Role of Infections in the Pathogenesis of Rheumatoid Arthritis: Focus on Mycobacteria
Abstract
1. Introduction
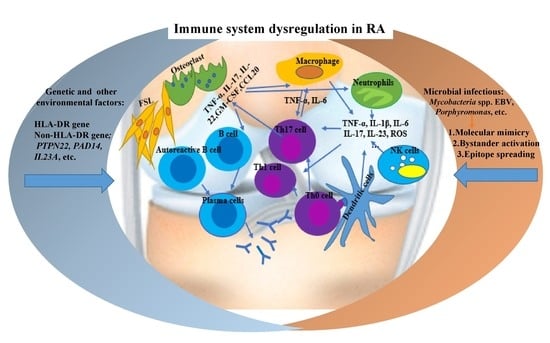
2. RA Immunopathogenesis
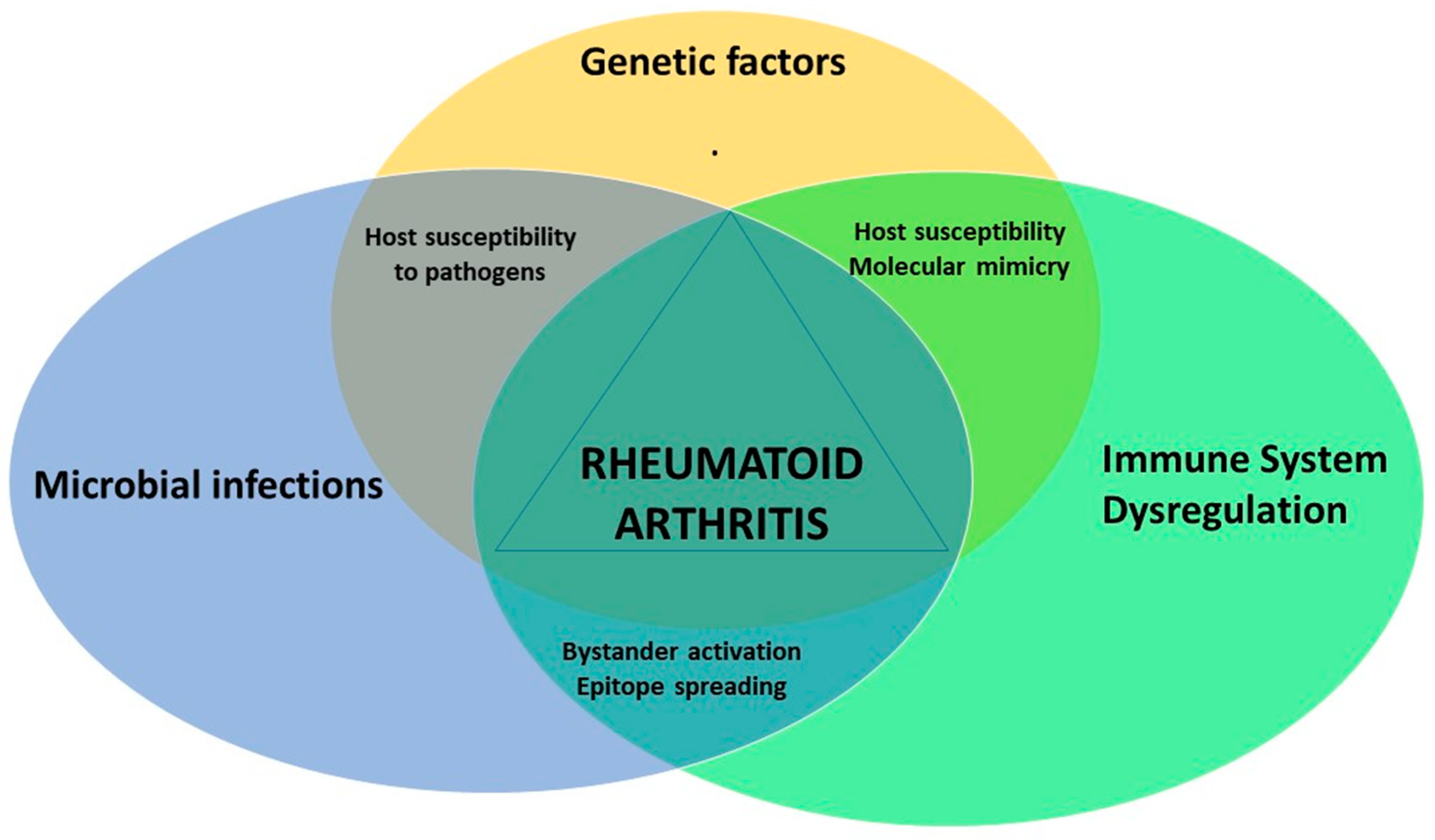
3. The Role of Mycobacterial Infections in Rheumatoid Arthritis
4. Other Infections Associated with RA
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Simon, T.A.; Kawabata, H.; Ray, N.; Baheti, A.; Suissa, S.; Esdaile, J.M. Prevalence of co-existing autoimmune disease in rheumatoid arthritis: A cross-sectional study. Adv. Ther. 2017, 34, 2481–2490. [Google Scholar] [CrossRef]
- Alivernini, S.; Tolusso, B.; Petricca, L.; Ferraccioli, G.; Gremese, E. Chapter 46—Rheumatoid arthritis. In Mosaic of Autoimmunity; Elsevier: Amsterdam, The Netherlands, 2019; pp. 501–526. [Google Scholar]
- de Brito Rocha, S.; Baldo, D.C.; Andrade, L.E.C. Clinical and pathophysiologic relevance of autoantibodies in rheumatoid arthritis. Adv. Rheumatol. 2019, 59, 2. [Google Scholar] [CrossRef]
- Huizinga, T.W.; Pincus, T. Rheumatoid arthritis. Ann. Intern. Med. 2010, 153, ITC1-1. [Google Scholar] [CrossRef]
- Grassi, W.; De Angelis, R.; Lamanna, G.; Cervini, C. The clinical featuRes. of rheumatoid arthritis. Eur. J. Radiol. 1998, 27, S18–S24. [Google Scholar] [CrossRef]
- van Delft, M.A.M.; Huizinga, T.W.J. An overview of autoantibodies in rheumatoid arthritis. J. Autoimmun. 2020, 110, 102392. [Google Scholar] [CrossRef]
- Ma, X.; Xu, S. TNF inhibitor therapy for rheumatoid arthritis. Biomed. Rep. 2013, 1, 177–184. [Google Scholar] [CrossRef]
- Croia, C.; Bursi, R.; Sutera, D.; Petrelli, F.; Alunno, A.; Puxeddu, I. One year in review 2019: Pathogenesis of rheumatoid arthritis. Clin. Exp. Rheumatol. 2019, 37, 347–357. [Google Scholar]
- Hussein, H.M.; Rahal, E.A. The role of viral infections in the development of autoimmune diseases. Crit Rev. Microbiol. 2019, 45, 394–412. [Google Scholar] [CrossRef]
- Atkin, S.L.; Welbury, R.R.; Stanfield, E.; Beavis, D.; Iwais, B.; Dick, W.C. Clinical and laboratory studies of inflammatory polyarthritis in patients with leprosy in Papua New Guinea. Ann. Rheum. Dis. 1987, 46, 688–690. [Google Scholar] [CrossRef]
- Rook, G.A. Rheumatoid arthritis, mycobacterial antigens and agalactosyl IgG. Scand. J. Immunol. 1988, 28, 487–493. [Google Scholar] [CrossRef]
- Shoenfeld, Y.; Isenberg, D.A. Mycobacteria and autoimmunity. Immunol. Today 1988, 9, 178–182. [Google Scholar] [CrossRef]
- Liao, T.L.; Lin, C.H.; Shen, G.H.; Chang, C.L.; Lin, C.F.; Chen, D.Y. Risk for mycobacterial disease among patients with rheumatoid arthritis, Taiwan, 2001–2011. Emerg. Infect. Dis. 2015, 21, 1387–1395. [Google Scholar] [CrossRef]
- Listing, J.; Gerhold, K.; Zink, A. The risk of infections associated with rheumatoid arthritis, with its comorbidity and treatment. Rheumatology 2012, 52, 53–61. [Google Scholar] [CrossRef]
- Mehta, B.; Pedro, S.; Ozen, G.; Kalil, A.; Wolfe, F.; Mikuls, T.; Michaud, K. Serious infection risk in rheumatoid arthritis compared with non-inflammatory rheumatic and musculoskeletal diseases: A US national cohort study. RMD Open 2019, 5, e000935. [Google Scholar] [CrossRef]
- McInnes, I.B.; Schett, G. Cytokines in the pathogenesis of rheumatoid arthritis. Nat. Rev. Immunol. 2007, 7, 429–442. [Google Scholar] [CrossRef]
- Brennan, F.M.; McInnes, I.B. Evidence that cytokines play a role in rheumatoid arthritis. J. Clin. Investig. 2008, 118, 3537–3545. [Google Scholar] [CrossRef]
- Coutant, F.; Miossec, P. Evolving concepts of the pathogenesis of rheumatoid arthritis with focus on the early and late stages. Curr. Opin. Rheumatol. 2020, 32, 57–63. [Google Scholar] [CrossRef]
- Karami, J.; Aslani, S.; Jamshidi, A.; Garshasbi, M.; Mahmoudi, M. Genetic implications in the pathogenesis of rheumatoid arthritis; an updated review. Gene 2019, 702, 8–16. [Google Scholar] [CrossRef]
- Lee, J.C.; Espéli, M.; Anderson, C.A.; Linterman, M.A.; Pocock, J.M.; Williams, N.J.; Roberts, R.; Viatte, S.; Fu, B.; Peshu, N.; et al. Human SNP links differential outcomes in inflammatory and infectious disease to a FOXO3-regulated pathway. Cell 2013, 155, 57–69. [Google Scholar] [CrossRef]
- Gregersen, P.K.; Silver, J.; Winchester, R.J. The shared epitope hypothesis. an approach to understanding the molecular genetics of susceptibility to rheumatoid arthritis. Arthritis Rheum. 1987, 30, 1205–1213. [Google Scholar] [CrossRef]
