Retrospective Analysis on Antimicrobial Resistance Trends and Prevalence of β-lactamases in Escherichia coli and ESKAPE Pathogens Isolated from Arabian Patients during 2000–2020
Abstract
:1. Introduction
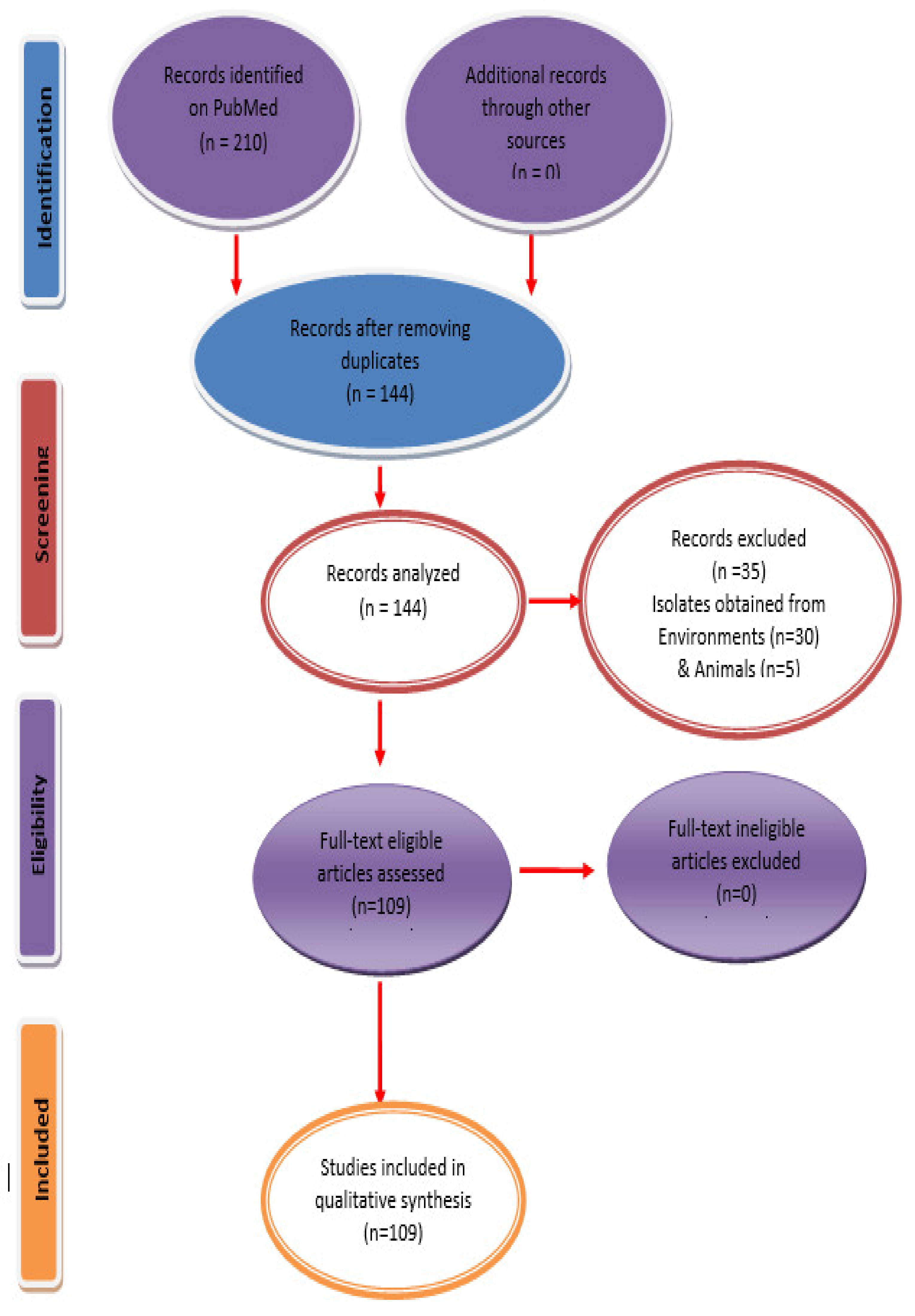
2. Materials and Methods
3. Results
3.1. Saudi Arabia
3.2. United Arab Emirates
3.3. Qatar
3.4. Kuwait
3.5. Oman
3.6. Bahrain
3.7. Yemen
3.8. Jordan
3.9. Iraq
3.10. Syria
3.11. Lebanon
3.12. Palestine
3.13. Egypt
3.14. Libya
3.15. Algeria
3.16. Tunisia
3.17. Morocco
3.18. Sudan
3.19. Djibouti
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Adedeji, W.A. The Treasure Called Antibiotics. Ann. Ibadan. Postgrad. Med. 2016, 14, 56–57. [Google Scholar]
- Spellberg, B.; Taylor-Blake, B. On the exoneration of Dr. William, H. Stewart: Debunking an urban legend. Infect. Dis. Poverty 2013, 2, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davies, J.; Davies, D. Origins and evolution of antibiotic resistance. Microbiologia 1996, 12, 9–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hancock, R.E.W. Mechanisms of action of newer antibiotics for Gram-positive pathogens. Lancet Infect. Dis. 2005, 5, 209–218. [Google Scholar] [CrossRef]
- Neu, H.C. Infections caused by gram-negative pathogens: New clinical considerations. Bull. N. Y. Acad. Med. 1975, 51, 1075–1083. [Google Scholar] [PubMed]
- Rice, L.B. Federal Funding for the Study of Antimicrobial Resistance in Nosocomial Pathogens: No ESKAPE. J. Infect. Dis. 2008, 197, 1079–1081. [Google Scholar] [CrossRef]
- Founou, R.C.; Founou, L.L.; Essack, S.Y. Clinical and economic impact of antibiotic resistance in developing countries: A systematic review and meta-analysis. PLoS ONE 2017, 12, e0189621. [Google Scholar] [CrossRef] [Green Version]
- New Report Calls for Urgent Action to Avert Antimicrobial Resistance Crisis. Available online: https://www.who.int/news-room/detail/29-04-2019-new-report-calls-for-urgent-action-to-avert-antimicrobial-resistance-crisis (accessed on 29 April 2020).
- WHO. Antimicrobial Resistance; WHO: Pari, France, 2014; Volume 61, ISBN 978-92-4-156474-8. [Google Scholar]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [Green Version]
- Uz Zaman, T.; Alrodayyan, M.; Albladi, M.; Aldrees, M.; Siddique, M.I.; Aljohani, S.; Balkhy, H.H. Clonal diversity and genetic profiling of antibiotic resistance among multidrug/carbapenem-resistant Klebsiella pneumoniae isolates from a tertiary care hospital in Saudi Arabia. BMC Infect. Dis. 2018, 18, 205. [Google Scholar] [CrossRef] [Green Version]
- Elabd, F.M.; Al-Ayed, M.S.Z.; Asaad, A.M.; Alsareii, S.A.; Qureshi, M.A.; Musa, H.A.A. Molecular characterization of oxacillinases among carbapenem-resistant Acinetobacter baumannii nosocomial isolates in a Saudi hospital. J. Infect. Public Health 2015, 8, 242–247. [Google Scholar] [CrossRef] [Green Version]
- Ibrahim, M.E.; Abbas, M.; Al-Shahrai, A.M.; Elamin, B.K. Phenotypic Characterization and Antibiotic Resistance Patterns of Extended-Spectrum β-Lactamase- and AmpC β-Lactamase-Producing Gram-Negative Bacteria in a Referral Hospital, Saudi Arabia. Can. J. Infect. Dis. Med. Microbiol. = J. Can. Mal. Infec. Microbiol. Med. 2019, 6054694. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Somily, A.M.; Habib, H.A.; Absar, M.M.; Arshad, M.Z.; Manneh, K.; Al Subaie, S.S.; Al Hedaithy, M.A.; Sayyed, S.B.; Shakoor, Z.; Murray, T.S. ESBL-producing Escherichia coli and Klebsiella pneumoniae at a tertiary care hospital in Saudi Arabia. J. Infect. Dev. Ctries. 2014, 8, 1129–1136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Agamy, M.H.; Shibl, A.M.; Ali, M.S.; Khubnani, H.; Radwan, H.H.; Livermore, D.M. Distribution of β-lactamases in carbapenem-non-susceptible Acinetobacter baumannii in Riyadh, Saudi Arabia. J. Glob. Antimicrob. Resist. 2014, 2, 17–21. [Google Scholar] [CrossRef]
- Al-Agamy, M.H.; Shibl, A.M.; Tawfik, A.F.; Elkhizzi, N.A.; Livermore, D.M. Extended-spectrum and metallo-beta-lactamases among ceftazidime-resistant Pseudomonas aeruginosa in Riyadh, Saudi Arabia. J. Chemother. 2012, 24, 97–100. [Google Scholar] [CrossRef]
- Abdalhamid, B.; Hassan, H.; Itbaileh, A.; Shorman, M. Characterization of carbapenem-resistant Acinetobacter baumannii clinical isolates in a tertiary care hospital in Saudi Arabia. New Microbiol. 2014, 37, 65–73. [Google Scholar] [PubMed]
- Al-Zahrani, I.A.; Alasiri, B.A. The emergence of carbapenem-resistant Klebsiella pneumoniae isolates producing OXA-48 and NDM in the Southern (Asir) Province, Saudi Arabia. Saudi Med. J. 2018, 39, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Al-Qahtani, A.A.; Al-Agamy, M.H.; Ali, M.S.; Al-Ahdal, M.N.; Aljohi, M.A.; Shibl, A.M. Characterization of extended-spectrum betalactamase-producing klebsiella pneumoniae from Riyadh, Saudi Arabia. J. Chemother. 2014, 26, 139–145. [Google Scholar] [CrossRef]
- Alyamani, E.J.; Khiyami, A.M.; Booq, R.Y.; Majrashi, M.A.; Bahwerth, F.S.; Rechkina, E. The occurrence of ESBL-producing Escherichia coli carrying aminoglycoside resistance genes in urinary tract infections in Saudi Arabia. Ann. Clin. Microbiol. Antimicrob. 2017, 16, 1. [Google Scholar] [CrossRef] [Green Version]
- Al-Agamy, M.H.M.; Shibl, A.M.; Tawfik, A.F. Prevalence and molecular characterization of extended-spectrum β-lactamase-producing Klebsiella pneumoniae in Riyadh, Saudi Arabia. Ann. Saudi Med. 2009, 29, 253–257. [Google Scholar] [CrossRef] [Green Version]
- Kader, A.A.; Kumar, A. Prevalence and antimicrobial susceptibility of extended-spectrum β-lactamase-producing Escherichia coli and Klebsiella pneumoniae in a general hospital. Ann. Saudi Med. 2005, 25, 239–242. [Google Scholar] [CrossRef]
- Yezli, S.; Shibl, A.M.; Livermore, D.M.; Memish, Z.A. Prevalence and antimicrobial resistance among Gram-negative pathogens in Saudi Arabia. J. Chemorther. 2014, 26, 257–272. [Google Scholar] [CrossRef] [PubMed]
- Tawfik, A.F.; Alswailem, A.M.; Shibl, A.M.; Al-Agamy, M.H.M. Prevalence and genetic characteristics of TEM, SHV, and CTX-M in clinical Klebsiella pneumoniae isolates from Saudi Arabia. Microb. Drug. Resist. 2011, 17, 383–388. [Google Scholar] [CrossRef] [PubMed]
