High-Titer Lactic Acid Production by Pediococcus acidilactici PA204 from Corn Stover through Fed-Batch Simultaneous Saccharification and Fermentation
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials and Enzyme
2.2. Medium and Strain
2.3. Simultaneous Saccharification and Fermentation (SSF), and NaOH-Pretreated Corn Stover
2.4. Analysis of Sugars, Acids, and Biomass
3. Results and Discussion
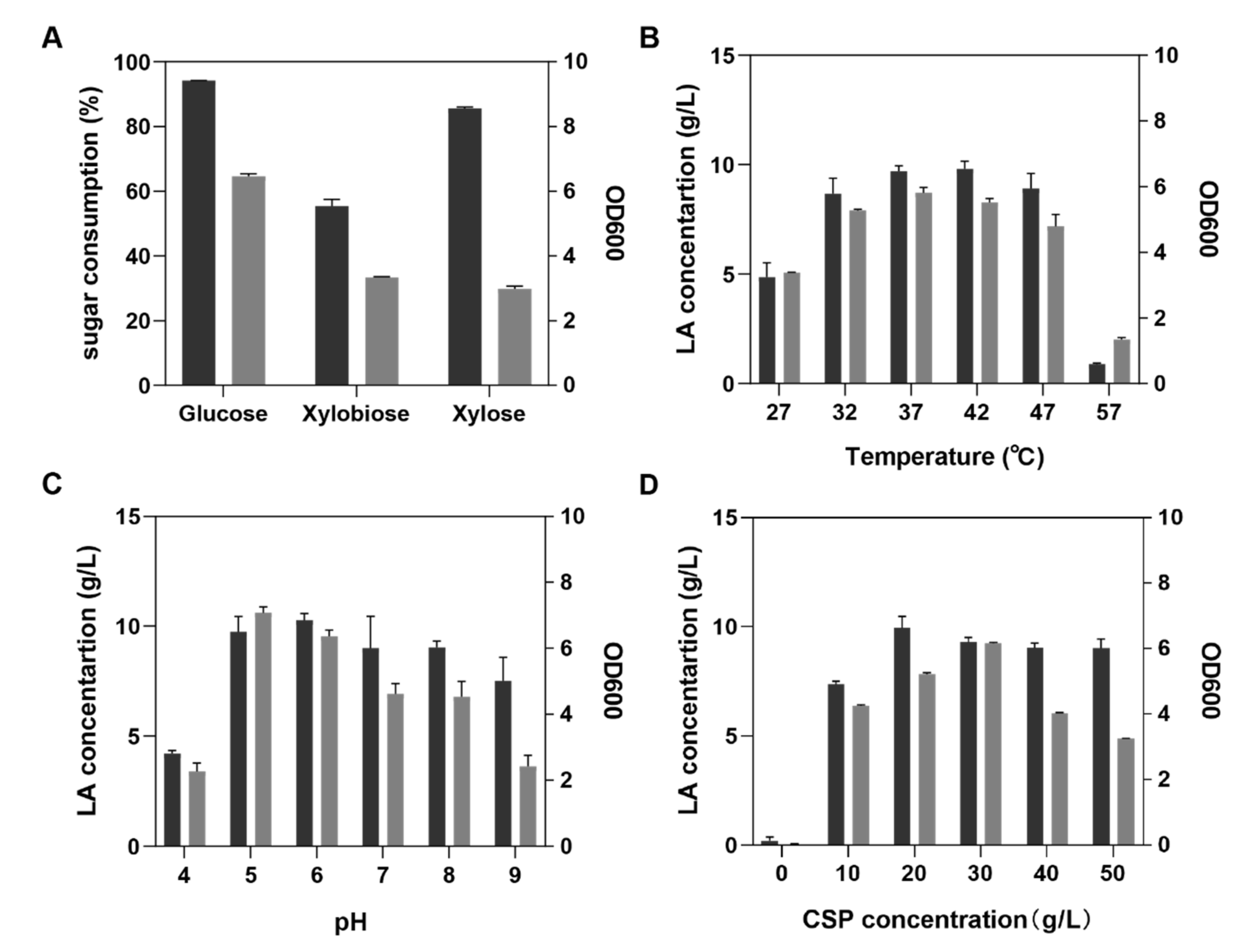
3.1. Effects of Temperature, pH, Carbon Source, and Nitrogen Source on LA Production
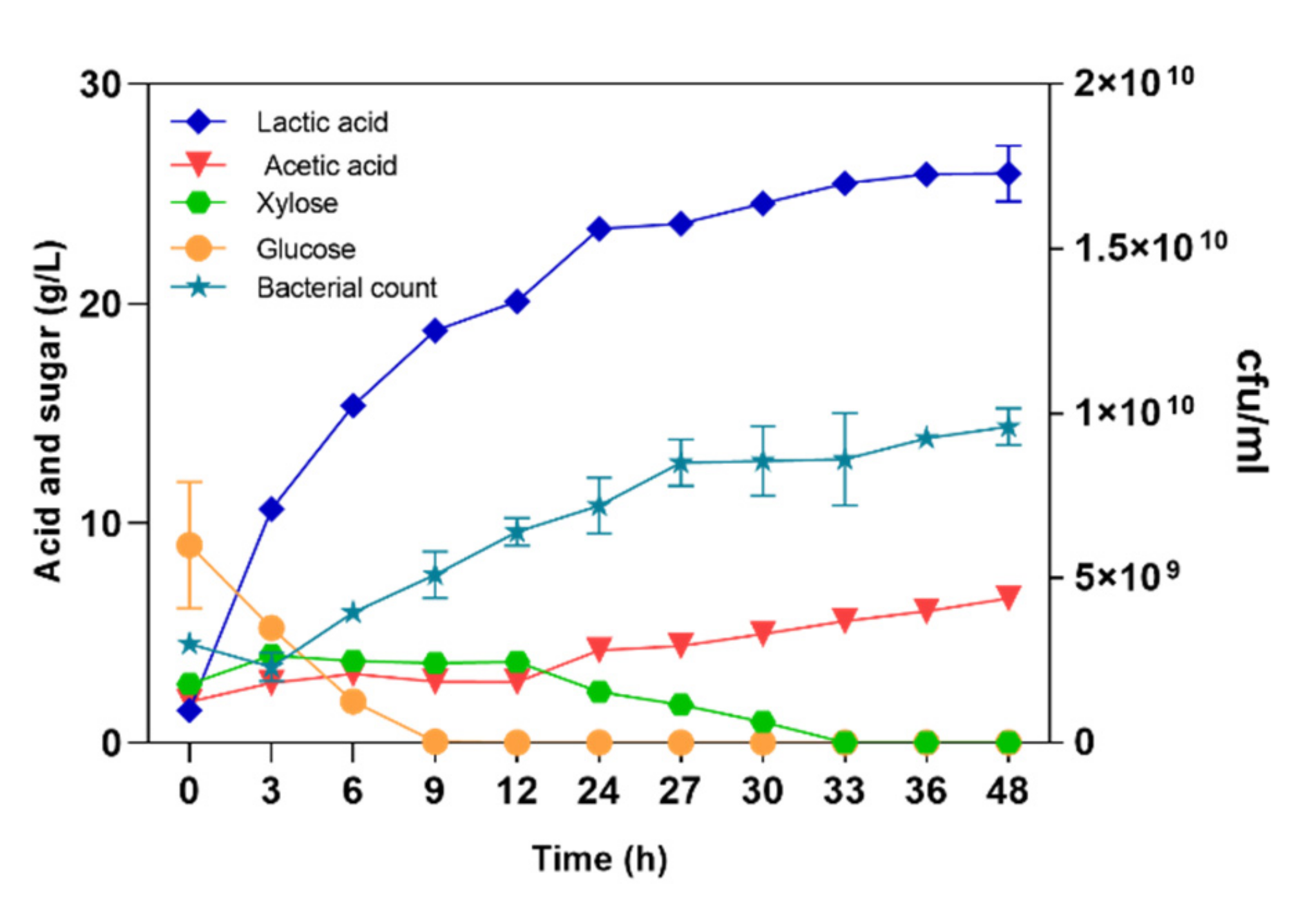
3.2. High-Titer and High-Yield LA Production from NaOH-Pretreated Corn Stover through SSF
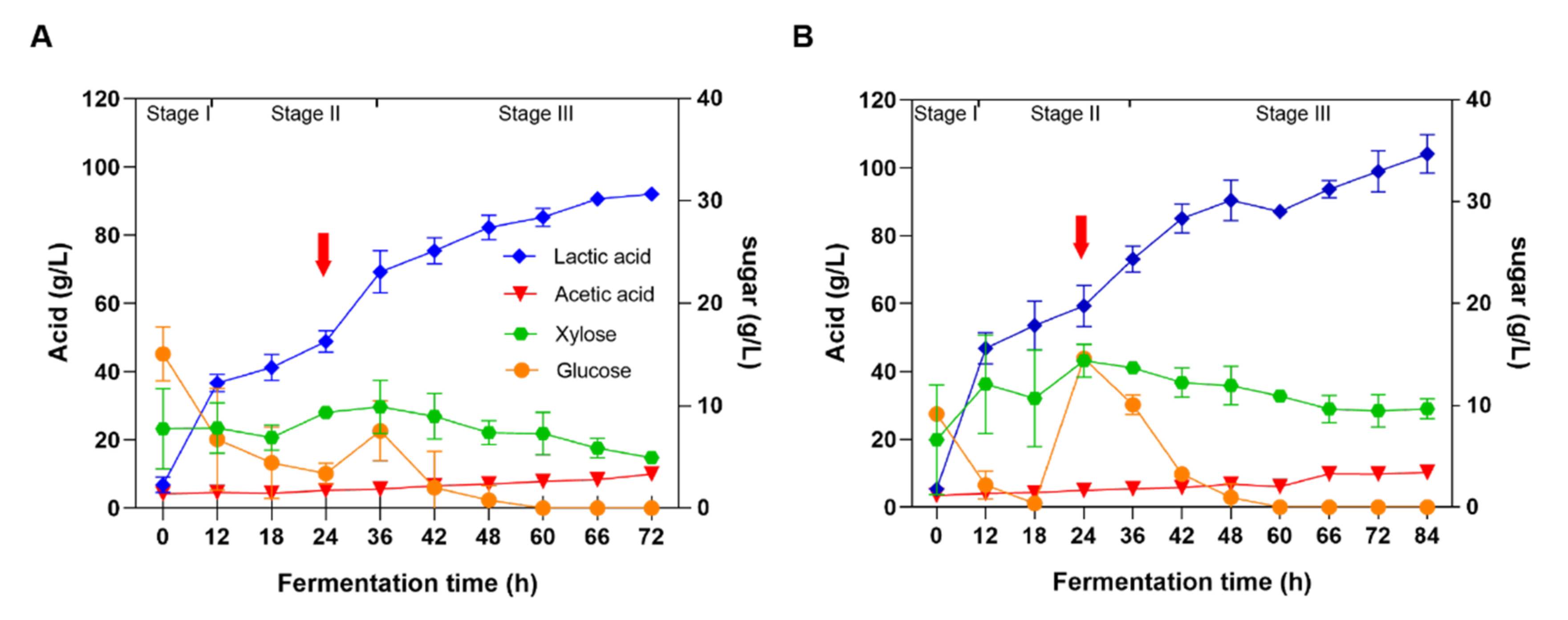
3.3. High-Titer and High-Yield LA Production through Fed-Batch Fermentation
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lin, Y.; Tanaka, S. Ethanol fermentation from biomass resources: Current state and prospects. Appl. Microbiol. Biotechnol. 2006, 69, 627–642. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Feng, Y.; Wang, H.; Qu, Y.; Yu, Y.; Ren, N.; Li, N.; Wang, E.; Lee, H.; Logan, B.E. Bioaugmentation for Electricity Generation from Com Stover Biomass Using Microbial Fuel Cells. Environ. Sci. Technol. 2009, 43, 6088–6093. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Xu, Z.; Li, X.; Yu, J.; Cai, M. Integrated bioethanol production from mixtures of corn and corn stover. Bioresour. Technol. 2018, 258, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Lin, Y.; Zhang, Z.; Xiang, T.; Mei, Y.; Zhao, S.; Liang, Y.; Peng, N. High-titer lactic acid production by Lactobacillus pentosus FL0421 from corn stover using fed-batch simultaneous saccharification and fermentation. Bioresour. Technol. 