- van der Helm-van Mil, A.H.M.; Verpoort, K.N.; le Cessie, S.; Huizinga, T.W.J.; de Vries, R.R.P.; Toes, R.E.M. The HLA–DRB1 shared epitope alleles differ in the interaction with smoking and predisposition to antibodies to cyclic citrullinated peptide. Arthritis Rheum. 2007, 56, 425–432. [Google Scholar] [CrossRef]
- Albani, S.; Keystone, E.C.; Nelson, J.L.; Ollier, W.E.; La Cava, A.; Montemayor, A.C.; Weber, D.A.; Montecucco, C.; Martini, A.; Carson, D.A. Positive selection in autoimmunity: Abnormal immune responses to a bacterial dnaJ antigenic determinant in patients with early rheumatoid arthritis. Nat. Med. 1995, 1, 448–452. [Google Scholar] [CrossRef]
- Bax, M.; van Heemst, J.; Huizinga, T.W.J.; Toes, R.E.M. Genetics of rheumatoid arthritis: What have we learned? Immunogenetics 2011, 63, 459–466. [Google Scholar] [CrossRef]
- Cha, S.; Choi, C.-B.; Han, T.-U.; Kang, C.P.; Kang, C.; Bae, S.-C. Association of Anti–Cyclic citrullinated peptide antibody levels with PADI4 haplotypes in early rheumatoid arthritis and with shared epitope alleles in very late rheumatoid arthritis. Arthritis Rheum. 2007, 56, 1454–1463. [Google Scholar] [CrossRef]
- Faragó, B.; Magyari, L.; Sáfrány, E.; Csöngei, V.; Járomi, L.; Horvatovich, K.; Sipeky, C.; Maász, A.; Radics, J.; Gyetvai, Á.; et al. Functional variants of interleukin-23 receptor gene confer risk for rheumatoid arthritis but not for systemic sclerosis. Ann. Rheum. Dis. 2008, 67, 248–250. [Google Scholar] [CrossRef]
- Farago, B.; Talian, G.C.; Komlosi, K.; Nagy, G.; Berki, T.; Gyetvai, A.; Szekanecz, Z.; Nyarady, Z.; Kiss, C.G.; Nemeth, P.; et al. Protein tyrosine phosphatase gene C1858T allele confers risk for rheumatoid arthritis in Hungarian subjects. Rheumatol. Int. 2009, 29, 793–796. [Google Scholar] [CrossRef]
- Alivernini, S.; Tolusso, B.; Petricca, L.; Ferraccioli, G.; Gremese, E. Chapter 16—Rheumatoid arthritis. In Mosaic of Autoimmunity; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Klareskog, L.; Catrina, A.I.; Paget, S. Rheumatoid arthritis. Lancet 2009, 373, 659–672. [Google Scholar] [CrossRef]
- McInnes, I.B.; Schett, G. The pathogenesis of rheumatoid arthritis. N. Engl. J. Med. 2011, 365, 2205–2219. [Google Scholar] [CrossRef]
- Farrugia, M.; Baron, B. The role of TNF-α in rheumatoid arthritis: A focus on regulatory T cells. J. Clin. Transl. Res. 2016, 2, 84–90. [Google Scholar] [CrossRef]
- Calabresi, E.; Petrelli, F.; Bonifacio, A.F.; Puxeddu, I.; Alunno, A. One year in review 2018: Pathogenesis of rheumatoid arthritis. Clin. Exp. Rheumatol. 2018, 36, 175–184. [Google Scholar]
- Huang, Q.Q.; Pope, R.M. The role of toll-like receptors in rheumatoid arthritis. Curr. Rheumatol. Rep. 2009, 11, 357–364. [Google Scholar] [CrossRef]
- Elshabrawy, H.A.; Essani, A.E.; Szekanecz, Z.; Fox, D.A.; Shahrara, S. TLRs, future potential therapeutic targets for RA. AutoImmun. Rev. 2017, 16, 103–113. [Google Scholar] [CrossRef]
- Ospelt, C.; Brentano, F.; Rengel, Y.; Stanczyk, J.; Kolling, C.; Tak, P.P.; Gay, R.E.; Gay, S.; Kyburz, D. Overexpression of toll-like receptors 3 and 4 in synovial tissue from patients with early rheumatoid arthritis: Toll-like receptor expression in early and longstanding arthritis. Arthritis Rheum. 2008, 58, 3684–3692. [Google Scholar] [CrossRef]
- Huang, Q.; Ma, Y.; Adebayo, A.; Pope, R.M. Increased macrophage activation mediated through toll-like receptors in rheumatoid arthritis. Arthritis Rheum. 2007, 56, 2192–2201. [Google Scholar] [CrossRef]
- Kowalski, M.L.; Wolska, A.; Grzegorczyk, J.; Hilt, J.; Jarzebska, M.; Drobniewski, M.; Synder, M.; Kurowski, M. Increased responsiveness to toll-like receptor 4 stimulation in peripheral blood mononuclear cells from patients with recent onset rheumatoid arthritis. Mediat. Inflamm. 2008, 2008, 132732. [Google Scholar] [CrossRef]
- Sacre, S.M.; Lo, A.; Gregory, B.; Simmonds, R.E.; Williams, L.; Feldmann, M.; Brennan, F.M.; Foxwell, B.M. Inhibitors of TLR8 reduce TNF production from human rheumatoid synovial membrane cultures. J. Immunol. 2008, 181, 8002–8009. [Google Scholar] [CrossRef]
- Doorenspleet, M.E.; Klarenbeek, P.L.; de Hair, M.J.; van Schaik, B.D.; Esveldt, R.E.; van Kampen, A.H.; Gerlag, D.M.; Musters, A.; Baas, F.; Tak, P.P.; et al. Rheumatoid arthritis synovial tissue harbours dominant B-cell and plasma-cell clones associated with autoreactivity. Ann. Rheum. Dis. 2014, 73, 756–762. [Google Scholar] [CrossRef]
- Lubberts, E. The IL-23-IL-17 axis in inflammatory arthritis. Nat. Rev. Rheumatol. 2015, 11, 415–429. [Google Scholar] [CrossRef] [PubMed]
- Gaffen, S.L.; Jain, R.; Garg, A.V.; Cua, D.J. The IL-23-IL-17 immune axis: From mechanisms to therapeutic testing. Nat. Rev. Immunol. 2014, 14, 585–600. [Google Scholar] [CrossRef]
