Isolation and Identification of Potentially Pathogenic Microorganisms Associated with Dental Caries in Human Teeth Biofilms
Abstract
1. Introduction
2. Materials and Methods
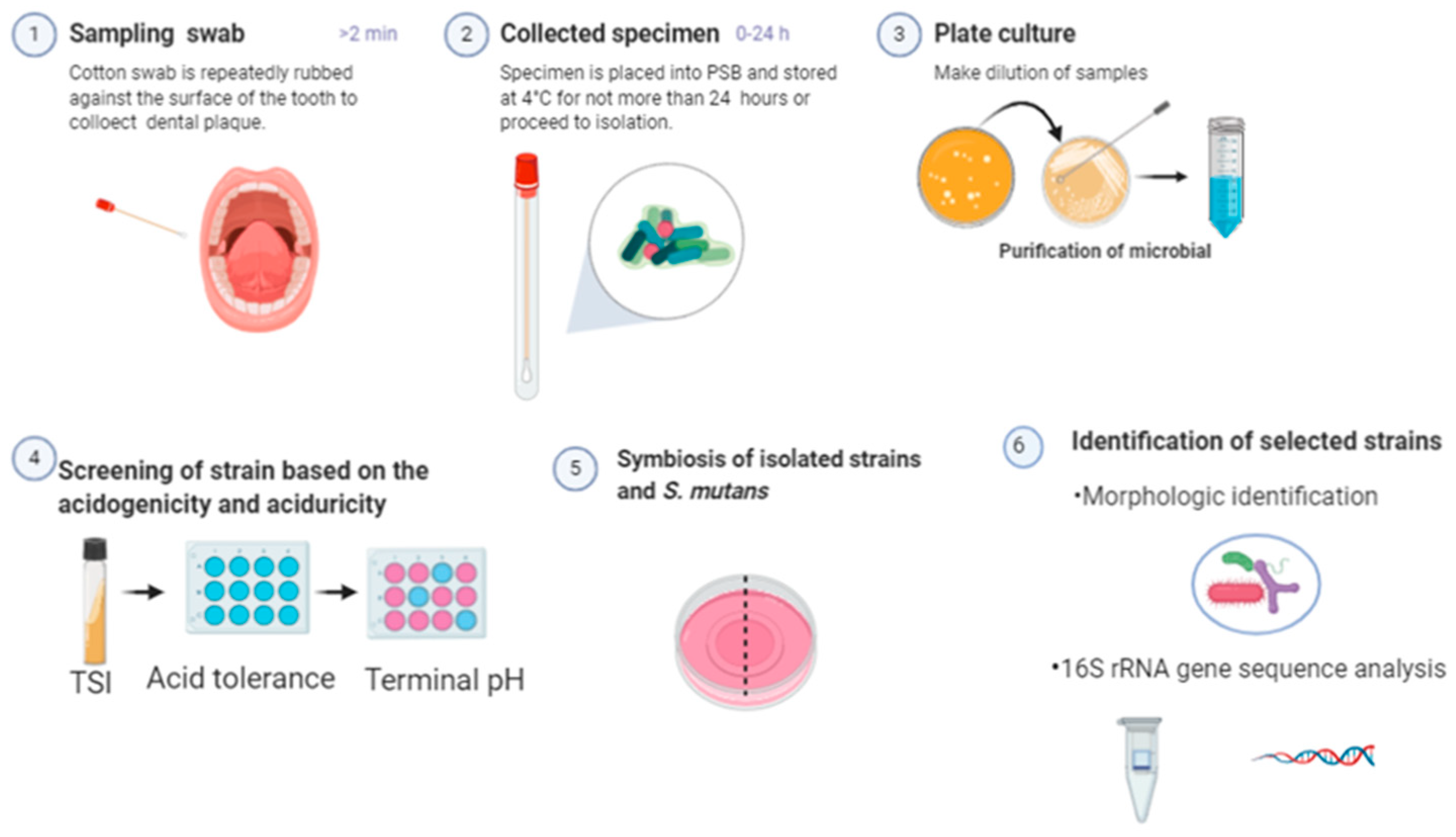
2.1. Isolation and Identification of Potentially Cariogenic Microorganisms
2.1.1. Sample Collection and Isolation of Bacterial Strain
2.1.2. Screening of Strains Based on Cariogenicity
2.1.3. Symbiosis of Isolated Strains and S. mutans
2.1.4. Bacterial Identification—Morphological and Biotyping
2.2. Evaluation of Cariogenicity of Isolated Oral Microorganisms
2.2.1. Measurement of Acidogenicity
2.2.2. Measurement of Acid Tolerance
2.2.3. In Vitro Biofilm Formation and Quantification
2.2.4. Dissolution of Tooth Enamel
2.3. Statistical Analysis
3. Results and Discussion
3.1. Isolation and Identification of Potentially Cariogenic Microorganisms
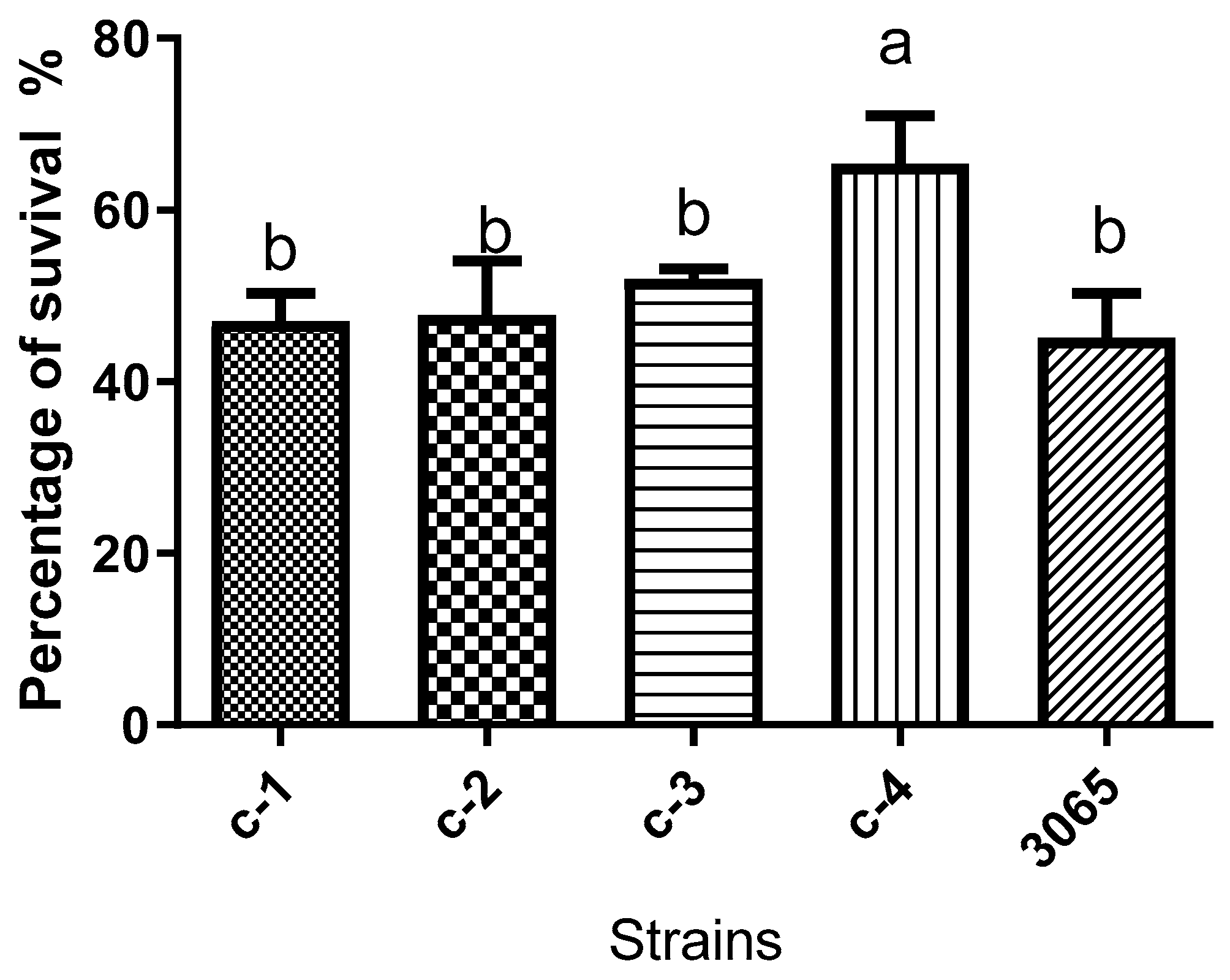
3.2. Cariogenicity of Isolated Oral Microorganisms
3.2.1. Acidogenic Potential of Isolated Oral Microorganisms and Their Acid Tolerance
3.2.2. In Vitro Biofilm Formation and Quantification
3.2.3. Dissolution of Tooth
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Pitts, N.B.; Zero, D.T.; Marsh, P.D.; Ekstrand, K.; Weintraub, J.A.; Ramos-Gomez, F.; Tagami, J.; Twetman, S.; Tsakos, G.; Ismail, A. Dental caries. Nat. Rev. Dis. Primers 2017, 3, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Shao, C.; Jin, B.; Mu, Z.; Lu, H.; Zhao, Y.; Wu, Z.; Yan, L.; Zhang, Z.; Zhou, Y.; Pan, H.; et al. Repair of tooth enamel by a biomimetic mineralization frontier ensuring epitaxial growth. Sci. Adv. 2019, 5, eaaw9569. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Yang, B.; He, L.; Li, R.; Liao, Y.; Zhang, S.; Yang, Y.; Xu, X.; Zhang, D.; Tan, H.; et al. Bioinspired peptide-decorated tannic acid for in situ remineralization of tooth enamel: In vitro and in vivo evaluation. ACS Biomater. Sci. Eng. 2017, 3, 3553–3562. [Google Scholar] [CrossRef]
- Peres, M.A.; Macpherson, L.M.D.; Weyant, R.J.; Daly, B.; Venturelli, R.; Mathur, M.R.; Listl, S.; Celeste, R.K.; Guarnizo-Herreño, C.C.; Kearns, C.; et al. Oral diseases: A global public health challenge. Lancet 2019, 394, 249–260. [Google Scholar] [CrossRef]
- WHO. Sugars and Dental Caries. 2017. Available online: https://www.who.int/news-room/fact-sheets/detail/sugars-and-dental-caries-90k (accessed on 17 March 2020).