2016, 214, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xu, F.; Li, Y.; Lu, J.; Li, S.; Shah, A.; Zhang, X.; Zhang, H.; Gong, X.; Li, G. Reactor performance and energy analysis of solid state anaerobic co-digestion of dairy manure with corn stover and tomato residues. Waste Manag. 2018, 73, 130–139. [Google Scholar] [CrossRef]
- Abdel-Rahman, M.A.; Tashiro, Y.; Sonomoto, K. Recent advances in lactic acid production by microbial fermentation processes. Biotechnol. Adv. 2013, 31, 877–902. [Google Scholar] [CrossRef]
- Zhu, Y.; Lee, Y.Y.; Elander, R.T. Conversion of aqueous ammonia-treated corn stover to lactic acid by simultaneous saccharification and cofermentation. Appl. Microbiol. Biotechnol. 2007, 136, 721–738. [Google Scholar]
- Li, Y.; Lei, L.; Zheng, L.; Xiao, X.; Tang, H.; Luo, C. Genome sequencing of gut symbiotic Bacillus velezensis LC1 for bioethanol production from bamboo shoots. Biotechnol. Biofuels 2020, 13, 34. [Google Scholar] [CrossRef]
- Zhang, J.; Shao, S.; Bao, J. Long term storage of dilute acid pretreated corn stover feedstock and ethanol fermentability evaluation. Bioresour. Technol. 2016, 201, 355–359. [Google Scholar] [CrossRef]
- Talebnia, F.; Karakashev, D.; Angelidaki, I. Production of bioethanol from wheat straw: An overview on pretreatment, hydrolysis and fermentation. Bioresour. Technol. 2010, 101, 4744–4753. [Google Scholar] [CrossRef]
- Lloyd, T.A.; Wyman, C.E. Combined sugar yields for dilute sulfuric acid pretreatment of corn stover followed by enzymatic hydrolysis of the remaining solids. Bioresour. Technol. 2005, 96, 1967–1977. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Zhang, Z.; Lin, Y.; Zhao, S.; Mei, Y.; Liang, Y.; Peng, N. High-titer lactic acid production from NaOH-pretreated corn stover by Bacillus coagulans LA204 using fed-batch simultaneous saccharification and fermentation under non-sterile condition. Bioresour. Technol. 2015, 182, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Xie, Y.; He, X.; Li, X.; Hu, J.; Ruan, Z.; Zhao, S.; Peng, N.; Liang, Y. Comparison of high-titer lactic acid fermentation from NaOH- and NH3-H2O2-pretreated corncob by Bacillus coagulans using simultaneous saccharification and fermentation. Sci. Rep. 2016, 6, 37245. [Google Scholar] [CrossRef] [PubMed]
- Daiana, W.; Arias, J.M.; Modesto, L.F.; de França Passos, D.; Pereira, N., Jr. Lactic acid production from sugarcane bagasse hydrolysates by Lactobacillus pentosus: Integrating xylose and glucose fermentation. Biotechnol. Prog. 2018, 35, e2718. [Google Scholar]
- Zhang, Y.; Vadlani, P.V. Lactic acid production from biomass-derived sugars via co-fermentation of Lactobacillus brevis and Lactobacillus plantarum. J. Biosci. Bioeng. 2015, 119, 694–699. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Rahman, M.A.; Tashiro, Y.; Zendo, T.; Hanada, K.; Shibata, K.; Sonomoto, K. Efficient homofermentative L-(+)-lactic acid production from xylose by a novel lactic acid bacterium, Enterococcus mundtii QU 25. Appl. Environ. Microbiol. 2011, 77, 1892–1895. [Google Scholar] [CrossRef]
- Abdel-Rahman, M.A.; Tashiro, Y.; Zendo, T.; Sakai, K.; Sonomoto, K. Enterococcus faecium QU 50: A novel thermophilic lactic acid bacterium for high-yield l-lactic acid production from xylose. FEMS Microbiol. Lett. 2015, 362, 1–7. [Google Scholar] [CrossRef]
- Cui, F.; Li, Y.; Wan, C. Lactic acid production from corn stover using mixed cultures of Lactobacillus rhamnosus and Lactobacillus brevis. Bioresour. Technol. 2011, 102, 1831–1836. [Google Scholar] [CrossRef]