- Paulissen, S.M.; van Hamburg, J.P.; Dankers, W.; Lubberts, E. The role and modulation of CCR6+ Th17 cell populations in rheumatoid arthritis. Cytokine 2015, 74, 43–53. [Google Scholar] [CrossRef]
- Andersson, A.K.; Li, C.; Brennan, F.M. Recent developments in the immunobiology of rheumatoid arthritis. Arthritis Res. Ther. 2008, 10, 204. [Google Scholar] [CrossRef] [PubMed]
- Anaya, J.M.; Shoenfeld, Y.; Rojas-Villarraga, A.; Levy, R.A.; Cervera, R. (Eds.) Autoimmunity: From Bench to Bedside; El Rosario University Press© 2020 Universidad del Rosario: Bogota, Colombia, 2013. [Google Scholar]
- Abbas, A.K.L.A.; Pillai, S. Cellular and Molecularimmunology, 7th ed.; Elsevier: Philadelphia, PA, USA, 2012. [Google Scholar]
- Demoruelle, M.K.; Deane, K.D.; Holers, V.M. When and where does inflammation begin in rheumatoid arthritis? Curr. Opin. Rheumatol. 2014, 26, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Deane, K.D.; Demoruelle, M.K.; Kelmenson, L.B.; Kuhn, K.A.; Norris, J.M.; Holers, V.M. Genetic and environmental risk factors for rheumatoid arthritis. Best Pract. Res. Clin. Rheumatol. 2017, 31, 3–18. [Google Scholar] [CrossRef] [PubMed]
- Klareskog, L.; Stolt, P.; Lundberg, K.; Källberg, H.; Bengtsson, C.; Grunewald, J.; Rönnelid, J.; Harris, H.E.; Ulfgren, A.K.; Rantapää-Dahlqvist, S.; et al. A new model for an etiology of rheumatoid arthritis: Smoking may trigger HLA-DR (shared epitope)-restricted immune reactions to autoantigens modified by citrullination. Arthritis Rheum. 2006, 54, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Rosenstein, E.D.; Greenwald, R.A.; Kushner, L.J.; Weissmann, G. Hypothesis: The humoral immune response to oral bacteria provides a stimulus for the development of rheumatoid arthritis. Inflammation 2004, 28, 311–318. [Google Scholar] [CrossRef]
- Malmström, V.; Catrina, A.I.; Klareskog, L. The immunopathogenesis of seropositive rheumatoid arthritis: From triggering to targeting. Nat. Rev. Immunol. 2017, 17, 60–75. [Google Scholar] [CrossRef]
- Gizinski, A.M.; Mascolo, M.; Loucks, J.L.; Kervitsky, A.; Meehan, R.T.; Brown, K.K.; Holers, V.M.; Deane, K.D. Rheumatoid arthritis (RA)-specific autoantibodies in patients with interstitial lung disease and absence of clinically apparent articular RA. Clin. Rheumatol. 2009, 28, 611–613. [Google Scholar] [CrossRef]
- Oldstone, M.B. Molecular mimicry and autoimmune disease. Cell 1987, 50, 819–820. [Google Scholar] [CrossRef]
- Venigalla, S.S.K.; Premakumar, S.; Janakiraman, V. A possible role for autoimmunity through molecular mimicry in alphavirus mediated arthritis. Sci. Rep. 2020, 10, 938. [Google Scholar] [CrossRef]
- Root-Bernstein, R.; Fairweather, D. Complexities in the relationship between infection and autoimmunity. Curr. Allergy Asthma Rep. 2014, 14, 407. [Google Scholar] [CrossRef]
- Thaper, D.; Prabha, V. Molecular mimicry: An explanation for autoimmune diseases and infertility. Scand. J. Immunol. 2018, 88, e12697. [Google Scholar] [CrossRef] [PubMed]
- Arleevskaya, M.I.; Kravtsova, O.A.; Lemerle, J.; Renaudineau, Y.; Tsibulkin, A.P. How rheumatoid arthritis can result from provocation of the immune system by microorganisms and viruses. Front. Microbiol. 2016, 7. [Google Scholar] [CrossRef]
- Ercolini, A.M.; Miller, S.D. The role of infections in autoimmune disease. Clin. Exp. Immunol. 2009, 155, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Fujinami, R.S.; von Herrath, M.G.; Christen, U.; Whitton, J.L. Molecular mimicry, bystander activation, or viral persistence: Infections and autoimmune disease. Clin. Microbiol. Rev. 2006, 19, 80–94. [Google Scholar] [CrossRef] [PubMed]
- Bo, M.; Niegowska, M.; Erre, G.L.; Piras, M.; Longu, M.G.; Manchia, P.; Manca, M.; Passiu, G.; Sechi, L.A. Rheumatoid arthritis patient antibodies highly recognize IL-2 in the immune response pathway involving IRF5 and EBV antigens. Sci. Rep. 2018, 8, 1789. [Google Scholar] [CrossRef]
- Bo, M.; Niegowska, M.; Eames, H.L.; Almuttaqi, H.; Arru, G.; Erre, G.L.; Passiu, G.; Khoyratty, T.E.; van Grinsven, E.; Udalova, I.A.; et al. Antibody response to homologous epitopes of Epstein-Barr virus, Mycobacterium avium subsp. paratuberculosis and IRF5 in patients with different connective tissue diseases and in mouse model of antigen-induced arthritis. J. Transl. Autoimmun. 2020, 3, 100048. [Google Scholar] [CrossRef]
- Gagneux, S. Ecology and evolution of Mycobacterium tuberculosis. Nat. Rev. Microbiol. 2018, 16, 202–213. [Google Scholar] [CrossRef]
