Effects of Wormwood (Artemisia montana) Essential Oils on Digestibility, Fermentation Indices, and Microbial Diversity in the Rumen
Abstract
1. Introduction
2. Materials and Methods
2.1. Substrate and Essential Oil
2.2. In Vitro Incubation
2.3. Laboratory Analysis
2.4. Statistical Analysis
3. Results
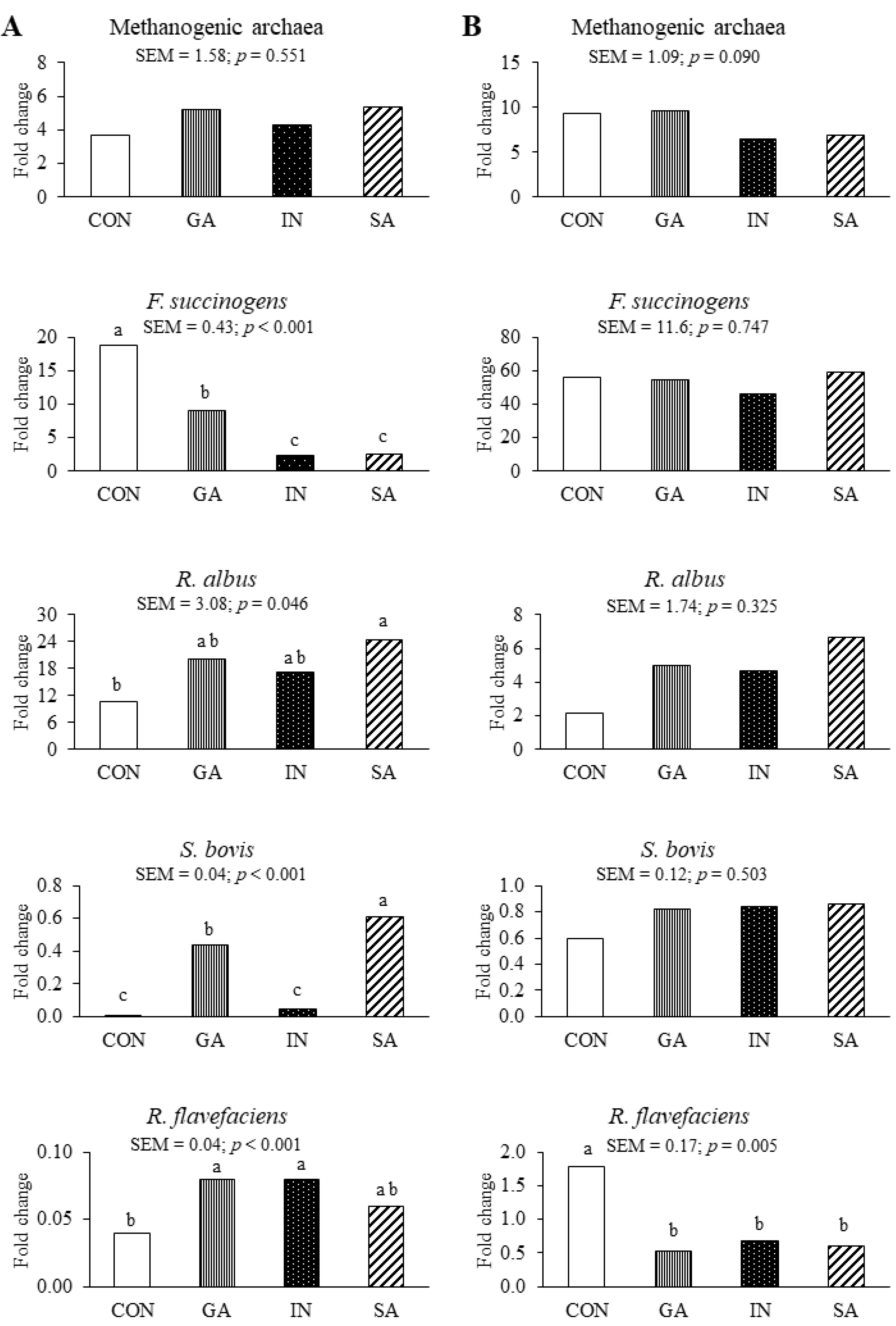
3.1. Experiment 1
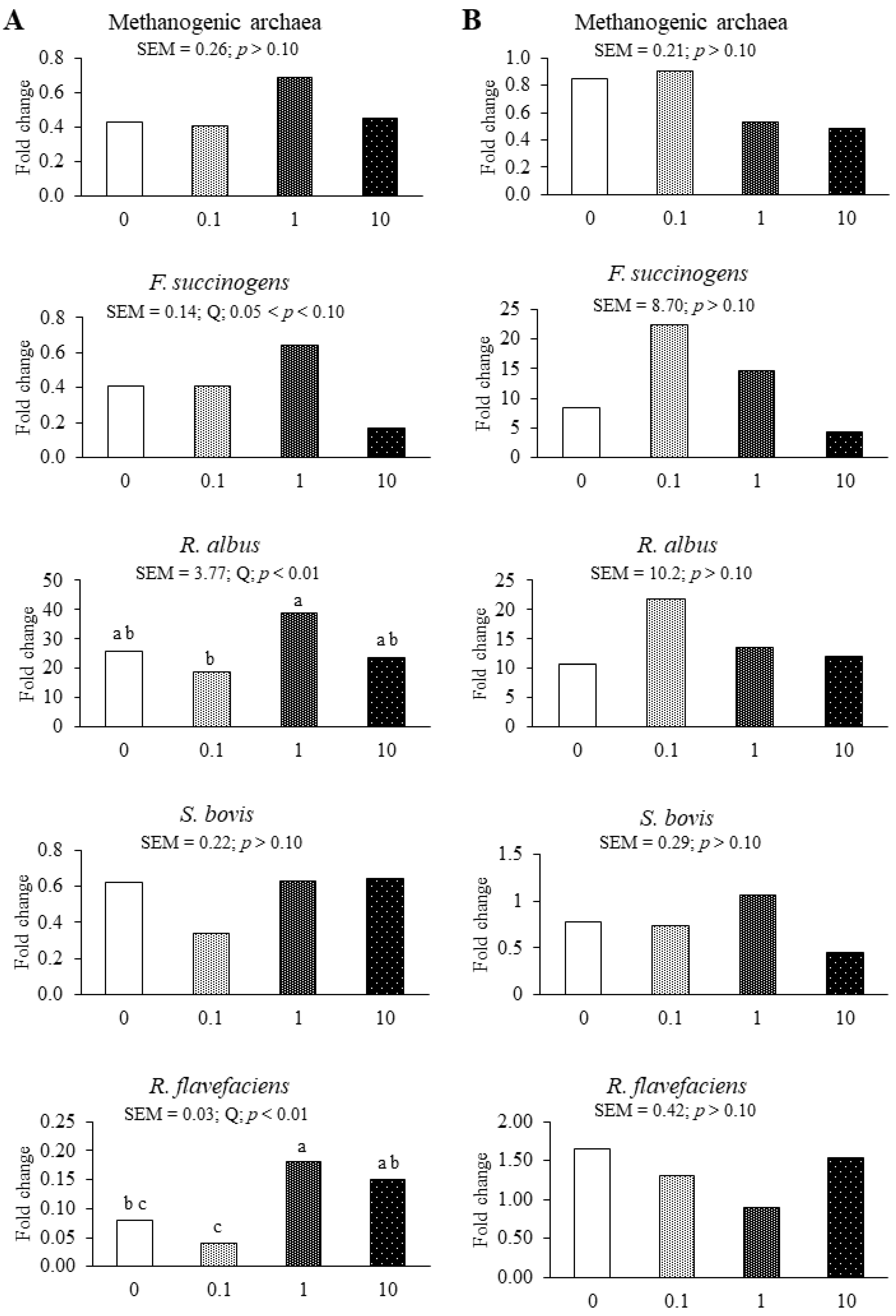
3.2. Experiment 2
4. Discussion
4.1. Experiment 1
4.2. Experiment 2
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Calsamiglia, S.; Busquet, M.; Cardozo, P.; Castillejos, L.; Ferret, A. Invited Review: Essential Oils as Modifiers of Rumen Microbial Fermentation. J. Dairy Sci. 2007, 90, 2580–2595. [Google Scholar] [CrossRef]
- Benchaar, C.; Calsamiglia, S.; Chaves, A.; Fraser, G.; Colombatto, D.; McAllister, T.; Beauchemin, K. A Review of Plant-Derived Essential Oils in Ruminant Nutrition and Production. Anim. Feed Sci. Technol. 2008, 145, 209–228. [Google Scholar] [CrossRef]
- Nanon, A.; Suksombat, W.; Yang, W. Effects of Essential Oils Supplementation on in Vitro and in Situ Feed Digestion in Beef Cattle. Anim. Feed. Sci. Technol. 2014, 196, 50–59. [Google Scholar] [CrossRef]
- Benchaar, C.; Chaves, A.V.; Fraser, G.R.; Beauchemin, K.; McAllister, T.A. Effects of Essential Oils and Their Components on In Vitro Rumen Microbial Fermentation. Can. J. Anim. Sci. 2007, 87, 413–419. [Google Scholar] [CrossRef]
- Szulc, P.; Mravčáková, D.; Szumacher-Strabel, M.; Varadyova, Z.; Várady, M.; Čobanová, K.; Syahrulawal, L.; Patra, A.K.; Cieslak, A. Ruminal Fermentation, Microbial Population and Lipid Metabolism in Gastrointestinal Nematode-Infected Lambs Fed a Diet Supplemented with Herbal Mixtures. PLoS ONE 2020, 15, e0231516. [Google Scholar] [CrossRef]
- Petrič, D.; Mravčáková, D.; Kucková, K.; Čobanová, K.; Kišidayová, S.; Cieslak, A.; Ślusarczyk, S.; Varadyova, Z. Effect of Dry Medicinal Plants (Wormwood, Chamomile, Fumitory and Mallow) on in Vitro Ruminal Antioxidant Capacity and Fermentation Patterns of Sheep. J. Anim. Physiol. Anim. Nutr. 2020, 104, 1219–1232. [Google Scholar] [CrossRef]