- Al-Agamy, M.H.; Jeannot, K.; El-Mahdy, T.S.; Samaha, H.A.; Shibl, A.M.; Plésiat, P.; Courvalin, P. Diversity of Molecular Mechanisms Conferring Carbapenem Resistance to Pseudomonas aeruginosa Isolates from Saudi Arabia. Can. J. Infect. Dis. Med. Microbiol. 2016, 2016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, M.A.; Faiz, A. Antimicrobial resistance patterns of Pseudomonas aeruginosa in tertiary care hospitals of Makkah and Jeddah. Ann. Saudi Med. 2016, 36, 23–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamid, M.E.; Mustafa, F.Y.; Alwaily, A.; Abdelrahman, S.; Al Azragi, T. Prevalence of bacterial pathogens in Aseer region, Kingdom of Saudi Arabia: Emphasis on antimicrobial susceptibility of staphylococcus aureus. Oman Med. J. 2011, 26, 368–370. [Google Scholar] [CrossRef] [PubMed]
- Mashwal, F.A.; El Safi, S.H.; George, S.K.; Adam, A.A.; Jebakumar, A.Z. Incidence and molecular characterization of the extended spectrum beta lactamase-producing Escherichia coli isolated from urinary tract infections in Eastern Saudi Arabia. Saudi Med. J. 2017, 38, 811–815. [Google Scholar] [CrossRef] [PubMed]
- Al-Garni, S.M.; Ghonaim, M.M.; Ahmed, M.M.M.; Al-Ghamdi, A.S.; Ganai, F.A. Risk factors and molecular features of extended-spectrum beta-lactamase producing bacteria at southwest of Saudi Arabia. Saudi Med. J. 2018, 39, 1186–1194. [Google Scholar] [CrossRef]
- Hameed, T.; Al Nafeesah, A.; Chishti, S.; Al Shaalan, M.; Al Fakeeh, K. Community-acquired urinary tract infections in children: Resistance patterns of uropathogens in a tertiary care center in Saudi Arabia. Int. J. Pediatr. Adolesc. Med. 2019, 6, 51–54. [Google Scholar] [CrossRef]
- Kader, A.A.; Kumar, A.K. Prevalence of extended spectrum beta-lactamase among multidrug resistant gram-negative isolates from a general hospital in Saudi Arabia. Saudi Med. J. 2004, 25, 570–574. [Google Scholar]
- Ahmad, S.; Al-Juaid, N.F.; Alenzi, F.Q.; Mattar, E.H.; Bakheet, O.E.S. Prevalence, antibiotic susceptibility pattern and production of extended-spectrum β-lactamases amongst clinical isolates of Klebsiella pneumoniae at Armed Forces Hospital in Saudi Arabia. J. Coll. Phys. Surg. Pak. 2009, 19, 264–265. [Google Scholar]
- Marie, M.A.; John, J.; Krishnappa, L.G.; Gopalkrishnan, S. Molecular characterization of the β-lactamases in Escherichia coli and Klebsiella pneumoniae from a tertiary care hospital in Riyadh, Saudi Arabia. Microbiol. Immunol. 2013, 57, 805–810. [Google Scholar] [CrossRef] [PubMed]
- Aly, M.M.; Abu Alsoud, N.M.; Elrobh, M.S.; Al Johani, S.M.; Balkhy, H.H. High prevalence of the PER-1 gene among carbapenem-resistant Acinetobacter baumannii in Riyadh, Saudi Arabia. Eur. J. Clin. Microbiol. Infect. Dis. 2016, 35, 1759–1766. [Google Scholar] [CrossRef] [PubMed]
- Bindayna, K.M.; Khanfar, H.S.; Senok, A.C.; Botta, G.A. Predominance of CTX-M genotype among extended spectrum beta lactamase isolates in a tertiary hospital in Saudi Arabia. Saudi Med. J. 2010, 31, 859–863. [Google Scholar]
- Azim, N.S.A.; Nofal, M.Y.; Alharbi, M.A.; Al-Zaban, M.I.; Somily, A.M. Molecular-diversity, prevalence and antibiotic susceptibility of pathogenic klebsiella pneumoniae under Saudi condition. Pak. J. Biol. Sci. 2019, 22, 174–179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farah, S.M.; Alshehri, M.A.; Alfawaz, T.S.; Alasmeri, F.A.; Alageel, A.A.; Alshahrani, D.A. Trends in antimicrobial susceptibility patterns in King Fahad Medical City, Riyadh, Saudi Arabia. Saudi Med. J. 2019, 40, 252–259. [Google Scholar] [CrossRef]
- Ranjan Dash, N.; Albataineh, M.T.; Alhourani, N.; Khoudeir, A.M.; Ghanim, M.; Wasim, M.; Mahmoud, I. Community-acquired urinary tract infections due to extended-spectrum β-lactamase-producing organisms in United Arab Emirates. Travel Med. Infect. Dis. 2018, 22, 46–50. [Google Scholar] [CrossRef]
- Dash, N.; Panigrahi, D.; Zarouni, M.A.; Darwish, D.; Ghazawi, A.; Sonnevend, A.; Pal, T.; Yasin, F.; Hadi, S. Al High Incidence of New Delhi Metallo-Beta-Lactamase-Producing Klebsiella pneumoniae Isolates in Sharjah, United Arab Emirates. Microb. Drug Resist. 2014, 20, 52–56. [Google Scholar] [CrossRef]
- Al-Zarouni, M.; Senok, A.; Rashid, F.; Al-Jesmi, S.M.; Panigrahi, D. Prevalence and antimicrobial susceptibility pattern of extended-spectrum beta-lactamase-producing enterobacteriaceae in the United Arab Emirates. Med. Princ. Pract. 2008, 17, 32–36. [Google Scholar] [CrossRef]
- Alfaresi, M.S.; Elkoush, A.A.; Alshehhi, H.M.; Abdulsalam, A.I. Molecular characterization and epidemiology of extended-spectrum beta-lactamase-producing escherichia coli and klebsiella pneumoniae isolates in the United Arab Emirates. Med. Princ. Pract. 2011, 20, 177–180. [Google Scholar] [CrossRef]
- Moubareck, C.A.; Mouftah, S.F.; Pál, T.; Ghazawi, A.; Halat, D.H.; Nabi, A.; AlSharhan, M.A.; AlDeesi, Z.O.; Peters, C.C.; Celiloglu, H.; et al. Clonal emergence of Klebsiella pneumoniae ST14 co-producing OXA-48-type and NDM carbapenemases with high rate of colistin resistance in Dubai, United Arab Emirates. Int. J. Antimicrob. Agents. 2018, 52, 90–95. [Google Scholar] [CrossRef]
- Abdulwahab, A.; Zahraldin, K.; Sid Ahmed, M.A.; Jarir, S.A.; Muneer, M.; Mohamed, S.F.; Hamid, J.M.; Hassan, A.A.I.; Ibrahim, E.B. The emergence of multidrug-resistant Pseudomonas aeruginosa in cystic fibrosis patients on inhaled antibiotics. Lung India 2017, 34, 527–531. [Google Scholar] [CrossRef] [PubMed]
- Sid Ahmed, M.A.; Bansal, D.; Acharya, A.; Elmi, A.A.; Hamid, J.M.; Sid Ahmed, A.M.; Chandra, P.; Ibrahim, E.; Sultan, A.A.; Doiphode, S.; et al. Antimicrobial susceptibility and molecular epidemiology of extended-spectrum beta-lactamase-producing Enterobacteriaceae from intensive care units at Hamad Medical Corporation, Qatar. Antimicrob. Resist. Infect. Control. 2016, 5, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- AbdulWahab, A.; Taj-Aldeen, S.J.; Ibrahim, E.; Abdulla, S.H.; Muhammed, R.; Ahmed, I.; Abdeen, Y.; Sadek, O.; Abu-Madi, M. Genetic relatedness and host specificity of Pseudomonas aeruginosa isolates from cystic fibrosis and non-cystic fibrosis patients. Infect. Drug. Resist. 2014, 7, 309–316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eltai, N.O.; Al Thani, A.A.; Al-Ansari, K.; Deshmukh, A.S.; Wehedy, E.; Al-Hadidi, S.H.; Yassine, H.M. Molecular characterization of extended spectrum β-lactamases enterobacteriaceae causing lower urinary tract infection among pediatric population. Antimicrob. Resist. Infect. Control. 2018, 7, 90. [Google Scholar] [CrossRef]
- Eltai, N.O.; Yassine, H.M.; Al Thani, A.A.; Abu Madi, M.A.; Ismail, A.; Ibrahim, E.; Alali, W.Q. Prevalence of antibiotic resistant Escherichia coli isolates from fecal samples of food handlers in Qatar. Antimicrob. Resist. Infect. Control 2018, 7, 78. [Google Scholar] [CrossRef] [PubMed]