- Cronan, M.R.; Beerman, R.W.; Rosenberg, A.F.; Saelens, J.W.; Johnson, M.G.; Oehlers, S.H.; Sisk, D.M.; Jurcic Smith, K.L.; Medvitz, N.A.; Miller, S.E.; et al. Macrophage epithelial reprogramming underlies mycobacterial granuloma formation and promotes infection. Immunity 2016, 45, 861–876. [Google Scholar] [CrossRef]
- Birnbaum, G.; Kotilinek, L.; Albrecht, L. Spinal fluid lymphocytes from a subgroup of multiple sclerosis patients respond to mycobacterial antigens. Ann. Neurol. 1993, 34, 18–24. [Google Scholar] [CrossRef]
- Mor, F.; Cohen, I.R. T cells in the lesion of experimental autoimmune encephalomyelitis. Enrichment for reactivities to myelin basic protein and to heat shock proteins. J. Clin. Investig. 1992, 90, 2447–2455. [Google Scholar] [CrossRef]
- Res, P.C.; Schaar, C.G.; Breedveld, F.C.; van Eden, W.; van Embden, J.D.; Cohen, I.R.; de Vries, R.R. Synovial fluid T cell reactivity against 65 kD heat shock protein of mycobacteria in early chronic arthritis. Lancet 1988, 2, 478–480. [Google Scholar] [CrossRef]
- Salvetti, M.; Ristori, G.; Buttinelli, C.; Fiori, P.; Falcone, M.; Britton, W.; Adams, E.; Paone, G.; Grasso, M.G.; Pozzilli, C. The immune response to mycobacterial 70-kDa heat shock proteins frequently involves autoreactive T cells and is quantitatively disregulated in multiple sclerosis. J. NeuroImmunol. 1996, 65, 143–153. [Google Scholar] [CrossRef]
- Poncet, A. De la polyarthrite tuberculeuse deformante oupseudorheumatisme chronique tuberculeux. Congr. Fr. Chir. 1897, 1, 732–739. [Google Scholar]
- Torisu, M.; Miyahara, T.; Shinohara, N.; Ohsato, K.; Sonozaki, H. A new side effect of BCG immunotherapy —BCG-induced arthritis in man. Cancer Immunol. Immunother. 1978, 5, 77–83. [Google Scholar] [CrossRef]
- Kempsell, K.E.; Cox, C.J.; McColm, A.A.; Bagshaw, J.A.; Reece, R.; Veale, D.J.; Emery, P.; Isaacs, J.D.; Gaston, J.S.; Crowe, J.S. Detection of Mycobacterium tuberculosis group organisms in human and mouse joInt. tissue by reverse transcriptase PCR: Prevalence in diseased synovial tissue suggests lack of specific association with rheumatoid arthritis. Infect. Immun. 2001, 69, 1821–1831. [Google Scholar] [CrossRef] [PubMed]
- van der Heijden, I.M.; Wilbrink, B.; Schouls, L.M.; van Embden, J.D.; Breedveld, F.C.; Tak, P.P. Detection of mycobacteria in joInt. samples from patients with arthritis using a genus-specific polymerase chain reaction and sequence analysis. Rheumatology 1999, 38, 547–553. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wu, C.H.; Jeng, K.C.; Lan, J.L. Mycobacterium tuberculosis antigen, interleukin 2 and interleukin 2 inhibitor in patients with rheumatoid arthritis. Immunol. Invest. 1995, 24, 957–964. [Google Scholar] [CrossRef]
- Erre, G.L.; Cossu, D.; Masala, S.; Mameli, G.; Cadoni, M.L.; Serdino, S.; Longu, M.G.; Passiu, G.; Sechi, L.A. Mycobacterium tuberculosis lipoarabinomannan antibodies are associated to rheumatoid arthritis in Sardinian patients. Clin. Rheumatol. 2014, 33, 1725–1729. [Google Scholar] [CrossRef]
- Bahr, G.M.; Rook, G.A.; al-Saffar, M.; Van Embden, J.; Stanford, J.L.; Behbehani, K. Antibody levels to mycobacteria in relation to HLA type: Evidence for non-HLA-linked high levels of antibody to the 65 kD heat shock protein of M. bovis in rheumatoid arthritis. Clin. Exp. Immunol. 1988, 74, 211–215. [Google Scholar]
- Tsoulfa, G.; Rook, G.A.; Bahr, G.M.; Sattar, M.A.; Behbehani, K.; Young, D.B.; Mehlert, A.; Van-Embden, J.D.; Hay, F.C.; Isenberg, D.A.; et al. Elevated IgG antibody levels to the mycobacterial 65-kDa heat shock protein are characteristic of patients with rheumatoid arthritis. Scand. J. Immunol. 1989, 30, 519–527. [Google Scholar] [CrossRef] [PubMed]
- Bo, M.; Erre, G.L.; Bach, H.; Slavin, Y.N.; Manchia, P.A.; Passiu, G.; Sechi, L.A. PtpA and PknG proteins secreted by Mycobacterium avium subsp. paratuberculosis are recognized by sera from patients with rheumatoid arthritis: A case-control study. J. Inflamm. Res. 2019, 12, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Gaston, J.S.; Life, P.F.; Bailey, L.C.; Bacon, P.A. In vitro responses to a 65-kilodalton mycobacterial protein by synovial T cells from inflammatory arthritis patients. J. Immunol. 1989, 143, 2494–2500. [Google Scholar] [PubMed]
- Holoshitz, J.; Koning, F.; Coligan, J.E.; De Bruyn, J.; Strober, S. Isolation of CD4- CD8- mycobacteria-reactive T lymphocyte clones from rheumatoid arthritis synovial fluid. Nature 1989, 339, 226–229. [Google Scholar] [CrossRef]