- Burcham, Z.M.; Garneau, N.L.; Comstock, S.S.; Tucker, R.M.; Knight, R.; Metcalf, J.L. Patterns of oral microbiota diversity in adults and children: A crowdsourced population study. Sci. Rep. 2020, 10, 2133. [Google Scholar] [CrossRef]
- Kim, K.; Choi, S.; Chang, J.; Kim, S.M.; Kim, S.J.; Kim, R.Y.; Cho, H.J.; Park, S.M. Severity of dental caries and risk of coronary heart disease in middle-aged men and women: A population-based cohort study of Korean adults, 2002–2013. Sci. Rep. 2019, 9, 10491. [Google Scholar] [CrossRef]
- Goh, C.E.; Trinh, P.; Colombo, P.C.; Genkinger, J.M.; Mathema, B.; Uhlemann, A.C.; LeDuc, C.; Leibel, R.; Rosenbaum, M.; Paster, B.J. Association between nitrate-reducing oral bacteria and cardiometabolic outcomes: Results from origins. J. Am. Heart Assoc. 2019, 8, e013324. [Google Scholar] [CrossRef]
- Misaki, T.; Fukunaga, A.; Shimizu, Y.; Ishikawa, A.; Nakano, K. Possible link between dental diseases and arteriosclerosis in patients on hemodialysis. PLoS ONE 2019, 14, e0225038. [Google Scholar] [CrossRef]
- Cho, G.J.; Kim, S.Y.; Lee, H.C.; Kim, H.Y.; Lee, K.M.; Han, S.W.; Oh, M.J. Association between dental caries and adverse pregnancy outcomes. Sci. Rep. 2020, 10, 5309. [Google Scholar] [CrossRef]
- Nyvad, B.; Takahashi, N. Integrated hypothesis of dental caries and periodontal diseases. J. Oral Microbiol. 2020, 12, 1710953. [Google Scholar] [CrossRef]
- Du, Q.; Fu, M.; Zhou, Y.; Cao, Y.; Guo, T.; Zhou, Z.; Li, M.; Peng, X.; Zheng, X.; Li, Y.; et al. Sucrose promotes caries progression by disrupting the microecological balance in oral biofilms: An in vitro study. Sci. Rep. 2020, 10, 2961. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, K.; Chen, T.; Paster, B.J. A practical guide to the oral microbiome and its relation to health and disease. Oral Dis. 2017, 23, 276–286. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Liu, J.; Chen, L.; Gan, N.; Yang, D. The oral microbiome in the elderly with dental caries and health. Front. Cell. Infect. Microbiol. 2019, 8, 442. [Google Scholar] [CrossRef] [PubMed]
- Fechney, J.M.; Browne, G.V.; Prabhu, N.; Irinyi, L.; Meyer, W.; Hughes, T.; Bockmann, M.; Townsend, G.; Salehi, H.; Adler, C.J. Preliminary study of the oral mycobiome of children with and without dental caries. J. Oral Microbiol. 2019, 11, 1536182. [Google Scholar] [CrossRef]