- Bischoff, K.M.; Liu, S.; Hughes, S.R.; Rich, J.O. Fermentation of corn fiber hydrolysate to lactic acid by the moderate thermophile Bacillus coagulans. Biotechnol. Lett. 2010, 32, 823–828. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, X.; Luo, J.; Qi, B.; Wan, Y. An efficient process for lactic acid production from wheat straw by a newly isolated Bacillus coagulans strain IPE22. Bioresour. Technol. 2014, 158, 396–399. [Google Scholar] [CrossRef]
- Ye, L.; Hudari, M.S.; Zhou, X.; Zhang, D.; Li, Z.; Wu, J.C. Conversion of acid hydrolysate of oil palm empty fruit bunch to L-lactic acid by newly isolated Bacillus coagulans JI12. Appl. Microbiol. Biotechnol. 2013, 97, 4831–4838. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Z.; Gao, Q.; Bao, J. Engineering Pediococcus acidilactici with xylose assimilation pathway for high titer cellulosic l-lactic acid fermentation. Bioresour. Technol. 2018, 249, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Z.; Gao, Q.; Bao, J. Constructing xylose-assimilating pathways in Pediococcus acidilactici for high titer d-lactic acid fermentation from corn stover feedstock. Bioresour. Technol. 2017, 245, 1369–1376. [Google Scholar] [CrossRef] [PubMed]
- Eom, I.Y.; Oh, Y.H.; Park, S.J.; Lee, S.H.; Yu, J.H. Fermentative l-lactic acid production from pretreated whole slurry of oil palm trunk treated by hydrothermolysis and subsequent enzymatic hydrolysis. Bioresour. Technol. 2015, 185, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.; Qiao, Q.; Chu, D.; Gu, H.; Dao, T.H.; Zhang, J.; Bao, J. Simultaneous saccharification and high titer lactic acid fermentation of corn stover using a newly isolated lactic acid bacterium Pediococcus acidilactici DQ2. Bioresour. Technol. 2013, 135, 481–489. [Google Scholar] [CrossRef] [PubMed]