- Kanagawa, H.; Niki, Y.; Kobayashi, T.; Sato, Y.; Katsuyama, E.; Fujie, A.; Hao, W.; Miyamoto, K.; Tando, T.; Watanabe, R.; et al. Mycobacterium tuberculosis promotes arthritis development through Toll-like receptor 2. J. Bone Min. Metab. 2015, 33, 135–141. [Google Scholar] [CrossRef]
- Brand, D.D.; Latham, K.A.; Rosloniec, E.F. Collagen-induced arthritis. Nat. Protoc. 2007, 2, 1269–1275. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; He, W.; Du, X.; Yang, J.; Wen, Q.; Zhong, X.P.; Ma, L. IL-17 Production of neutrophils enhances antibacteria ability but promotes arthritis development during mycobacterium tuberculosis infection. EBioMedicine 2017, 23, 88–99. [Google Scholar] [CrossRef]
- Billiau, A.; Matthys, P. Modes of action of Freund’s adjuvants in experimental models of autoimmune diseases. J. Leukoc. Biol. 2001, 70, 849–860. [Google Scholar]
- Celis, L.; Vandevyver, C.; Geusens, P.; Dequeker, J.; Raus, J.; Zhang, J. Clonal expansion of mycobacterial heat-shock protein-reactive T lymphocytes in the synovial fluid and blood of rheumatoid arthritis patients. Arthritis Rheum. 1997, 40, 510–519. [Google Scholar] [CrossRef]
- Kogure, A.; Miyata, M.; Nishimaki, T.; Kasukawa, R. Proliferative response of synovial fluid mononuclear cells of patients with rheumatoid arthritis to mycobacterial 65 kDa heat shock protein and its association with HLA-DR+.gamma delta+ T cells. J. Rheumatol. 1994, 21, 1403–1408. [Google Scholar]
- Wucherpfennig, K.W.; Strominger, J.L. Molecular mimicry in T cell-mediated autoimmunity: Viral peptides activate human T cell clones specific for myelin basic protein. Cell 1995, 80, 695–705. [Google Scholar] [CrossRef]
- Tsuchiya, N.; Williams, R.C., Jr. Molecular mimicry--hypothesis or reality? West. J. Med. 1992, 157, 133–138. [Google Scholar] [PubMed]
- Esaguy, N.; Aguas, A.P.; van Embden, J.D.; Silva, M.T. Mycobacteria and human autoimmune disease: Direct evidence of cross-reactivity between human lactoferrin and the 65-kilodalton protein of tubercle and leprosy bacilli. Infect. Immun. 1991, 59, 1117–1125. [Google Scholar] [CrossRef] [PubMed]
- Bizzaro, N.; Mazzanti, G.; Tonutti, E.; Villalta, D.; Tozzoli, R. Diagnostic accuracy of the anti-citrulline antibody assay for rheumatoid arthritis. Clin. Chem. 2001, 47, 1089–1093. [Google Scholar] [CrossRef] [PubMed]
- Lim, M.K.; Shim, T.S.; Sheen, D.H.; Na, D.J.; Min, S.S.; Shim, S.C. Anti-cyclic citrulline peptide antibody in non-tuberculous mycobacteria sera: A negative association. Clin. Rheumatol. 2010, 29, 335–336. [Google Scholar] [CrossRef]
- Elkayam, O.; Segal, R.; Bendayan, D.; van Uitert, R.; Onnekink, C.; Pruijn, G.J. The anti-cyclic citrullinated peptide response in tuberculosis patients is not citrulline-dependent and sensitive to treatment. Arthritis Res. Ther. 2010, 12, R12. [Google Scholar] [CrossRef]
- Silva, A.F.D.; Matos, A.N.; Lima, Á.M.S.; Lima, E.F.; Gaspar, A.P.; Braga, J.A.F.; Carvalho, E.M. Valor diagnóstico do anticorpo antipeptídeo citrulinado cíclico na artrite reumatóide. Revista Brasileira de Reumatologia 2006, 46, 174–180. [Google Scholar] [CrossRef]
- Aguas, A.; Esaguy, N.; Sunkel, C.E.; Silva, M.T. Cross-reactivity and sequence homology between the 65-kilodalton mycobacterial heat shock protein and human lactoferrin, transferrin, and DR beta subsets of major histocompatibility complex class II molecules. Infect. Immun. 1990, 58, 1461–1470. [Google Scholar] [CrossRef]
- van Eden, W.; Holoshitz, J.; Nevo, Z.; Frenkel, A.; Klajman, A.; Cohen, I.R. Arthritis induced by a T-lymphocyte clone that responds to Mycobacterium tuberculosis and to cartilage proteoglycans. Proc. Natl. Acad. Sci. USA 1985, 82, 5117–5120. [Google Scholar] [CrossRef]
- van Eden, W.; Hogervorst, E.J.; van der Zee, R.; van Embden, J.D.; Hensen, E.J.; Cohen, I.R. The mycobacterial 65 kD heat-shock protein and autoimmune arthritis. Rheumatol. Int. 1989, 9, 187–191. [Google Scholar] [CrossRef]
- Dow, C.T.M. paratuberculosis Heat Shock Protein 65 and Human Diseases: Bridging Infection and Autoimmunity. Autoimmune Dis. 2012, 2012, 150824. [Google Scholar] [CrossRef]
- Valdez, M.M.; Clark, J.I.; Wu, G.J.; Muchowski, P.J. Functional similarities between the small heat shock proteins Mycobacterium tuberculosis HSP 16.3 and human alphaB-crystallin. Eur. J. BioChem. 2002, 269, 1806–1813. [Google Scholar] [CrossRef] [PubMed]