- Ling, Z.; Kong, J.; Jia, P.; Wei, C.; Wang, Y.; Pan, Z.; Huang, W.; Li, L.; Chen, H.; Xiang, C. Analysis of oral microbiota in children with dental caries by PCR-DGGE and barcoded pyrosequencing. Microb. Ecol. 2010, 60, 677–690. [Google Scholar] [CrossRef]
- Widyarman, A.S.; Theodorea, C.F. Effect of reuterin on dual-species biofilm in vitro of streptococcus mutans and veillonella parvula. J. Int. Dent. Med. Res. 2019, 12, 77–83. [Google Scholar]
- Kressirer, C.A.; Smith, D.J.; King, W.F.; Dobeck, J.M.; Starr, J.R.; Tanner, A.C. Scardovia wiggsiae and its potential role as a caries pathogen. J. Oral Biosci. 2017, 59, 135–141. [Google Scholar] [CrossRef]
- Eşian, D.; Man, A.; Burlibasa, L.; Burlibasa, M.; Perieanu, M.V.; Bică, C. Salivary level of Streptococcus mutans and Lactobacillus spp. related to a high a risk of caries disease. Rom. Biotechnol. Lett. 2017, 22, 12496–12503. [Google Scholar]
- Obata, J.; Fujishima, K.; Nagata, E.; Oho, T. Pathogenic mechanisms of cariogenic Propionibacterium acidifaciens. Arch. Oral Biol. 2019, 105, 46–51. [Google Scholar] [CrossRef]
- Zeng, Y.; Youssef, M.; Wang, L.; Alkhars, N.; Thomas, M.; Cacciato, R.; Qing, S.; Ly-Mapes, O.; Xiao, J. Identification of non-streptococcus mutans bacteria from predente infant saliva grown on mitis-salivarius-bacitracin agar. J. Clin. Pediatr. Dent. 2020, 44, 28–34. [Google Scholar] [CrossRef]
- Chen, H.; Jiang, W. Application of high-throughput sequencing in understanding human oral microbiome related with health and disease. Front. Microbiol. 2014, 5, 508. [Google Scholar] [CrossRef]
- Banas, J.A.; Takanami, E.; Hemsley, R.M.; Villhauer, A.; Zhu, M.; Qian, F.; Marolf, A.; Drake, D.R. Evaluating the relationship between acidogenicity and acid tolerance for oral streptococci from children with or without a history of caries. J. Oral Microbiol. 2020, 12, 1688449. [Google Scholar] [CrossRef]
- Kim, D.; Barraza, J.P.; Arthur, R.A.; Hara, A.; Lewis, K.; Liu, Y.; Scisci, E.L.; Hajishengallis, E.; Whiteley, M.; Koo, H. Spatial mapping of polymicrobial communities reveals a precise biogeography associated with human dental caries. Proc. Natl. Acad. Sci. USA 2020, 10, 1073. [Google Scholar] [CrossRef] [PubMed]
- Krzyściak, W.; Papież, M.; Jurczak, A.; Kościelniak, D.; Vyhouskaya, P.; Zagórska-Świeży, K.; Skalniak, A. Relationship between pyruvate kinase activity and cariogenic biofilm formation in streptococcus mutans biotypes in caries patients. Front. Microbiol. 2017, 8, 856. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, N.; Nyvad, B. The role of bacteria in the caries process: Ecological perspectives. J. Dent. Res. 2011, 90, 294–303. [Google Scholar] [CrossRef]