- Yi, X.; Zhang, P.; Sun, J.; Tu, Y.; Gao, Q.; Zhang, J.; Bao, J. Engineering wild-type robust Pediococcus acidilactici strain for high titer L- and D-lactic acid production from corn stover feedstock. J. Biotechnol. 2015, 217, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Guo, P.; Mochidzuki, K.; Cheng, W.; Zhou, M.; Gao, H.; Zheng, D.; Wang, X.; Cui, Z. Effects of different pretreatment strategies on corn stalk acidogenic fermentation using a microbial consortium. Bioresour. Technol. 2011, 102, 7526–7531. [Google Scholar] [CrossRef]
- Toquero, C.; Bolado, S. Effect of four pretreatments on enzymatic hydrolysis and ethanol fermentation of wheat straw. Influence of inhibitors and washing. Bioresour. Technol. 2014, 157, 68–76. [Google Scholar] [CrossRef]
- Millette, M.; Dupont, C.; Shareck, F.; Ruiz, M.T.; Lacroix, M. Purification and identification of the pediocin produced by Pediococcus acidilactici MM33, a new human intestinal Strain. J. Appl. Microbiol. 2008, 104, 269–275. [Google Scholar]
- Porto, M.C.; Kuniyoshi, T.M.; Azevedo, P.O.; Vitolo, M.; Oliveira, R.P. Pediococcus spp.: An important genus of lactic acid bacteria and pediocin producers. Biotechnol. Adv. 2017, 35, 361–374. [Google Scholar] [CrossRef]
- De Man, J.C. Lactobacillus bulgaricus (Luerssen et Kuehn) Holland. Antonie Van Leeuwenhoek 1960, 26, 77–80. [Google Scholar] [CrossRef] [PubMed]
| Corn Stover | Cellulose (%) | Hemicellulose (%) | Lignin (%) |
|---|---|---|---|
| A | 37.12 ± 0.32 | 29.60 ± 0.26 | 20.80 ± 0.56 |
| B | 51.34 ± 0.57 | 27.20 ± 0.34 | 8.31 ± 0.89 |