- Dubaniewicz, A.; Trzonkowski, P.; Dubaniewicz-Wybieralska, M.; Dubaniewicz, A.; Singh, M.; Myśliwski, A. Comparative analysis of mycobacterial heat shock proteins-induced apoptosis of peripheral blood mononuclear cells in sarcoidosis and tuberculosis. J. Clin. Immunol. 2006, 26, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Hill, H.M.; Kirshbaum, J.D. Military tuberculosis developing during prolonged cortisone therapy of systemic lupus erythematosus. Ann. Intern. Med. 1956, 44, 781–790. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, F.M.; Szyper-Kravitz, M.; Klumb, E.M.; Lannes, G.; Ribeiro, F.R.; Albuquerque, E.M.; Shoenfeld, Y. Can lupus flaRes. be associated with tuberculosis infection? Clin. Rev. Allergy Immunol. 2010, 38, 163–168. [Google Scholar] [CrossRef]
- van Eden, W.; Thole, J.E.; van der Zee, R.; Noordzij, A.; van Embden, J.D.; Hensen, E.J.; Cohen, I.R. Cloning of the mycobacterial epitope recognized by T lymphocytes in adjuvant arthritis. Nature 1988, 331, 171–173. [Google Scholar] [CrossRef]
- Zügel, U.; Kaufmann, S.H. Role of heat shock proteins in protection from and pathogenesis of infectious diseases. Clin. Microbiol. Rev. 1999, 12, 19–39. [Google Scholar] [CrossRef]
- Dubaniewicz, A.; Dubaniewicz-Wybieralska, M.; Sternau, A.; Zwolska, Z.; Izycka-Swieszewska, E.; Augustynowicz-Kopec, E.; Skokowski, J.; Singh, M.; Zimnoch, L. Mycobacterium tuberculosis complex and mycobacterial heat shock proteins in lymph node tissue from patients with pulmonary sarcoidosis. J. Clin. Microbiol. 2006, 44, 3448–3451. [Google Scholar] [CrossRef]
- van Eden, W.; van der Zee, R.; Prakken, B. Heat-shock proteins induce T-cell regulation of chronic inflammation. Nat. Rev. Immunol. 2005, 5, 318–330. [Google Scholar] [CrossRef]
- Shoda, H.; Hanata, N.; Sumitomo, S.; Okamura, T.; Fujio, K.; Yamamoto, K. Immune responses to Mycobacterial heat shock protein 70 accompany self-reactivity to human BiP in rheumatoid arthritis. Sci. Rep. 2016, 6, 22486. [Google Scholar] [CrossRef]
- Chodisetti, S.B.; Rai, P.K.; Gowthaman, U.; Pahari, S.; Agrewala, J.N. Potential T cell epitopes of Mycobacterium tuberculosis that can instigate molecular mimicry against host: Implications in autoimmune pathogenesis. BMC Immunol. 2012, 13, 13. [Google Scholar] [CrossRef]
- Gutlapalli, V.R.; Sykam, A.; Nayarisseri, A.; Suneetha, S.; Suneetha, L.M. Insights from the predicted epitope similarity between Mycobacterium tuberculosis virulent factors and its human homologs. Bioinformation 2015, 11, 517–524. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, A.S.; Al-Attiyah, R.; Hanif, S.N.; Shaban, F.A. Efficient testing of large pools of Mycobacterium tuberculosis RD1 peptides and identification of major antigens and immunodominant peptides recognized by human Th1 cells. Clin. Vaccine Immunol. 2008, 15, 916–924. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mustafa, A.S. In silico binding predictions for identification of HLA-DR-promiscuous regions and epitopes of Mycobacterium tuberculosis protein MPT64 (Rv1980c) and their recognition by human Th1 cells. Med. Princ. Pract. 2010, 19, 367–372. [Google Scholar] [CrossRef] [PubMed]
- Gowthaman, U.; Agrewala, J.N. In silico methods for predicting T-cell epitopes: Dr Jekyll or Mr Hyde? Expert Rev. Proteom. 2009, 6, 527–537. [Google Scholar] [CrossRef] [PubMed]
- Ates, O.; Dalyan, L.; Müsellim, B.; Hatemi, G.; Türker, H.; Ongen, G.; Hamuryudan, V.; Topal-Sarikaya, A. NRAMP1 (SLC11A1) gene polymorphisms that correlate with autoimmune versus infectious disease susceptibility in tuberculosis and rheumatoid arthritis. Int. J. Immunogenet. 2009, 36, 15–19. [Google Scholar] [CrossRef]
- Sechi, L.A.; Gazouli, M.; Sieswerda, L.E.; Molicotti, P.; Ahmed, N.; Ikonomopoulos, J.; Scanu, A.M.; Paccagnini, D.; Zanetti, S. Relationship between Crohn’s disease, infection with Mycobacterium avium subspecies paratuberculosis and SLC11A1 gene polymorphisms in Sardinian patients. World J. Gastroenterol. 2006, 12, 7161–7164. [Google Scholar] [CrossRef]
- Paccagnini, D.; Sieswerda, L.; Rosu, V.; Masala, S.; Pacifico, A.; Gazouli, M.; Ikonomopoulos, J.; Ahmed, N.; Zanetti, S.; Sechi, L.A. Linking chronic infection and autoimmune diseases: Mycobacterium avium subspecies paratuberculosis, SLC11A1 polymorphisms and type-1 diabetes mellitus. PLoS ONE 2009, 4, e7109. [Google Scholar] [CrossRef]
- Sharp, R.C.; Beg, S.A.; Naser, S.A. Polymorphisms in protein tyrosine phosphatase non-receptor type 2 and 22 (PTPN2/22) are linked to Hyper-Proliferative T-Cells and susceptibility to mycobacteria in rheumatoid arthritis. Front. Cell. Infect. Microbiol. 2018, 8, 11. [Google Scholar] [CrossRef]
- Wyllie, S.; Seu, P.; Goss, J.A. The natural resistance-associated macrophage protein 1 Slc11a1 (formerly Nramp1) and iron metabolism in macrophages. Microbes Infect. 2002, 4, 351–359. [Google Scholar] [CrossRef]