- Senneby, A.; Davies, J.; Svensäter, G.; Neilands, J. Acid tolerance properties of dental biofilms in vivo. BMC Microbiol. 2017, 17, 165. [Google Scholar] [CrossRef] [PubMed]
- Nikawa, H.; Yamashiro, H.; Makihira, S.; Nishimura, M.; Egusa, H.; Furukawa, M.; Setijanto, D.; Hamada, T. In vitro cariogenic potential of Candida albicans. Mycoses 2003, 46, 471–478. [Google Scholar] [CrossRef]
- Lapirattanakul, J.; Takashima, Y.; Tantivitayakul, P.; Maudcheingka, T.; Leelataweewud, P.; Nakano, K.; Matsumoto-Nakano, M. Cariogenic properties of Streptococcus mutans clinical isolates with sortase defects. Arch. Oral Biol. 2017, 81, 7–14. [Google Scholar] [CrossRef]
- Hussain, M.S.; Kwon, M.; Tango, C.N.; Oh, D.H. Effect of electrolyzed water on the disinfection of bacillus cereus biofilms: The mechanism of enhanced resistance of sessile cells in the biofilm matrix. J. Food Prot. 2018, 81, 860–869. [Google Scholar] [CrossRef]
- Park, E.-j.; Hussain, M.S.; Wei, S.; Kwon, M.; Oh, D.H. Genotypic and phenotypic characteristics of biofilm formation of emetic toxin producing Bacillus cereus strains. Food Control. 2019, 96, 527–534. [Google Scholar] [CrossRef]
- Featherstone, J.D. Dental caries: A dynamic disease process. Aust. Dent. J. 2008, 53, 286–291. [Google Scholar] [CrossRef] [PubMed]
- Lamont, R.J.; Koo, H.; Hajishengallis, G. The oral microbiota: Dynamic communities and host interactions. Nat. Rev. Microbiol. 2018, 16, 745–759. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulou, O.S.; Iliopoulos, V.; Mallouchos, A.; Panagou, E.Z.; Chorianopoulos, N.; Tassou, C.C.; Nychas, G.E. Spoilage potential of pseudomonas (p. fragi, p. putida) and lab (leuconostoc mesenteroides, lactobacillus sakei) strains and their volatilome profile during storage of sterile pork meat using gc/ms and data analytics. Foods 2020, 9, 633. [Google Scholar] [CrossRef] [PubMed]
- Rubab, M.; Chellia, R.; Saravanakumar, K.; Mandava, S.; Khan, I.; Tango, C.N.; Wang, M.H.; Oh, D.H. Preservative effect of Chinese cabbage (Brassica rapa subsp. pekinensis) extract on their molecular docking, antioxidant and antimicrobial properties. PLoS ONE 2018, 13, e0203306. [Google Scholar] [CrossRef]