- Kim, S.C.; Adesogan, A.; Ko, Y.D. The Respective Effects of Shoot Height and Conservation Method on the Yield and Nutritive Value, and Essential Oils of Wormwood (Artemisia montana Pampan). Asian-Australas. J. Anim. Sci. 2006, 19, 816–824. [Google Scholar] [CrossRef]
- Ko, Y.; Kim, J.; Adesogan, A.; Ha, H.; Kim, S. The Effect of Replacing Rice Straw with Dry Wormwood (Artemisia sp.) on Intake, Digestibility, Nitrogen Balance and Ruminal Fermentation Characteristics in Sheep. Anim. Feed Sci. Technol. 2006, 125, 99–110. [Google Scholar] [CrossRef]
- Kim, S.; Adesogan, A.; Kim, J.; Ko, Y. Influence of Replacing Rice Straw with Wormwood (Artemisia montana) Silage on Feed Intake, Digestibility and Ruminal Fermentation Characteristics of Sheep. Anim. Feed Sci. Technol. 2006, 128, 1–13. [Google Scholar] [CrossRef]
- Kepner, R.E.; Maarse, H. Changes in Composition of Volatile Terpenes in Douglas Fir Needles During Maturation. J. Agric. Food Chem. 1970, 18, 1095–1101. [Google Scholar] [CrossRef]
- Likens, S.T.; Nickerson, G.B. Detection of Certain Hop Oil Constituents in Brewing Products. Proc. Am. Soc. Brew. Chem. 1964, 22, 5–13. [Google Scholar] [CrossRef]
- Adesogan, A.; Krueger, N.; Kim, S. A Novel, Wireless, Automated System for Measuring Fermentation Gas Production Kinetics of Feeds and Its Application to Feed Characterization. Anim. Feed Sci. Technol. 2005, 123, 211–223. [Google Scholar] [CrossRef]
- Paradhipta, D.H.V.; Joo, Y.-H.; Lee, H.-J.; Lee, S.S.; Kwak, Y.-S.; Han, O.K.; Kim, D.; Kim, S.-C. Effects of Wild or Mutated Inoculants on Rye Silage and Its Rumen Fermentation Indices. Asian-Australas. J. Anim. Sci. 2020, 33, 949–956. [Google Scholar] [CrossRef]
- Van Soest, P.; Robertson, J.; Lewis, B. Methods for Dietary Fiber, Neutral Detergent Fiber, and Nonstarch Polysaccharides in Relation to Animal Nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Paradhipta, D.H.V.; Lee, S.S.; Kang, B.; Joo, Y.H.; Lee, H.J.; Lee, Y.; Kim, J.; Kim, S.-C. Dual-Purpose Inoculants and Their Effects on Corn Silage. Microorganisms 2020, 8, 765. [Google Scholar] [CrossRef]
