplasmic |
| YceI family protein | Bmul_2669 | EED98206.1 | Unknown |
| Thiol: disulfide interchange protein DsbA/DsbL | dsbA | WP_006398112.1 | Periplasmic |
| Redoxin family protein | Bmul_1331 | KOS88719.1 | Cytoplasmic |
| Hypothetical protein CA831_04455 partial | Bmul_4929 | OXH91981.1 | Unknown |
| Acyl carrier protein | acpP | ACP_BURCA | Cytoplasmic |
| Thioredoxin | Bmul_1445 | ABC39250.1 | Cytoplasmic |
| Conserved hypothetical protein | Bmul_0436 | EBA48742.1 | Unknown |
| Endoribonuclease L-PSP | Bmul_0927 | WP_006398680.1 | Unknown |
| 50S ribosomal protein | rplL | RL7_BURM1 | Cytoplasmic |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Terán, L.C.; Distefano, M.; Bellich, B.; Petrosino, S.; Bertoncin, P.; Cescutti, P.; Sblattero, D. Proteomic Studies of the Biofilm Matrix including Outer Membrane Vesicles of Burkholderia multivorans C1576, a Strain of Clinical Importance for Cystic Fibrosis. Microorganisms 2020, 8, 1826. https://doi.org/10.3390/microorganisms8111826
Terán LC, Distefano M, Bellich B, Petrosino S, Bertoncin P, Cescutti P, Sblattero D. Proteomic Studies of the Biofilm Matrix including Outer Membrane Vesicles of Burkholderia multivorans C1576, a Strain of Clinical Importance for Cystic Fibrosis. Microorganisms. 2020; 8(11):1826. https://doi.org/10.3390/microorganisms8111826
Chicago/Turabian StyleTerán, Lucrecia C., Marco Distefano, Barbara Bellich, Sara Petrosino, Paolo Bertoncin, Paola Cescutti, and Daniele Sblattero. 2020. "Proteomic Studies of the Biofilm Matrix including Outer Membrane Vesicles of Burkholderia multivorans C1576, a Strain of Clinical Importance for Cystic Fibrosis" Microorganisms 8, no. 11: 1826. https://doi.org/10.3390/microorganisms8111826
APA StyleTerán, L. C., Distefano, M., Bellich, B., Petrosino, S., Bertoncin, P., Cescutti, P., & Sblattero, D. (2020). Proteomic Studies of the Biofilm Matrix including Outer Membrane Vesicles of Burkholderia multivorans C1576, a Strain of Clinical Importance for Cystic Fibrosis. Microorganisms, 8(11), 1826. https://doi.org/10.3390/microorganisms8111826