- Rolain, J.M.; Loucif, L.; Al-Maslamani, M.; Elmagboul, E.; Al-Ansari, N.; Taj-Aldeen, S.; Shaukat, A.; Ahmedullah, H.; Hamed, M. Emergence of multidrug-resistant Acinetobacter baumannii producing OXA-23 Carbapenemase in Qatar. New Microbes New Infect. 2016, 11, 47–51. [Google Scholar] [CrossRef] [Green Version]
- Bonnin, R.A.; Rotimi, V.O.; Al Hubail, M.; Gasiorowski, E.; Al Sweih, N.; Nordmann, P.; Poirela, L. Wide dissemination of GES-type carbapenemases in Acinetobacter baumannii isolates in Kuwait. Antimicrob. Agents Chemother. 2013, 57, 183–188. [Google Scholar] [CrossRef] [Green Version]
- Jamal, W.Y.; Albert, M.J.; Rotimi, V.O. High prevalence of New Delhi metallo-β-lactamase-1 (NDM-1) producers among carbapenem-resistant Enterobacteriaceae in Kuwait. PLoS ONE 2016, 11, e0152638. [Google Scholar] [CrossRef] [Green Version]
- Dimitrov, T.S.; Udo, E.E.; Awni, F.; Emara, M.; Passadilla, R. Etiology and antibiotic susceptibility patterns of community-acquired urinary tract Infections in a Kuwait Hospital. Med. Princ. Pract. 2004, 13, 334–339. [Google Scholar] [CrossRef]
- Mokaddas, E.M.; Abdulla, A.A.; Shati, S.; Rotimi, V.O. The technical aspects and clinical significance of detecting extended-spectrum β-lactamase-producing Enterobacteriaceae at a tertiary-care hospital in Kuwait. J. Chemother. 2008, 20, 445–451. [Google Scholar] [CrossRef]
- Ensor, V.M.; Jamal, W.; Rotimi, V.O.; Evans, J.T.; Hawkey, P.M. Predominance of CTX-M-15 extended spectrum β-lactamases in diverse Escherichia coli and Klebsiella pneumoniae from hospital and community patients in Kuwait. Int. J. Antimicrob. Agents 2009, 33, 487–489. [Google Scholar] [CrossRef] [PubMed]
- Poirel, L.; Carbonnelle, E.; Bernabeu, S.; Gutmann, L.; Rotimi, V.; Nordmann, P. Importation of OXA-48-producing Klebsiella pneumoniae from Kuwait. J. Antimicrob. Chemother. 2012, 67, 2051–2052. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poirel, L.; Rotimi, V.O.; Mokaddas, E.M.; Karim, A.; Nordmann, P. VEB-1-like Extended-Spectrum ß-Lactamases in Pseudomonas aeruginosa, Kuwait. Emerg. Infect. Dis. 2001, 7, 468–470. [Google Scholar] [CrossRef] [PubMed]
- Udo, E.E.; Al-Lawati, B.A.H.; Al-Muharmi, Z.; Thukral, S.S. Genotyping of methicillin-resistant Staphylococcus aureus in the Sultan Qaboos University Hospital, Oman reveals the dominance of Panton-Valentine leucocidin-negative ST6-IV/t304 clone. New Microbes New Infect. 2014, 2, 100–105. [Google Scholar] [CrossRef] [Green Version]
- Al-Lawati, A.M.; Crouch, N.D.; Elhag, K.M. Antibiotic consumption and development of resistance among gram-negative bacilli in intensive care units in Oman. Ann. Saudi Med. 2000, 20, 324–327. [Google Scholar] [CrossRef] [Green Version]
- Al Muharrmi, Z.; Rafay, A.M.; Balkhair, A.; Al-Tamemi, S.; Al Mawali, A.; Al Sadiri, H. Extended-spectrum β-lactamase (ESBL) in Omani Children: Study of prevalence, risk factors and clinical outcomes at Sultan Qaboos University Hospital, Sultanate of Oman. Sultan Qaboos Univ. Med. J. 2008, 8, 171–177. [Google Scholar]
- Dortet, L.; Poirel, L.; Al Yaqoubi, F.; Nordmann, P. NDM-1, OXA-48 and OXA-181 carbapenemase-producing Enterobacteriaceae in Sultanate of Oman. Clin. Microbiol. Infect. 2012, 18, E144–E148. [Google Scholar] [CrossRef] [Green Version]
- Joji, R.M.; Al-Rashed, N.; Saeed, N.K.; Bindayna, K.M. Detection of VIM and NDM-1 metallo-beta-lactamase genes in carbapenem-resistant Pseudomonas aeruginosa clinical strains in Bahrain. J. Lab. Physicians 2019, 11, 138–143. [Google Scholar] [CrossRef]
- Bindayna, K.M.; Senok, A.C.; Jamsheer, A.E. Prevalence of extended-spectrum beta-lactamase-producing Enterobacteriaceae in Bahrain. J. Infect. Public Health 2009, 2, 129–135. [Google Scholar] [CrossRef] [Green Version]
- Bindayna, K.M.; Murtadha, M. High prevalence of blaCTX-M in Enterobacteriaceae isolates from the Kingdom of Bahrain. Asian Pac. J. Trop. Med. 2011, 4, 937–940. [Google Scholar] [CrossRef] [Green Version]
- Gharout-Sait, A.; Alsharapy, S.A.; Brasme, L.; Touati, A.; Kermas, R.; Bakour, S.; Guillard, T.; de Champs, C. Enterobacteriaceae isolates carrying the New Delhi metallo-β-lactamase gene in Yemen. J. Med. Microbiol. 2014, 63, 1316–1323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nasher, S.; Alsharapy, S.; Al-Madhagi, A.; Zakham, F. Epidemiology of extended-spectrum β-lactamase producing escherichia coli from hospital settings in yemen. J. Infect. Dev. Ctries. 2018, 12, 953–959. [Google Scholar] [CrossRef] [PubMed]
- Bakour, S.; Alsharapy, S.A.; Touati, A.; Rolain, J.M. Characterization of acinetobacter baumannii clinical isolates carrying blaOXA-23 carbapenemase and 16S rRNA methylase armA genes in Yemen. Microb. Drug Resist. 2014, 20, 604–609. [Google Scholar] [CrossRef] [PubMed]
- Wolterlink-Van Loo, S.; Siemerink, M.A.J.; Perrakis, G.; Kaper, T.; Kengen, S.W.M.; Van der Oost, J. Improving low-temperature activity of Sulfolobus acidocaldarius 2-keto-3-deoxygluconate aldolase. Archaea 2009, 2, 233–239. [Google Scholar] [CrossRef]
- Al-Jamei, S.A.; Albsoul, A.Y.; Bakri, F.G.; Al-Bakri, A.G. Extended-spectrum β-lactamase producing E. coli in urinary tract infections: A two-center, cross-sectional study of prevalence, genotypes and risk factors in Amman, Jordan. J. Infect. Public Health 2019, 12, 21–25. [Google Scholar] [CrossRef]
- Al Dawodeyah, H.Y.; Obeidat, N.; Abu-Qatouseh, L.F.; Shehabi, A.A. Antimicrobial resistance and putative virulence genes of Pseudomonas aeruginosa isolates from patients with respiratory tract infection. Germs 2018, 8, 31–40. [Google Scholar] [CrossRef]
- Aqel, A.A.; Giakkoupi, P.; Alzoubi, H.; Masalha, I.; Ellington, M.J.; Vatopoulos, A. Detection of OXA-48-like and NDM carbapenemases producing Klebsiella pneumoniae in Jordan: A pilot study. J. Infect. Public Health 2017, 10, 150–155. [Google Scholar] [CrossRef] [Green Version]
- Ronat, J.B.; Kakol, J.; Khoury, M.N.; Berthelot, M.; Yun, O.; Brown, V.; Murphy, R.A. Highly drug-resistant pathogens implicated in burn-associated bacteremia in an Iraqi burn care unit. PLoS ONE 2014, 9, e101017. [Google Scholar] [CrossRef] [Green Version]
- Al-Charrakh, A.H.; Al-Awadi, S.J.; Mohammed, A.S. Detection of Metallo-β-Lactamase Producing Pseudomonas aeruginosa Isolated from Public and Private Hospitals in Baghdad, Iraq. Acta Med. Iran. 2016, 54, 107–113. [Google Scholar]