- Chaney, A.L.; Marbach, E.P. Modified Reagents for Determination of Urea and Ammonia. Clin. Chem. 1962, 8, 130–132. [Google Scholar] [CrossRef]
- Muck, R.E.; Dickerson, J.T. Storage Temperature Effects on Proteolysis in Alfalfa Silage. Trans. ASAE 1988, 31, 1005–1009. [Google Scholar] [CrossRef]
- Denman, S.E.; McSweeney, C.S. Development of a Real-Time PCR Assay for Monitoring Anaerobic Fungal and Cellulolytic Bacterial Populations within the Rumen. FEMS Microbiol. Ecol. 2006, 58, 572–582. [Google Scholar] [CrossRef]
- Denman, S.E.; Tomkins, N.W.; McSweeney, C.S. Quantitation and Diversity Analysis of Ruminal Methanogenic Populations in Response to the Antimethanogenic Compound Bromochloromethane. FEMS Microbiol. Ecol. 2007, 62, 313–322. [Google Scholar] [CrossRef]
- Koike, S.; Kobayashi, Y. Development and Use of Competitive PCR Assays for the Rumen Cellulolytic Bacteria: Fibrobacter succinogenes, Ruminococcus albus and Ruminococcus flavefaciens. FEMS Microbiol. Lett. 2001, 204, 361–366. [Google Scholar] [CrossRef]
- Tajima, K.; Aminov, R.I.; Nagamine, T.; Matsui, H.; Nakamura, M.; Benno, Y. Diet-Dependent Shifts in the Bacterial Population of the Rumen Revealed with Real-Time PCR. Appl. Environ. Microbiol. 2001, 67, 2766–2774. [Google Scholar] [CrossRef]
- SAS Institute Inc. SAS/STAT User’s Guide, Version 9; SAS Institute Inc.: Cary, NC, USA, 2002. [Google Scholar]
- Busquet, M.; Calsamiglia, S.; Ferret, A.; Kamel, C. Plant Extracts Affect in Vitro Rumen Microbial Fermentation. J. Dairy Sci. 2006, 89, 761–771. [Google Scholar] [CrossRef]
- Castillejos, L.; Calsamiglia, S.; Ferret, A. Effect of Essential Oil Active Compounds on Rumen Microbial Fermentation and Nutrient Flow in in Vitro Systems. J. Dairy Sci. 2006, 89, 2649–2658. [Google Scholar] [CrossRef]
- Tager, L.; Krause, K. Effects of Essential Oils on Rumen Fermentation, Milk Production, and Feeding Behavior in Lactating Dairy Cows. J. Dairy Sci. 2011, 94, 2455–2464. [Google Scholar] [CrossRef]
- Torres, R.; Moura, D.; Ghedini, C.; Ezequiel, J.; Almeida, M. Meta-Analysis of the Effects of Essential Oils on Ruminal Fermentation and Performance of Sheep. Small Rumin. Res. 2020, 189, 106148. [Google Scholar] [CrossRef]