- Hackam, D.J.; Rotstein, O.D.; Zhang, W.; Gruenheid, S.; Gros, P.; Grinstein, S. Host resistance to intracellular infection: Mutation of natural resistance-associated macrophage protein 1 (Nramp1) impairs phagosomal acidification. J. Exp. Med. 1998, 188, 351–364. [Google Scholar] [CrossRef]
- Yang, Y.S.; Kim, S.J.; Kim, J.W.; Koh, E.M. NRAMP1 gene polymorphisms in patients with rheumatoid arthritis in Koreans. J. Korean Med. Sci. 2000, 15, 83–87. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kotze, M.J.; de Villiers, J.N.; Rooney, R.N.; Grobbelaar, J.J.; Mansvelt, E.P.; Bouwens, C.S.; Carr, J.; Stander, I.; du Plessis, L. Analysis of the NRAMP1 gene implicated in iron transport: Association with multiple sclerosis and age effects. Blood Cells Mol. Dis. 2001, 27, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Gazouli, M.; Sechi, L.; Paccagnini, D.; Sotgiu, S.; Arru, G.; Nasioulas, G.; Vassilopoulos, D. NRAMP1 polymorphism and viral factors in Sardinian multiple sclerosis patients. Can. J. Neurol. Sci. 2008, 35, 491–494. [Google Scholar] [CrossRef]
- Kotlowski, R.; Bernstein, C.N.; Silverberg, M.S.; Krause, D.O. Population-based case-control study of alpha 1-antitrypsin and SLC11A1 in Crohn’s disease and ulcerative colitis. Inflamm. Bowel Dis. 2008, 14, 1112–1117. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Satoh, J.; Kojima, Y.; Negoro, K.; Hirai, M.; Hinokio, Y.; Kinouchi, Y.; Suzuki, S.; Matsuura, N.; Shimosegawa, T.; et al. Promoter polymorphism of SLC11A1 (formerly NRAMP1) confers susceptibility to autoimmune type 1 diabetes mellitus in Japanese. Tissue Antigens 2004, 63, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Qasem, A.; Ramesh, S.; Naser, S.A. Genetic polymorphisms in tumour necrosis factor receptors (TNFRSF1A/1B) illustrate differential treatment response to TNFα inhibitors in patients with Crohn’s disease. BMJ Open Gastroenterol. 2019, 6, e000246. [Google Scholar] [CrossRef]
- Matsui, T.; Ohsumi, K.; Ozawa, N.; Shimada, K.; Sumitomo, S.; Shimane, K.; Kawakami, M.; Nakayama, H.; Sugii, S.; Ozawa, Y.; et al. CD64 on neutrophils is a sensitive and specific marker for detection of infection in patients with rheumatoid arthritis. J. Rheumatol. 2006, 33, 2416–2424. [Google Scholar]
- Caporali, R.; Caprioli, M.; Bobbio-Pallavicini, F.; Montecucco, C. DMARDS and infections in rheumatoid arthritis. AutoImmun. Rev. 2008, 8, 139–143. [Google Scholar] [CrossRef]
- Solovic, I.; Sester, M.; Gomez-Reino, J.J.; Rieder, H.L.; Ehlers, S.; Milburn, H.J.; Kampmann, B.; Hellmich, B.; Groves, R.; Schreiber, S.; et al. The risk of tuberculosis related to tumour necrosis factor antagonist therapies: A TBNET consensus statement. Eur. Respir. J. 2010, 36, 1185–1206. [Google Scholar] [CrossRef] [PubMed]
- Askling, J.; Fored, C.M.; Brandt, L.; Baecklund, E.; Bertilsson, L.; Cöster, L.; Geborek, P.; Jacobsson, L.T.; Lindblad, S.; Lysholm, J.; et al. Risk and case characteristics of tuberculosis in rheumatoid arthritis associated with tumor necrosis factor antagonists in Sweden. Arthritis Rheum. 2005, 52, 1986–1992. [Google Scholar] [CrossRef]
- Ingraham, N.E.; Schneider, B.; Alpern, J.D. Prosthetic JoInt. Infection due to Mycobacterium avium-intracellulare in a Patient with Rheumatoid Arthritis: A Case Report and Review of the Literature. Case Rep. Infect. Dis. 2017, 2017, 8682354. [Google Scholar] [CrossRef] [PubMed]
- Iwata, K.; Oka, S.; Tsuno, H.; Furukawa, H.; Shimada, K.; Hashimoto, A.; Komiya, A.; Tsuchiya, N.; Katayama, M.; Tohma, S. Biomarker for nontuberculous mycobacterial pulmonary disease in patients with rheumatoid arthritis: Anti-glycopeptidolipid core antigen immunoglobulin A antibodies. Mod. Rheumatol. 2018, 28, 271–275. [Google Scholar] [CrossRef] [PubMed]
- Schubert, N.; Schill, T.; Plüß, M.; Korsten, P. Flare or foe?—Mycobacterium marinum infection mimicking rheumatoid arthritis tenosynovitis: Case report and literature review. BMC Rheumatol. 2020, 4, 11. [Google Scholar] [CrossRef]
- Chen, H.W.; Lai, C.C.; Tan, C.K. Arthritis caused by Mycobacterium terrae in a patient with rheumatoid arthritis. Int. J. Infect Dis. 2009, 13, e145–e147. [Google Scholar] [CrossRef] [PubMed]
- Lam, A.; Toma, W.; Schlesinger, N. Mycobacterium marinum arthritis mimicking rheumatoid arthritis. J. Rheumatol. 2006, 33, 817–819. [Google Scholar]
- DeMerieux, P.; Keystone, E.C.; Hutcheon, M.; Laskin, C. Polyarthritis due to Mycobacterium kansasii in a patient with rheumatoid arthritis. Ann. Rheum. Dis. 1980, 39, 90–94. [Google Scholar] [CrossRef]