- Van Burgh, S.; Maghdid, D.M.; Ganjo, A.R.; Mansoor, I.Y.; Kok, D.J.; Fatah, M.H.; Alnakshabandi, A.A.; Asad, D.; Hammerum, A.M.; Ng, K.; et al. PME and Other ESBL-Positive Multiresistant Pseudomonas aeruginosa Isolated from Hospitalized Patients in the Region of Kurdistan, Iraq. Microb. Drug Resist. 2019, 25, 32–38. [Google Scholar] [CrossRef]
- Majeed, H.T.; Aljanaby, A.A.J. Antibiotic Susceptibility Patterns and Prevalence of Some Extended Spectrum Beta-Lactamases Genes in Gram-Negative Bacteria Isolated from Patients Infected with Urinary Tract Infections in Al-Najaf City, Iraq. Avicenna J. Med. Biotechnol. 2019, 11, 192–201. [Google Scholar] [PubMed]
- Hussein, N.H. Emergence of NDM-1 among carbapenemresistant klebsiella pneumoniae in Iraqi hospitals. Acta Microbiol. Immunol. Hung. 2018, 65, 211–227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamzeh, A.R.; Al Najjar, M.; Mahfoud, M. Prevalence of antibiotic resistance among Acinetobacter baumannii isolates from Aleppo, Syria. Am. J. Infect. Control 2012, 40, 776–777. [Google Scholar] [CrossRef]
- Al-Assil, B.; Mahfoud, M.; Hamzeh, A.R. Resistance trends and risk factors of extended spectrum β-lactamases in Escherichia coli infections in Aleppo, Syria. Am. J. Infect. Control 2013, 41, 597–600. [Google Scholar] [CrossRef] [PubMed]
- Samaha-Kfoury, J.N.; Kanj, S.S.; Araj, G.F. In vitro activity of antimicrobial agents against extended-spectrum β-lactamase-producing Escherichia coli and Klebsiella pneumoniae at a tertiary care center in Lebanon. Am. J. Infect. Control 2005, 33, 134–136. [Google Scholar] [CrossRef] [PubMed]
- Obeid Charrouf, F.; Hamze, M.; Mallat, H.; Achkar, M.; Dabboussi, F. Characterization of resistance genes in 68ESBL-producing Klebsiella pneumonia in Lebanon. Med. Mal. Infect. 2014, 44, 535–538. [Google Scholar] [CrossRef]
- Dahdouh, E.; Hajjar, M.; Suarez, M.; Daoud, Z. Acinetobacter baumannii isolated from lebanese patients: Phenotypes and genotypes of resistance, clonality, and determinants of pathogenicity. Front. Cell. Infect. Microbiol. 2016, 25, 163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arabaghian, H.; Salloum, T.; Alousi, S.; Panossian, B.; Araj, G.F.; Tokajian, S. Molecular Characterization of Carbapenem Resistant Klebsiella pneumoniae and Klebsiella quasipneumoniae Isolated from Lebanon. Sci. Rep. 2019, 9, 531. [Google Scholar] [CrossRef]
- Yaghi, J.; Fattouh, N.; Akkawi, C.; El Chamy, L.; Maroun, R.G.; Khalil, G. Unusually High Prevalence of Cosecretion of Ambler Class A and B Carbapenemases and Nonenzymatic Mechanisms in Multidrug-Resistant Clinical Isolates of Pseudomonas aeruginosa in Lebanon. Microb. Drug Resist. 2020, 26, 150–159. [Google Scholar] [CrossRef]
- Tayh, G.; Al Laham, N.; Ben Yahia, H.; Ben Sallem, R.; Elotto, A.E.; Ben Slamm, K. Extended-Spectrum β-Lactamases Among Enterobacteriaceae Isolated From Urinary Tract Infections in Gaza Strip, Palestine. Biomed. Res. Int. 2019, 2019, 4041801. [Google Scholar] [CrossRef] [Green Version]
- Adwan, K.; Jarrar, N.; Abu-Hijleh, A.; Adwan, G.; Awwad, E. Molecular characterization of Escherichia coli isolates from patients with urinary tract infections in Palestine. J. Med. Microbiol. 2014, 63, 229–234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaballah, A.; Elbaradei, A.; Elsheredy, A.; Kader, O. Emergence of blaveB and blages among VIM-producing Pseudomonas aeruginosa clinical isolates in Alexandria, Egypt. Acta Microbiol. Immunol. Hung. 2019, 66, 131–142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Shouny, W.A.; Ali, S.S.; Sun, J.; Samy, S.M.; Ali, A. Drug resistance profile and molecular characterization of extended spectrum beta-lactamase (ESβL)-producing Pseudomonas aeruginosa isolated from burn wound infections. Essential oils and their potential for utilization. Microb. Pathog. 2018, 116, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Hashem, H.; Hanora, A.; Abdalla, S.; Shaeky, A.; Saad, A. Dissemination of metallo-β-lactamase in Pseudomonas aeruginosa isolates in Egypt: Mutation in bla VIM-4. Apmis 2017, 125, 499–505. [Google Scholar] [CrossRef]
- Zorgani, A.; Daw, H.; Sufya, N.; Bashein, A.; Elahmer, O.; Chouchani, C. Co-Occurrence of Plasmid-Mediated AmpC β-Lactamase Activity Among Klebsiella pneumoniae and Escherichia Coli. Open Microbiol. J. 2017, 11, 195–202. [Google Scholar] [CrossRef]
- Kamel, N.A.; El-tayeb, W.N.; El-Ansary, M.R.; Mansour, M.T.; Aboshanab, K.M. Phenotypic screening and molecular characterization of carbapenemase-producing Gram-negative bacilli recovered from febrile neutropenic pediatric cancer patients in Egypt. PLoS ONE 2018, 13, e0202119. [Google Scholar] [CrossRef]
- Al Yousef, S.A.; Younis, S.; Farrag, E.; Moussa, H.S.; Bayoumi, F.S.; Ali, A.M. Clinical and laboratory profile of urinary tract infections associated with extended spectrum ß-lactamase producing Escherichia coli and Klebsiella pneumoniae. Ann. Clin. Lab. Sci. 2016, 46, 393–400. [Google Scholar]
- Al-Agamy, M.H.; Khalaf, N.G.; Tawfick, M.M.; Shibl, A.M.; El Kholy, A.A. Molecular characterization of carbapenem-insensitive Acinetobacter baumannii in Egypt. Int. J. Infect. Dis. 2014, 22, 49–54. [Google Scholar] [CrossRef] [Green Version]
- Zafer, M.M.; Al-Agamy, M.H.; El-Mahallawy, H.A.; Amin, M.A.; Ashour, M.S.E.D. Antimicrobial resistance pattern and their beta-lactamase encoding genes among Pseudomonas aeruginosa strains isolated from cancer patients. Biomed. Res. Int. 2014, 101635. [Google Scholar] [CrossRef] [Green Version]
- Abdallah, H.M.; Wintermans, B.B.; Reuland, E.A.; Koek, A.; Al Naiemi, N.; Ammar, A.M.; Mohamed, A.A.; Vandenbroucke-Grauls, C.M.J.E. Extended-spectrum β-lactamase- and carbapenemase-producing enterobacteriaceae isolated from Egyptian patients with suspected blood stream infection. PLoS ONE 2015, 10, e0136052. [Google Scholar] [CrossRef]
- Abbas, H.A.; El-Ganiny, A.M.; Kamel, H.A. Phenotypic and genotypic detection of antibiotic resistance of Pseudomonas aeruginosa isolated from urinary tract infections. Afr. Health Sci. 2018, 18, 11–21. [Google Scholar] [CrossRef] [Green Version]