- John, A.J.; George, V.; Pradeep, N.S.; Sethuraman, M.G. Chemical Composition and Antibacterial Activity of the Leaf, Bark and Fruit Oils of Neolitsea fischeri Gamble. J. Essent. Oil Res. 2008, 20, 279–282. [Google Scholar] [CrossRef]
- Elzaawely, A.A.; Xuan, T.D.; Koyama, H.; Tawata, S. Antiooxidant Activity and Contents of Essential Oil and Phenolic Compounds in Flowers and Seed of Alpinia Zerumbet B.L. Burtt. & R.M. Sm. Food Chem. 2007, 104, 1648–1653. [Google Scholar] [CrossRef]
- Scollard, J.; Francis, G.A.; O’Beirne, D. Chemical Basis of Anti-Listerial Effects of Rosemary Herb during Stomaching with Fresh-Cut Vegetables. LWT Food Sci. Technol. 2014, 57, 16–21. [Google Scholar] [CrossRef]
- Lin, B.; Lu, Y.; Wang, J.H.; Liang, Q.; Liu, J.X. The Effects of Combined Essential Oils along with Fumarate on Rumen Fermentation and Methane Production in Vitro. J. Anim. Feed Sci. 2012, 21, 198–210. [Google Scholar] [CrossRef]
- Patra, A.K.; Yu, Z. Effects of Essential Oils on Methane Production and Fermentation by, and Abundance and Diversity of, Rumen Microbial Populations. Appl. Environ. Microbiol. 2012, 78, 4271–4280. [Google Scholar] [CrossRef]
- Wallace, R.J.; Onodera, R.; Cotta, M.A. Metabolism of Nitrogen-Containing Compounds. In The Rumen Microbial Ecosystem; Hobson, P.N., Stewart, C.S., Eds.; Blackie Academic and Professional: London, UK, 1997; pp. 283–328. [Google Scholar]
- Garcia, F.; Colombatto, D.; Brunetti, M.A.; Martínez, M.J.; Moreno, M.V.; Turcato, M.C.S.; Lucini, E.I.; Frossasco, G.; Ferrer, J.M. The Reduction of Methane Production in the in Vitro Ruminal Fermentation of Different Substrates is Linked with the Chemical Composition of the Essential Oil. Animals 2020, 10, 786. [Google Scholar] [CrossRef]
| Target Species | Primers | Size (bp) | PCR Condition (*modified from the ref.) | Reference |
|---|---|---|---|---|
| Methanogenic archaea | TTCGGTGGATCDCARAGRGC/ GBARGTCGWAWCCGTAGAATCC | 140 | *95 °C for 3 min, (95 °C for 15 s, 60 °C for 30 s, 72 °C for 30 s)- 48 cycles. | [19] |