| C | 60.01 ± 0.51 | 27.33 ± 0.21 | 6.87 ± 0.66 |
| Experiments | 4% Stover | 12% Stover | 15% Stover |
|---|---|---|---|
| Lactic acid titer (g/L) | 25.92 | 92.01 | 104.11 |
| Lactic acid yield (g/g stover) | 0.65 | 0.77 | 0.69 |
| Lactic acid productivity (g/L/h) | 0.54 | 1.28 | 1.24 |
| Acetic acid titer (g/L) | 6.57 | 10.03 | 10.28 |
| Acetic acid yield (g/g stover) | 0.16 | 0.08 | 0.07 |
| Acetic acid productivity (g/L/h) | 0.14 | 0.14 | 0.12 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Li, Y.; Zhang, J.; Peng, N.; Liang, Y.; Zhao, S. High-Titer Lactic Acid Production by Pediococcus acidilactici PA204 from Corn Stover through Fed-Batch Simultaneous Saccharification and Fermentation. Microorganisms 2020, 8, 1491. https://doi.org/10.3390/microorganisms8101491
Zhang Z, Li Y, Zhang J, Peng N, Liang Y, Zhao S. High-Titer Lactic Acid Production by Pediococcus acidilactici PA204 from Corn Stover through Fed-Batch Simultaneous Saccharification and Fermentation. Microorganisms. 2020; 8(10):1491. https://doi.org/10.3390/microorganisms8101491
Chicago/Turabian StyleZhang, Zhenting, Yanan Li, Jianguo Zhang, Nan Peng, Yunxiang Liang, and Shumiao Zhao. 2020. "High-Titer Lactic Acid Production by Pediococcus acidilactici PA204 from Corn Stover through Fed-Batch Simultaneous Saccharification and Fermentation" Microorganisms 8, no. 10: 1491. https://doi.org/10.3390/microorganisms8101491
APA StyleZhang, Z., Li, Y., Zhang, J., Peng, N., Liang, Y., & Zhao, S. (2020). High-Titer Lactic Acid Production by Pediococcus acidilactici PA204 from Corn Stover through Fed-Batch Simultaneous Saccharification and Fermentation. Microorganisms, 8(10), 1491. https://doi.org/10.3390/microorganisms8101491