- Mathlouthi, N.; Al-Bayssari, C.; El Salabi, A.; Bakour, S.; Ben Gwierif, S.; Zorgani, A.A.; Jridi, Y.; Ben Slama, K.; Rolain, J.M.; Chouchani, C. Carbapenemases and extended-spectrum β-lactamases producing enterobacteriaceae isolated from Tunisian and Libyan hospitals. J. Infect. Dev. Ctries. 2016, 10, 718–727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Messai, Y.; Iabadene, H.; Benhassine, T.; Alouache, S.; Tazir, M.; Gautier, V.; Arlet, G.; Bakour, R. Prevalence and characterization of extended-spectrum β-lactamases in Klebsiella pneumoniae in Algiers hospitals (Algeria). Pathol. Biol. 2008, 56, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Nedjai, S.; Barguigua, A.; Djahmi, N.; Jamali, L.; Zerouali, K.; Dekhil, M.; Timinouni, M. Prevalence and characterization of extended spectrum beta-lactamase-producing Enterobacter cloacae strains in Algeria. J. Infect. Dev. Ctries. 2013, 7, 804–811. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nabti, L.Z.; Sahli, F.; Radji, N.; Mezaghcha, W.; Semara, L.; Aberkane, S.; Lounnas, M.; Solassol, J.; Didelot, M.N.; Jean-Pierre, H.; et al. High Prevalence of Multidrug-Resistant Escherichia coli in Urine Samples from Inpatients and Outpatients at a Tertiary Care Hospital in Sétif, Algeria. Microb. Drug Resist. 2019, 25, 386–393. [Google Scholar] [CrossRef] [PubMed]
- Baba Ahmed-Kazi Tani, Z.; Decré, D.; Genel, N.; Boucherit-Otmani, Z.; Arlet, G.; Drissi, M. Molecular and epidemiological characterization of enterobacterial multidrug-resistant strains in Tlemcen Hospital (Algeria) (2008–2010). Microb. Drug Resist. 2013, 19, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Touati, M.; Diene, S.M.; Dekhil, M.; Djahoudi, A.; Racherache, A.; Rolain, J.M. Dissemination of a class i integron carrying VIM-2 carbapenemase in Pseudomonas Aeruginosa clinical isolates from a hospital intensive care unit in annaba Algeria. Antimicrob. Agents Chemother. 2013, 57, 2426–2427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dziri, O.; Dziri, R.; Maraoub, A.; Chouchani, C. First report of SHV-148-Type ESBL and CMY-42-type AmpC β-lactamase in klebsiella pneumoniae clinical isolates in Tunisia. Microb. Drug. Resist. 2018, 24, 1483–1488. [Google Scholar] [CrossRef]
- Ben Tanfous, F.; Achour, W.; Raddaoui, A.; Ben Hassen, A. Molecular characterisation and epidemiology of extended-spectrum β-lactamase-producing Klebsiella pneumoniae isolates from immunocompromised patients in Tunisia. J. Glob. Antimicrob. Resist. 2018, 13, 154–160. [Google Scholar] [CrossRef]
- Elhani, D.; Bakir, L.; Aouni, M.; Passet, V.; Arlet, G.; Brisse, S.; Weill, F.X. Molecular epidemiology of extended-spectrum β-lactamase-producing Klebsiella pneumoniae strains in a university hospital in Tunis, Tunisia, 1999–2005. Clin. Microbiol. Infect. 2010, 16, 157–164. [Google Scholar] [CrossRef] [Green Version]
- Ben Sallem, R.; Ben Slama, K.; Estepa, V.; Jouini, A.; Gharsa, H.; Klibi, N.; Sáenz, Y.; Ruiz-Larrea, F.; Boudabous, A.; Torres, C. Prevalence and characterisation of extended-spectrum beta-lactamase (ESBL)-producing Escherichia coli isolates in healthy volunteers in Tunisia. Eur. J. Clin. Microbiol. Infect. Dis. 2012, 31, 1511–1516. [Google Scholar] [CrossRef] [PubMed]
- Mechergui, A.; Achour, W.; Mathlouthi, S.; Hassen, A.B. Prevalence of infectious multi-drug resistant bacteria isolated from immunocompromised patients in Tunisia. Afr. Health Sci. 2019, 19, 2021–2025. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jaidane, N.; Naas, T.; Oueslati, S.; Bernabeu, S.; Boujaafar, N.; Bouallegue, O.; Bonnin, R.A. Whole-genome sequencing of NDM-1-producing ST85 Acinetobacter baumannii isolates from Tunisia. Int. J. Antimicrob. Agents 2018, 52, 916–921. [Google Scholar] [CrossRef] [PubMed]
- Maroui, I.; Barguigua, A.; Aboulkacem, A.; Ouarrak, K.; Sbiti, M.; Louzi, H.; Timinouni, M.; Belhaj, A. First report of VIM-2 metallo-β-lactamases producing Pseudomonas aeruginosa isolates in Morocco. J. Infect. Chemother. 2016, 22, 127–132. [Google Scholar] [CrossRef]
- Bourjilat, F.; Bouchrif, B.; Dersi, N.; Claude, J.D.P.G.; Amarouch, H.; Timinouni, M. Emergence of extended-spectrum beta-lactamase-producing Escherichia coli in community-acquired urinary infections in Casablanca, Morocco. J. Infect. Dev. Ctries. 2011, 5, 850–855. [Google Scholar] [CrossRef] [PubMed]
- Arhoune, B.; Oumokhtar, B.; Hmami, F.; Barguigua, A.; Timinouni, M.; el Fakir, S.; Chami, F.; Bouharrou, A. Rectal carriage of extended-spectrum β-lactamase- and carbapenemase-producing Enterobacteriaceae among hospitalised neonates in a neonatal intensive care unit in Fez, Morocco. J. Glob. Antimicrob. Resist. 2017, 8, 90–96. [Google Scholar] [CrossRef]
- Girlich, D.; Bouihat, N.; Poirel, L.; Benouda, A.; Nordmann, P. High rate of faecal carriage of extended-spectrum β-lactamase and OXA-48 carbapenemase-producing Enterobacteriaceae at a University hospital in Morocco. Clin. Microbiol. Infect. 2014, 20, 350–354. [Google Scholar] [CrossRef] [Green Version]
- Mohammed, I.; Abass, E. Phenotypic detection of Extended Spectrum β-Lactamases (ESBL) among gram negative uropathogens reveals highly susceptibility to imipenem. Pak. J. Med. Sci. 2019, 35, 1104–1109. [Google Scholar] [CrossRef] [Green Version]
- Ibrahim, M.E.; Bilal, N.E.; Magzoub, M.A.; Hamid, M.E. Prevalence of extended-spectrum β-lactamases-producing Escherichia coli from hospitals in Khartoum State, Sudan. Oman. Med. J. 2013, 28, 116–120. [Google Scholar] [CrossRef]
- Adam, M.A.; Elhag, W.I. Prevalence of metallo-β-lactamase acquired genes among carbapenems susceptible and resistant Gram-negative clinical isolates using multiplex PCR, Khartoum hospitals, Khartoum Sudan. BMC Infect. Dis. 2018, 18, 668. [Google Scholar] [CrossRef]
- Plantamura, J.; Bousquet, A.; Védy, S.; Larréché, S.; Bigaillon, C.; Delacour, H.; Mérens, A. Molecular epidemiological of extended-spectrum β-lactamase producing Escherichia coli isolated in Djibouti. J. Infect. Dev. Ctries 2019, 13, 753–758. [Google Scholar] [CrossRef] [PubMed]
- SENTRY 2013-2018 JMI MVP. Available online: https://sentry-mvp.jmilabs.com/app/sentry-public/heatmap (accessed on 13 May 2020).
- Cantón, R.; González-Alba, J.M.; Galán, J.C. CTX-M enzymes: Origin and diffusion. Front. Microbiol. 2012, 3, 110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kazmierczak, K.M.; de Jonge, B.L.M.; Stone, G.G.; Sahm, D.F. Longitudinal analysis of ESBL and carbapenemase carriage among Enterobacterales and Pseudomonas aeruginosa isolates collected in Europe as part of the International Network for Optimal Resistance Monitoring (INFORM) global surveillance programme, 2013–2017. J. Antimicrob. Chemother. 2020, 75, 1165–1173. [Google Scholar] [CrossRef] [PubMed]
- Antimicrobial Resistance Surveillance in Europe. 2016. Available online: https://www.ecdc.europa.eu/en/publications-data/antimicrobial-resistance-surveillance-europe-2016 (accessed on 25 March 2020).