- Lee, S.Y.; Sekhon, S.S.; Ko, J.H.; Kim, H.C.; Kim, S.Y.; Won, K.; Ahn, J.Y.; Lee, K.; Kim, Y.H. Lactic acid bacteria isolated from Kimchi to evaluate anti-obesity effect in high fat diet-induced obese mice. J. Toxicol. 2018, 10, 11–16. [Google Scholar] [CrossRef]
- Seo, D.J.; Jung, D.; Jung, S.; Yeo, D.; Choi, C. Inhibitory effect of lactic acid bacteria isolated from kimchi against murine norovirus. Food Control. 2020, 109, 106881. [Google Scholar] [CrossRef]
- Pérez-Ibarreche, M.; Mendoza, L.M.; Vignolo, G.; Fadda, S. Proteomic and genetics insights on the response of the bacteriocinogenic Lactobacillus sakei CRL1862 during biofilm formation on stainless steel surface at 10 °C. Int. J. Food Microbiol. 2017, 258, 18–27. [Google Scholar] [CrossRef]
- Shao, X.; Fang, K.; Medina, D.; Wan, J.; Lee, J.L.; Hong, S.H. The probiotic, Leuconostoc mesenteroides, inhibits Listeria monocytogenes biofilm formation. J. Food Saf. 2020, 40, e12750. [Google Scholar] [CrossRef]
- Ananieva, M.; Faustova, M.; Basarab, Y.O.; Loban, G. Kocuria rosea, kocuria kristinae, leuconostoc mesenteroides as caries-causing representatives of oral microflora. Wiad. Lek. 2017, 70, 296–298. [Google Scholar]
- Kirilova, J.N.; Topalova-Pirinska, S.Z.; Kirov, D.N.; Deliverska, E.G.; Doichinova, L.B. Types of microorganisms in proximal caries lesion and ozone treatment. Biotechnol. Biotechnol. Equip. 2019, 33, 683–688. [Google Scholar] [CrossRef]
- Bin, C.; Al-Dhabi, N.A.; Esmail, G.A.; Arokiyaraj, S.; Arasu, M.V. Potential effect of Allium sativum bulb for the treatment of biofilm forming clinical pathogens recovered from periodontal and dental caries. Saudi J. Biol. Sci. 2020, 27, 1428–1434. [Google Scholar] [CrossRef] [PubMed]
- Nadell, C.D.; Xavier, J.B.; Levin, S.A.; Foster, K.R. The evolution of quorum sensing in bacterial biofilms. PLoS Biol. 2008, 6, e14. [Google Scholar] [CrossRef] [PubMed]
- Gross, E.L.; Beall, C.J.; Kutsch, S.R.; Firestone, N.D.; Leys, E.J.; Griffen, A.L. Beyond streptococcus mutans: Dental caries onset linked to multiple species by 16s rRNA community analysis. PLoS ONE 2012, 7, 11. [Google Scholar] [CrossRef] [PubMed]
- Shaw, L.; Murray, J.; Burchell, C.; Best, J. Calcium and phosphorus content of plaque and saliva in relation to dental caries. Caries Res. 1983, 17, 543–548. [Google Scholar] [CrossRef]