| F. succinogens | GTTCGGAATTACTGGGCGTAAA/ CGCCTGCCCCTGAACTATC | 121 | *95 °C for 3 min, (95 °C for 30 s, 57 °C for 15 s, 72 °C for 30 s)- 40 cycles. | [18] |
| R. albus | CCCTAAAAGCAGTCTTAGTTCG/ CCTCCTTGCGGTTAGAACA | 175 | *95 °C for 9 min, (95 °C for 30 s, 55 °C for 30 s, 72 °C for 30 s)- 48 cycles, 72 °C for 10 min. | [20] |
| S. bovis | CTAATACCGCATAACAGCAT/ AGAAACTTCCTATCTCTAGG | 869 | 95 °C for 3 min, (95 °C for 30 s, 57 °C for 30 s, 72 °C for 1 min)- 35 cycles. | [21] |
| R. flavefaciens | CGAACGGAGATAATTTGAGTTTACTTAGG/ CGGTCTCTGTATGTTATGAGGTATTACC | 132 | *95 °C for 3 min, (95 °C for 15 s, 57 °C for 30 s, 72 °C for 30 s)- 40 cycles. | [18] |
| Item | GA | IN | SA |
|---|---|---|---|
| (-)-Caryophyllene oxide | 26.4 | 38.0 | 44.3 |
| endo-Borneol | 185 | ND 1 | 48.2 |
| 1,8-Cineole | ND | 83.6 | 56.7 |
| 3-Cyclohexen-1-ol | 102 | ND | ND |
| Naphthalene (CAS) | 44.0 | ND | ND |
| Phenol, 2-methoxy-4-(2-propenyl)- (CAS) | 28.6 | ND | ND |
| Bicyclo [2.2.2] oct-2-ene | 30.1 | ND | ND |
| Pentan-1,3-Dioldiisobutyrate | 511 | ND | ND |
| Caryophyllenol-II | 34.2 | ND | ND |
| 2-Pentadecanone, 6,10,14-trimethyl- (CAS) | 38.7 | ND | ND |
| Benzene, 1-methoxy-4-nitro-(CAS) | ND | ND | 25.6 |
| trans-Caryophyllene | ND | ND | 162 |
| α-Humulene | ND | ND | 55.8 |
| Naphthalene | ND | ND | 57.7 |
| α-amorphene | ND | ND | 31.3 |
| α-Muurolene-(-) | ND | ND | 31.9 |
| ɣ-Cadinene | ND | ND | 39.7 |
| δ-Cadinene | ND | ND | 109 |
| α-Cadinol | ND | ND | 131 |
| T-Muurolol | ND | ND | 151 |
| Hexadecanoic acid, ethyl ester(CAS) | ND | ND | 33.3 |
| Ethyl Linoleate | ND | ND | 22.5 |
| Camphor | ND | 332 | ND |
| 1-Borneol | ND | 299 | ND |
| α-Copaene | ND | 20.6 | ND |
| Trans(β)-Caryophyllene | ND | 107 | ND |
| Germacrene-d | ND | 47.6 | ND |
| β-bisabolene | ND | 47.6 | ND |
| selin-11-en-4-α-ol | ND | 24.6 | ND |
| Item 1 | Soybean Meal | Bermudagrass Hay | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CON 2 | GA | IN | SA | SEM | p-Value | CON | GA | IN | SA | SEM | p-Value | |
| IVDMD, g kg−1 DM | 658 | 642 | 625 | 630 | 19.4 | 0.486 | 308 b | 323 b | 440 a | 411 a | 26.8 | 0.004 |