- Xu, Y.; Gu, B.; Huang, M.; Liu, H.; Xu, T.; Xia, W.; Wang, T. Epidemiology of carbapenem resistant Enterobacteriaceae (CRE) during 2000-2012 in Asia. J. Thorac. Dis. 2015, 7, 376–385. [Google Scholar] [CrossRef] [PubMed]
- Almohammed, R.A.; Bird, E.L. Public knowledge and behaviours relating to antibiotic use in Gulf Cooperation Council countries: A systematic review. J. Infect. Public Health 2019, 12, 159–166. [Google Scholar] [CrossRef]
- Al Baz, M.; Law, M.R.; Saadeh, R. Antibiotics use among Palestine refugees attending UNRWA primary health care centers in Jordan—A cross-sectional study. Travel Med. Infect. Dis. 2018, 22, 25–29. [Google Scholar] [CrossRef]
- Barah, F.; Goncalves, V. Antibiotic use and knowledge in the community in Kalamoon, Syrian Arab Republic: A cross-sectional study. East. Mediterr. Health J. 2010, 16, 516–521. [Google Scholar] [CrossRef]
- Albawani, S.; Gnanasan, S.; Abd, A.N.; Hassan, Y. Self-Medication with Antibiotics in Children in Sana’a City, Yemen. Oman Med. J. 2010, 25, 41–43. [Google Scholar] [CrossRef]
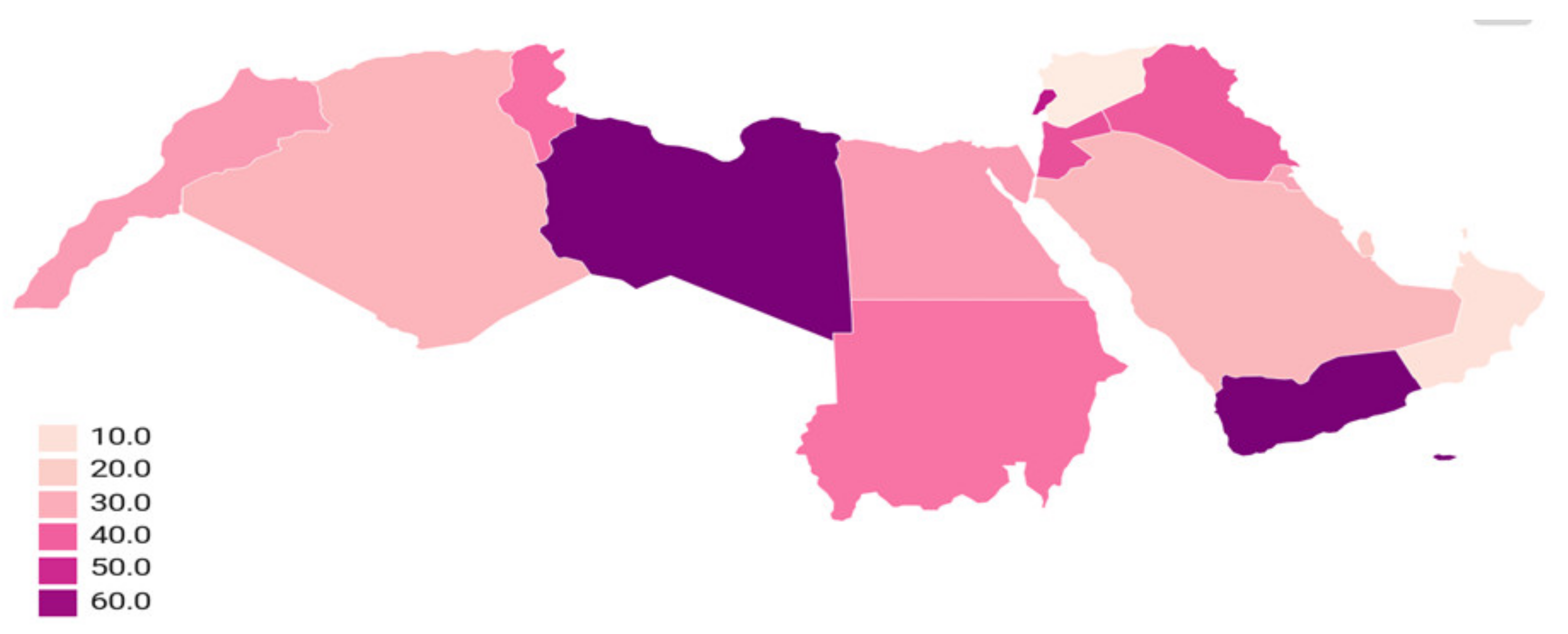

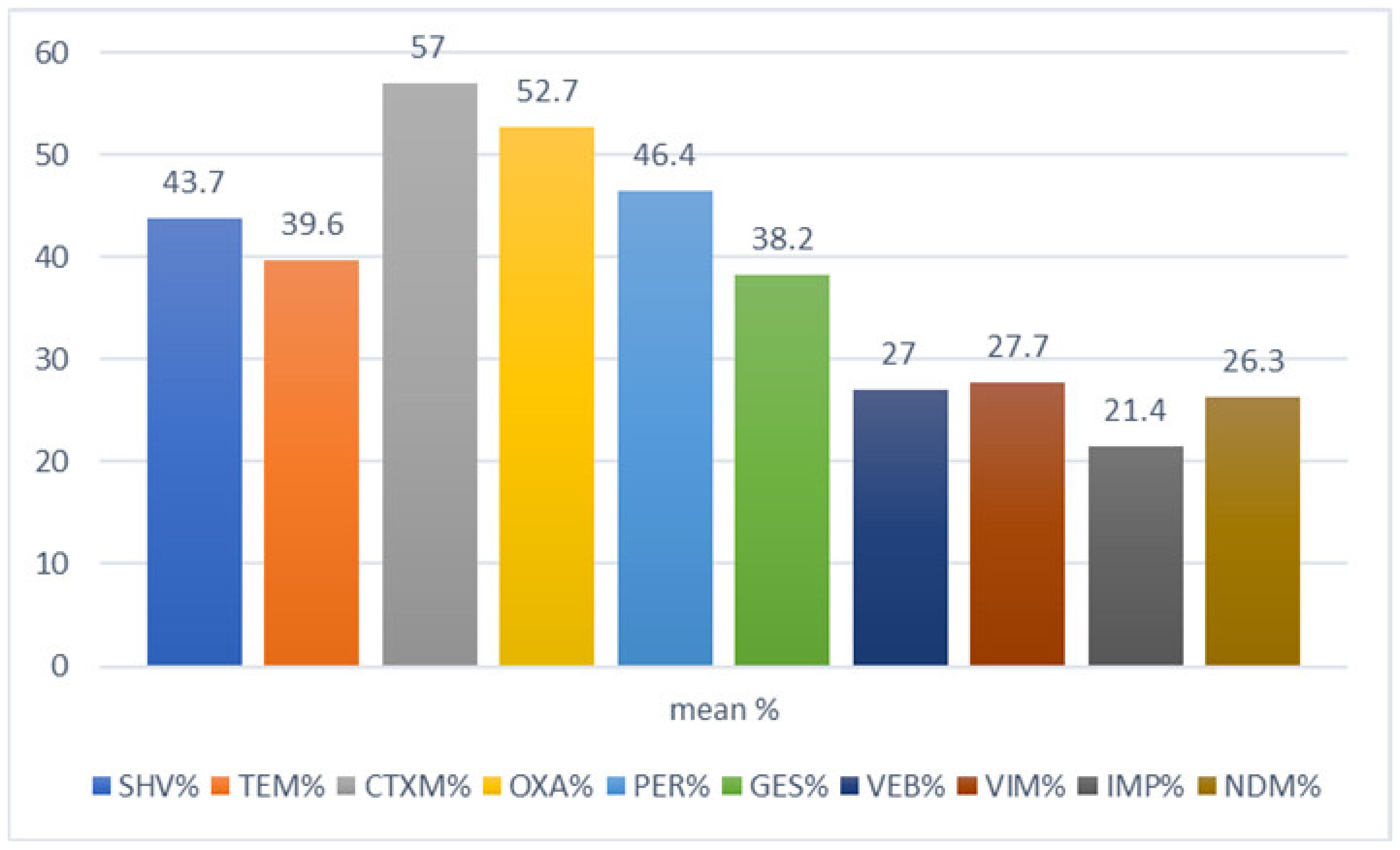
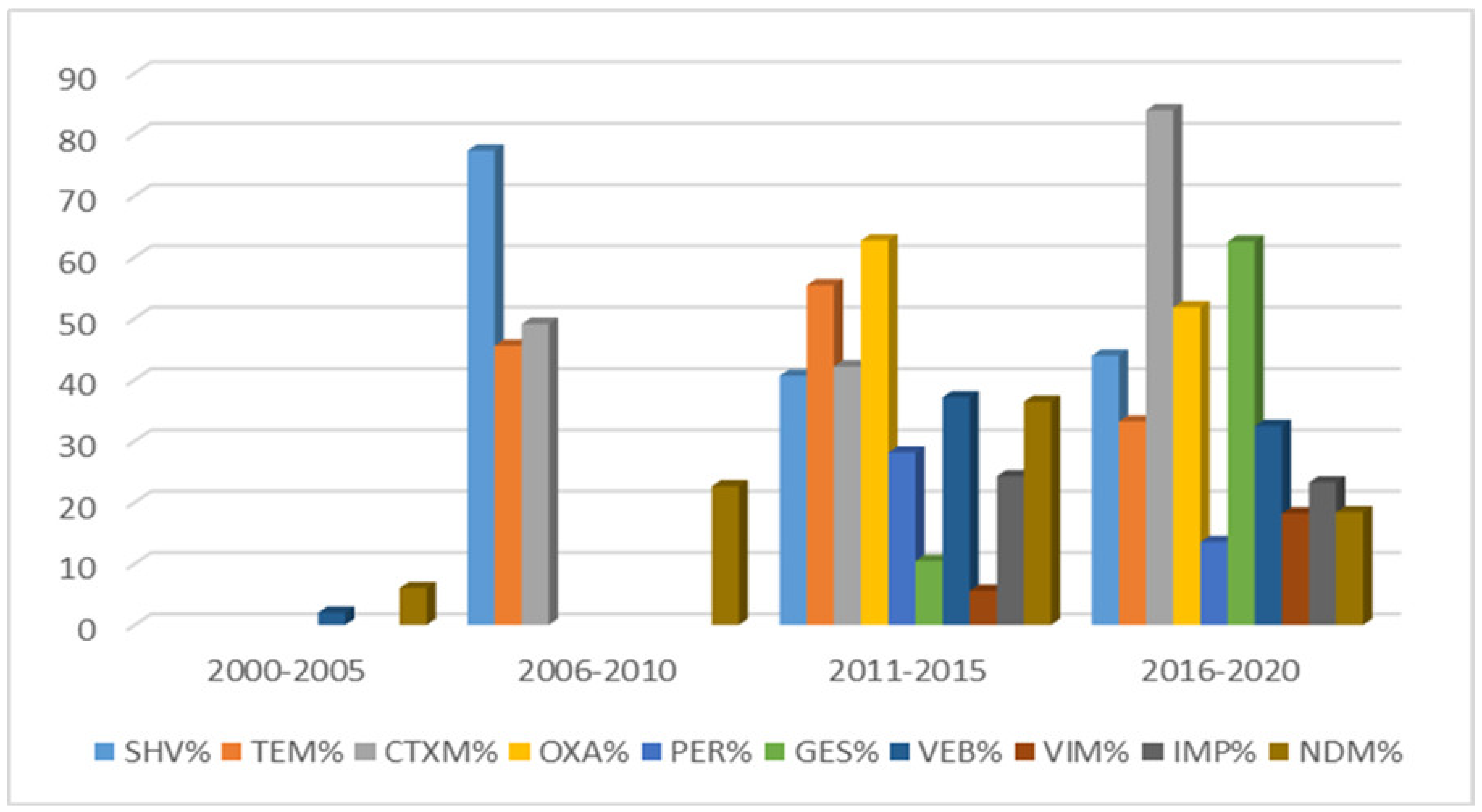
| Country | Increased Antimicrobial Resistance Rates during 2000–2020 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CXM | GAZ | CPM | CTX | AUG | TPZ | CIP | AK | CN | MEM | IMP | ATM | |
| Saudi Arabia | 54% to 80% | 16.6% to 95% | 8.3% to 83% | 4.4% to 97% | 25% to 86% | 4.9% to 33.3% | 24% to 94.5% | 23.5% to 82% | 29% to 91.6% | 0% to 57.4% | 0% to 63.6% | 4.9% to 99% |
| Qatar | - | 93.6% | 99% | - | 92% | 22% to 50% | 34.4% to 91.7% | 3% to 99% | 28% to 99% | 0% to 58.3% | - | - |
| Kuwait | 100% | - | 32.8% | - | - | 32.8% to 97.8% | 31.1% to 100% | 23% to 93.5% | 45% | 54% | 32.8% to 100% | - |
| Oman | - | - | - | 24% to 45% | 66% | 22% to 99% | 17.7% to 25% | 12.7% | 12.7% | - | - | - |
| Iraq | 36.3% to 87% | 50% to 78% | - | 37.5% to 50% | 86% to 87% | 40% to 86% | 57.1% to 75% | 38.3% to 95% | 37.5% to 56% | - | - | |
| Lebanon | - | 86.7% to 99% | 12% to 94.1% | 87.8% to 97.5% | - | 17% to 51.2% | 35% to 93.3% | 4% to 17.7% | 63.2% to 85.6% | 2.9% to 90% | 4.4% to 90% | 98.5% |
| Palestine | 99%, | 55% | - | 71% | 32% to 33.3% | - | 56% to 66.7% | 33.3% | 27% to 33.3% | - | 20% to 22% | - |