- Traisaeng, S.; Batsukh, A.; Chuang, T.H.; Herr, D.R.; Huang, Y.F.; Chimeddorj, B.; Huang, C.M. Leuconostoc mesenteroides fermentation produces butyric acid and mediates Ffar2 to regulate blood glucose and insulin in type 1 diabetic mice. Sci. Rep. 2020, 10, 7928. [Google Scholar] [CrossRef] [PubMed]
- Banas, J.A.; Drake, D.R. Are the mutans streptococci still considered relevant to understanding the microbial etiology of dental caries? BMC Oral Health 2018, 18, 129. [Google Scholar] [CrossRef]
- Pereira, D.F.A.; Seneviratne, C.J.; Koga-Ito, C.Y.; Samaranayake, L.P. Is the oral fungal pathogen Candida albicans a cariogen? Oral Dis. 2018, 24, 518–526. [Google Scholar] [CrossRef]
- Aas, J.A.; Griffen, A.L.; Dardis, S.R.; Lee, A.M.; Olsen, I.; Dewhirst, F.E.; Leys, E.J.; Paster, B.J. Bacteria of dental caries in primary and permanent teeth in children and young adults. J. Clin. Microbiol. 2008, 46, 1407–1417. [Google Scholar] [CrossRef]



| No. | Gram | Hemolytic | Isolated Source | NCBI Blast Sequencing Results |
|---|---|---|---|---|
| C-1 | + | γ-hemolysis | Human mouth (caries) | Streptococcus salivarius |
| C-2 | + | β-hemolysis | Infected teeth | Streptococcus anginosus |
| C-3 | + | γ-hemolysis | Human mouth (caries) | Leuconostoc mesenteroides |
| C-4 | + | β-hemolysis | Human mouth(caries-free) | Lactobacillus sakei |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Daliri, E.B.-M.; Chelliah, R.; Oh, D.-H. Isolation and Identification of Potentially Pathogenic Microorganisms Associated with Dental Caries in Human Teeth Biofilms. Microorganisms 2020, 8, 1596. https://doi.org/10.3390/microorganisms8101596
Chen X, Daliri EB-M, Chelliah R, Oh D-H. Isolation and Identification of Potentially Pathogenic Microorganisms Associated with Dental Caries in Human Teeth Biofilms. Microorganisms. 2020; 8(10):1596. https://doi.org/10.3390/microorganisms8101596
Chicago/Turabian StyleChen, Xiuqin, Eric Banan-Mwine Daliri, Ramachandran Chelliah, and Deog-Hwan Oh. 2020. "Isolation and Identification of Potentially Pathogenic Microorganisms Associated with Dental Caries in Human Teeth Biofilms" Microorganisms 8, no. 10: 1596. https://doi.org/10.3390/microorganisms8101596
APA StyleChen, X., Daliri, E. B.-M., Chelliah, R., & Oh, D.-H. (2020). Isolation and Identification of Potentially Pathogenic Microorganisms Associated with Dental Caries in Human Teeth Biofilms. Microorganisms, 8(10), 1596. https://doi.org/10.3390/microorganisms8101596