| IVNDFD, g kg−1 DM | 1000 | 1000 | 1000 | 1000 | 0.000 | 1.000 | 312 a | 217 b | 370 a | 356 a | 22.6 | 0.001 |
| pH | 7.42 | 7.51 | 7.48 | 7.49 | 0.068 | 0.458 | 7.20 | 7.31 | 7.29 | 7.17 | 0.129 | 0.588 |
| Ammonia-N, mg N dL−1 | 36.6 | 35.9 | 35.3 | 35.2 | 1.44 | 0.664 | 17.4 b | 17.0 b | 17.9 b | 20.5 a | 0.26 | 0.001 |
| Total SCFA, mM L−1 | 154.2 a | 142.6 b | 148.8 a,b | 145.7 a,b | 3.18 | 0.026 | 136.1 a | 119.8 b | 130.2 a | 131.7 a | 2.87 | 0.001 |
| Acetate, mol 100 mol−1 | 58.2 | 58.0 | 58.3 | 57.9 | 0.85 | 0.922 | 67.6 | 67.7 | 68.4 | 68.1 | 0.72 | 0.501 |
| Propionate, mol 100 mol−1 | 16.6 | 17.2 | 16.3 | 16.3 | 0.44 | 0.097 | 14.8 | 15.1 | 15.4 | 15.2 | 0.26 | 0.157 |
| Isobutyrate, mol 100 mol−1 | 2.95 | 2.90 | 2.98 | 2.94 | 0.206 | 0.976 | 1.84 | 1.74 | 1.57 | 1.57 | 0.182 | 0.269 |
| Butyrate, mol 100 mol−1 | 12.9 | 12.8 | 13.0 | 13.0 | 0.23 | 0.759 | 10.2 | 9.76 | 9.60 | 9.87 | 0.273 | 0.223 |
| Isovalerate, mol 100 mol−1 | 5.30 | 5.43 | 5.37 | 5.77 | 0.194 | 0.072 | 3.23 | 3.10 | 2.70 | 2.83 | 0.216 | 0.579 |
| Valerate, mol 100 mol−1 | 4.17 | 4.60 | 4.13 | 4.20 | 0.183 | 0.096 | 2.23 | 2.53 | 2.10 | 2.37 | 0.206 | 0.141 |
| A:P ratio | 3.51 | 3.37 | 3.58 | 3.56 | 0.109 | 0.156 | 4.55 | 4.49 | 4.45 | 4.47 | 0.101 | 0.649 |
| Item 1 | Soybean Meal | Bermudagrass Hay | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 2 | 0.1 | 1 | 10 | SEM | Effects 3 | 0 | 0.1 | 1 | 10 | SEM | Effects | |
| IVDMD, g kg−1 DM | 743 | 764 | 721 | 725 | 17.1 | †, Q | 422 b | 391 b | 465 a,b | 532 a | 28.2 | **, L |
| IVNDFD, g kg−1 DM | 1000 | 1000 | 1000 | 1000 | 0.000 | NS | 265 b,c | 225 c | 317 b | 428 a | 19.7 | *, Q |
| pH | 6.98 | 7.01 | 6.90 | 6.94 | 0.100 | NS | 6.77 | 6.76 | 6.75 | 6.73 | 0.034 | NS |
| Ammonia-N, mg N dL−1 | 98.6 b | 111.0 a | 101.9 a,b | 106.6 a,b | 3.05 | **, C | 50.0 | 49.9 | 48.8 | 49.0 | 2.06 | NS |
| Total SCFA, mM L−1 | 126.3 b | 127.0 b | 131.3 a,b | 135.5 a | 2.00 | **, L | 101.7 b | 102.0 b | 114.3 a | 107.7 a,b | 2.97 | **, Q |
| Acetate, mol 100 mol−1 | 53.8 | 53.5 | 54.8 | 55.5 | 1.06 | NS | 63.1 a | 63.6 a | 58.6 b | 62.1 a | 1.15 | **, Q |