| Egypt | 77% to 100% | 60.6% to 94.1% | 75% to 98.8% | 100% | 55% | 22.5% to 42.4% | 34.4% to 77.6% | 18.8% to 32.8% | 45% to 52.2% | 45.9% to 93.9% | 15% to 93.9% | 21.2% to 85.8% |
| Tunisia | 100% | 72.2% | - | - | - | - | 48.5% to 62% | 95% | 92% to 93% | - | 0.8% | - |
| Country | Increased β-lactamases Genes Rates during 2000–2020 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| blaSHV | blaTEM | blaCTX-M | blaOXA | blaPER | blaGES | blaVIM | blaIMP | blaNDM | blaVEB | |
| Saudi Arabia | 3.2% to 97.3% | 2.6% to 84.1% | 8.5% to 87% | 26.2% to 85.7% | 49.1% to 76.3% | 21.7% to 34.5% | 7% to 32% | 9% | 7.4% to 55% | |
| Emirates | 4.4% | 21.3% | 6.4% & 53.3% | 24.7%. | ||||||
| Qatar | 53.2% | 40.4% | 59% to 66.1% | 100% | ||||||
| Iraq | 24% | 20% to 46% | 26% | 58% | 17% | 5% | 9.1% to 50% | 67.2% | 30% | |
| Lebanon | 88.2% | 63.2% | 41.2% to 86.7% | 5.8% to 96.7% | 75% | 50% | 17% | 14.7 to 30% | ||
| Palestine | 3.3% | 22.8% to 33.3% | ||||||||
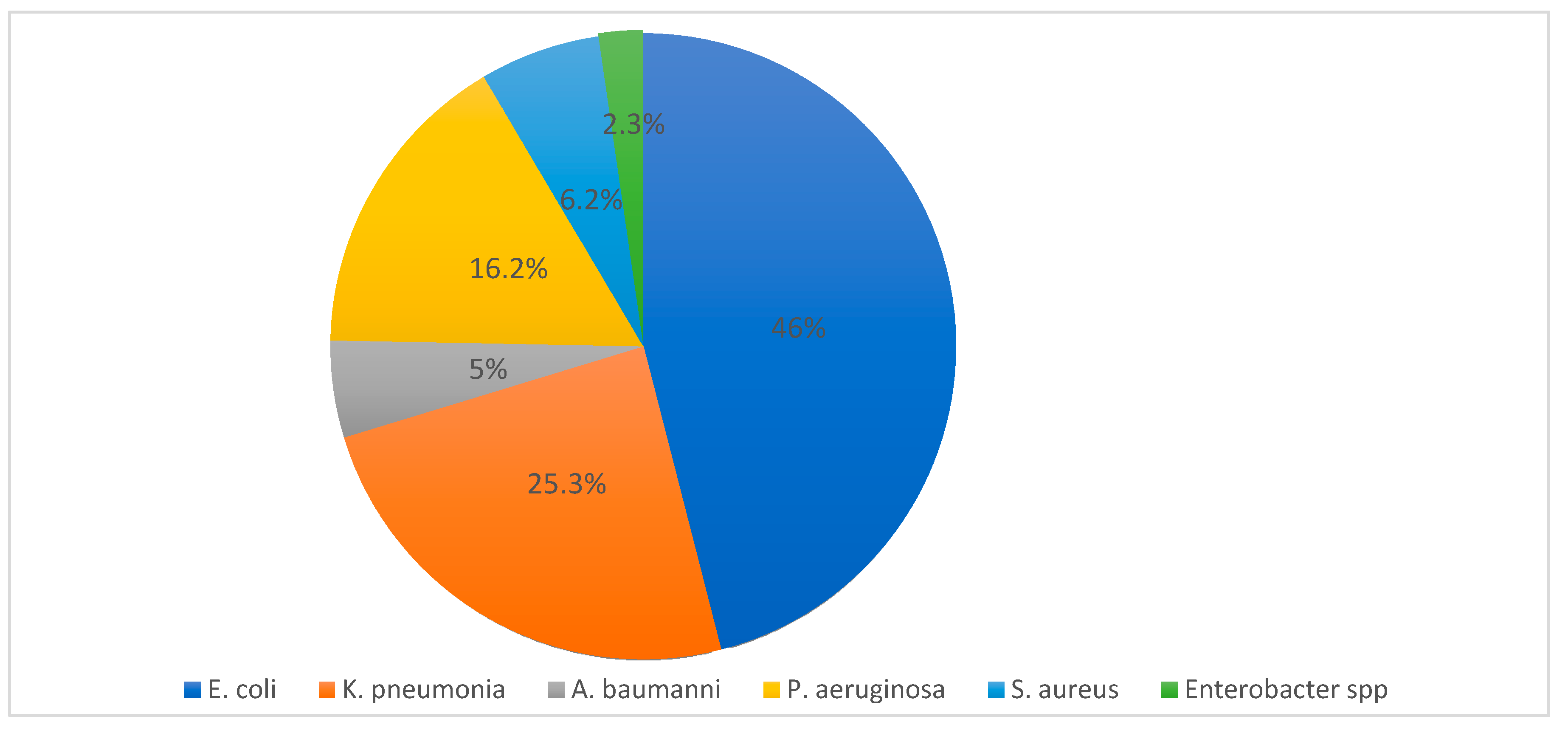
| Country | E. coli | K. pneumonia | P. aeruginosa | S. aureus | A. baumannii | Enterobacter spp. | Total | Reference |
|---|---|---|---|---|---|---|---|---|
| Saudi | 22,493 | 11,959 | 9854 | 3829 | 2582 | 1316 | 52,033 | [11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37] |
| Emirate | 654 | 524 | - | - | - | 13 | 1191 | [38,39,40,41,42] |
| Qatar | 448 | 168 | 106 | - | 48 | - | 770 | [43,44,45,46,47,48] |
| Kuwait | 1206 | 669 | 79 | 209 | 63 | 7 | 2233 | [49,50,51,52,53,54,55] |
| Oman | 165 | 112 | 48 | 155 | 107 | 20 | 607 | [56,57,58,59] |
| Bahrain | 1594 | 704 | 60 | - | - | 62 | 2420 | [60,61,62] |
| Yemen | 130 | 8 | - | - | 3 | 2 | 143 | [63,64,65] |
| Jordan | 316 | 428 | 125 | - | 18 | 37 | 924 | [66,67,68,69] |
| Iraq | 49 | 98 | 196 | 17 | 9 | 13 | 382 | [70,71,72,73,74] |
| Syria | 104 | - | - | - | 260 | - | 364 | [75,76] |
| Lebanon | 2000 | 676 | 40 | - | 121 | - | 2837 | [77,78,79,80,81] |
| Palestine | 101 | 15 | - | - | - | 3 | 119 | [82,83] |
| Egypt | 661 | 246 | 356 | - | 7 | 19 | 1289 | [84,88,90,91,92,93] |
| Libya | 397 | 36 | - | - | - | - | 433 | [87,94] |
| Algeria | 232 | 246 | - | 106 | 17 | 67 | 668 | [95,96,97,98,99] |
| Tunisia | 368 | 811 | 210 | - | 246 | 15 | 1650 | [100,101,102,104,105] |