| Propionate, mol 100 mol−1 | 18.9 a | 18.3 a,b | 17.8 a,b | 17.3 b | 0.39 | *, L | 17.9 | 17.4 | 18.4 | 16.8 | 0.43 | †, Q |
| Isobutyrate, mol 100 mol−1 | 3.71 | 3.33 | 3.30 | 2.66 | 0.551 | NS | 2.55 b | 2.34 b | 3.96 a | 2.06 b | 0.443 | **, Q |
| Butyrate, mol 100 mol−1 | 11.8 | 12.1 | 12.6 | 12.7 | 0.44 | NS | 9.6 a,b | 9.4 b | 10.4 a,b | 11.0 a | 0.53 | **, L |
| Isovalerate, mol 100 mol−1 | 6.50 a,b | 6.49 a,b | 6.39 b | 6.88 a | 0.165 | **, Q | 3.94 c | 3.94 c | 4.71 a | 4.43 b | 0.050 | **, Q |
| Valerate, mol 100 mol−1 | 5.35 | 5.73 | 5.10 | 4.96 | 0.581 | NS | 2.82 | 3.39 | 2.89 | 2.93 | 0.295 | *, C |
| A:P ratio | 2.85 b | 3.01 a,b | 3.08 a,b | 3.22 a | 0.071 | *, L | 3.52 a,b | 3.66 a | 3.18 b | 3.65 a | 0.116 | **, Q |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.S.; Kim, D.H.; Paradhipta, D.H.V.; Lee, H.J.; Yoon, H.; Joo, Y.H.; Adesogan, A.T.; Kim, S.C. Effects of Wormwood (Artemisia montana) Essential Oils on Digestibility, Fermentation Indices, and Microbial Diversity in the Rumen. Microorganisms 2020, 8, 1605. https://doi.org/10.3390/microorganisms8101605
Lee SS, Kim DH, Paradhipta DHV, Lee HJ, Yoon H, Joo YH, Adesogan AT, Kim SC. Effects of Wormwood (Artemisia montana) Essential Oils on Digestibility, Fermentation Indices, and Microbial Diversity in the Rumen. Microorganisms. 2020; 8(10):1605. https://doi.org/10.3390/microorganisms8101605
Chicago/Turabian StyleLee, Seong Shin, Dong Hyeon Kim, Dimas Hand Vidya Paradhipta, Hyuk Jun Lee, Hee Yoon, Young Ho Joo, Adegbola T. Adesogan, and Sam Churl Kim. 2020. "Effects of Wormwood (Artemisia montana) Essential Oils on Digestibility, Fermentation Indices, and Microbial Diversity in the Rumen" Microorganisms 8, no. 10: 1605. https://doi.org/10.3390/microorganisms8101605
APA StyleLee, S. S., Kim, D. H., Paradhipta, D. H. V., Lee, H. J., Yoon, H., Joo, Y. H., Adesogan, A. T., & Kim, S. C. (2020). Effects of Wormwood (Artemisia montana) Essential Oils on Digestibility, Fermentation Indices, and Microbial Diversity in the Rumen. Microorganisms, 8(10), 1605. https://doi.org/10.3390/microorganisms8101605