| Morocco | 639 | 113 | - | - | - | - | 752 | [106,107,108,109] |
| Sudan | 450 | 315 | - | 54 | 4 | - | 823 | [110,111,112] |
| Djibouti | 31 | - | - | - | - | - | 31 | [113] |
| Total | 32,038 | 17,128 | 11,074 | 4370 | 3485 | 1574 | 69,669 |
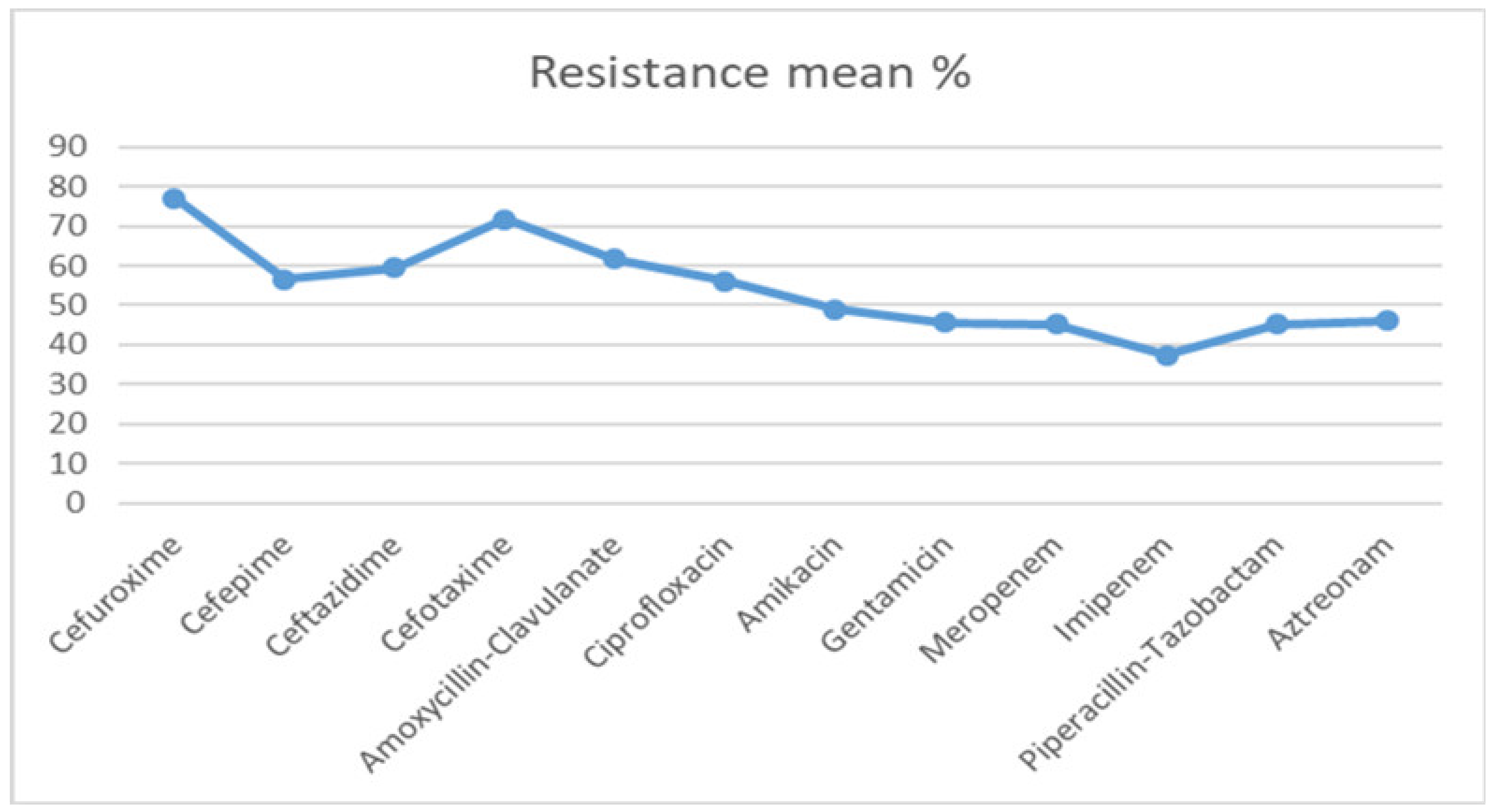
| Antibiotic | 2000–2005 Mean% | 2006–2010 Mean% | 2011–2015 Mean% | 2016–2020 Mean% |
|---|---|---|---|---|
| Cefuroxime | 44.6 | 87.2 | 89.2 | 87.5 |
| Cefepime | 39 | 47 | 66.5 | 73.6 |
| Ceftazidime | 49 | 53. | 62.8 | 72.3 |
| Cefotaxime | 62.5 | 78.0 | 74.3 | 72.5 |
| Amoxycillin-Clavulanate | 58.6 | 60 | 60.6 | 67.9 |
| Ciprofloxacin | 46.8 | 48.5 | 59.3 | 70.3 |
| Amikacin | 40.2 | 47.3 | 56.9 | 51.4 |
| Gentamicin | 49 | 50.4 | 55.2 | 64.4 |
| Meropenem | 30.5 | 40.2 | 64.9 | |
| Imipenem | 30.9 | 37.6 | 44.1 | |
| Piperacillin-Tazobactam | 49 | 52.7 | 34.3 | |
| Aztreonam | 30.6 | 33.3 | 73.6 | 46.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nasser, M.; Palwe, S.; Bhargava, R.N.; Feuilloley, M.G.J.; Kharat, A.S. Retrospective Analysis on Antimicrobial Resistance Trends and Prevalence of β-lactamases in Escherichia coli and ESKAPE Pathogens Isolated from Arabian Patients during 2000–2020. Microorganisms 2020, 8, 1626. https://doi.org/10.3390/microorganisms8101626
Nasser M, Palwe S, Bhargava RN, Feuilloley MGJ, Kharat AS. Retrospective Analysis on Antimicrobial Resistance Trends and Prevalence of β-lactamases in Escherichia coli and ESKAPE Pathogens Isolated from Arabian Patients during 2000–2020. Microorganisms. 2020; 8(10):1626. https://doi.org/10.3390/microorganisms8101626
Chicago/Turabian StyleNasser, Mahfouz, Snehal Palwe, Ram Naresh Bhargava, Marc G. J. Feuilloley, and Arun S. Kharat. 2020. "Retrospective Analysis on Antimicrobial Resistance Trends and Prevalence of β-lactamases in Escherichia coli and ESKAPE Pathogens Isolated from Arabian Patients during 2000–2020" Microorganisms 8, no. 10: 1626. https://doi.org/10.3390/microorganisms8101626
APA StyleNasser, M., Palwe, S., Bhargava, R. N., Feuilloley, M. G. J., & Kharat, A. S. (2020). Retrospective Analysis on Antimicrobial Resistance Trends and Prevalence of β-lactamases in Escherichia coli and ESKAPE Pathogens Isolated from Arabian Patients during 2000–2020. Microorganisms, 8(10), 1626. https://doi.org/10.3390/microorganisms8101626