- Dos Santos Sobrín, R.; Pérez Gómez, N.; Vilas, A.S.; Suárez, M.P.; Pampín, E.P.; Antúnez López, J.R.; Mera Varela, A. Infection by Mycobacterium chelonae at the site of administration of sarilumab for rheumatoid arthritis. Rheumatology 2020, 59, 265. [Google Scholar] [CrossRef]
- Dutertre, M.; Delobel, P.; Marchou, B.; Boyer, J.F.; Mougari, F.; Martin-Blondel, G. Olecranon bursitis secondary to Mycobacterium europaeum infection in a patient receiving immunosuppressive drugs for rheumatoid arthritis. Med. Et Mal. Infect. 2019, 49, 358–359. [Google Scholar] [CrossRef]
- Benedek, T.G. The history of bacteriologic concepts of rheumatic fever and rheumatoid arthritis. Semin Arthritis Rheum. 2006, 36, 109–123. [Google Scholar] [CrossRef]
- Martinez-Martinez, R.E.; Abud-Mendoza, C.; Patiño-Marin, N.; Rizo-Rodríguez, J.C.; Little, J.W.; Loyola-Rodríguez, J.P. Detection of periodontal bacterial DNA in serum and synovial fluid in refractory rheumatoid arthritis patients. J. Clin. Periodontol. 2009, 36, 1004–1010. [Google Scholar] [CrossRef]
- Totaro, M.C.; Cattani, P.; Ria, F.; Tolusso, B.; Gremese, E.; Fedele, A.L.; D’Onghia, S.; Marchetti, S.; Sante, G.D.; Canestri, S.; et al. Porphyromonas gingivalis and the pathogenesis of rheumatoid arthritis: Analysis of various compartments including the synovial tissue. Arthritis Res. Ther. 2013, 15, R66. [Google Scholar] [CrossRef] [PubMed]
- Kawahito, Y.; Ichinose, S.; Sano, H.; Tsubouchi, Y.; Kohno, M.; Yoshikawa, T.; Tokunaga, D.; Hojo, T.; Harasawa, R.; Nakano, T.; et al. Mycoplasma fermentans glycolipid-antigen as a pathogen of rheumatoid arthritis. BioChem. Biophys. Res. Commun. 2008, 369, 561–566. [Google Scholar] [CrossRef] [PubMed]
- Schaeverbeke, T.; Renaudin, H.; Clerc, M.; Lequen, L.; Vernhes, J.P.; De Barbeyrac, B.; Bannwarth, B.; Bébéar, C.; Dehais, J. Systematic detection of mycoplasmas by culture and polymerase chain reaction (PCR) proceduRes. in 209 synovial fluid samples. Rheumatology 1997, 36, 310–314. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hoffman, R.W.; O’Sullivan, F.X.; Schafermeyer, K.R.; Moore, T.L.; Roussell, D.; Watson-McKown, R.; Kim, M.F.; Wise, K.S. Mycoplasma infection and rheumatoid arthritis: Analysis of their relationship using immunoblotting and an ultrasensitive polymerase chain reaction detection method. Arthritis Rheum. 1997, 40, 1219–1228. [Google Scholar] [CrossRef]
- Wilkinson, N.Z.; Kingsley, G.H.; Jones, H.W.; Sieper, J.; Braun, J.; Ward, M.E. The detection of DNA from a range of bacterial species in the joints of patients with a variety of arthritides using a nested, broad-range polymerase chain reaction. Rheumatology 1999, 38, 260–266. [Google Scholar] [CrossRef]
- van der Heijden, I.M.; Wilbrink, B.; Tchetverikov, I.; Schrijver, I.A.; Schouls, L.M.; Hazenberg, M.P.; Breedveld, F.C.; Tak, P.P. Presence of bacterial DNA and bacterial peptidoglycans in joints of patients with rheumatoid arthritis and other arthritides. Arthritis Rheum. 2000, 43, 593–598. [Google Scholar] [CrossRef]
- Saal, J.G.; Steidle, M.; Einsele, H.; Müller, C.A.; Fritz, P.; Zacher, J. Persistence of B19 parvovirus in synovial membranes of patients with rheumatoid arthritis. Rheumatol. Int. 1992, 12, 147–151. [Google Scholar] [CrossRef]
- Jobanputra, P.; Davidson, F.; Graham, S.; O’Neill, H.; Simmonds, P.; Yap, P.L. High frequency of parvovirus B19 in patients tested for rheumatoid factor. BMJ 1995, 311, 1542. [Google Scholar] [CrossRef]
- Takeda, T.; Mizugaki, Y.; Matsubara, L.; Imai, S.; Koike, T.; Takada, K. Lytic Epstein-Barr virus infection in the synovial tissue of patients with rheumatoid arthritis. Arthritis Rheum. 2000, 43, 1218–1225. [Google Scholar] [CrossRef]
- Mehraein, Y.; Lennerz, C.; Ehlhardt, S.; Remberger, K.; Ojak, A.; Zang, K.D. Latent Epstein-Barr virus (EBV) infection and cytomegalovirus (CMV) infection in synovial tissue of autoimmune chronic arthritis determined by RNA- and DNA-in situ hybridization. Mod. Pathol. 2004, 17, 781–789. [Google Scholar] [CrossRef]
- Sorgato, C.C.; Lins, E.S.M.; Leão, J.C.; Vasconcelos, L.R.; Melo, T.R.; Duarte, A.L.; Gueiros, L.A. EBV and CMV Viral Load in Rheumatoid Arthritis and Their Role Associated Sjögren’s Syndrome. J. Oral. Pathol. Med. 2020. [Google Scholar] [CrossRef]
- Kuusela, E.; Kouri, V.P.; Olkkonen, J.; Koivuniemi, R.; Äyräväinen, L.; Rajamäki, K.; Valleala, H.; Nordström, D.; Leirisalo-Repo, M.; Ainola, M.; et al. Serum Epstein-Barr virus DNA, detected by droplet digital PCR, correlates with disease activity in patients with rheumatoid arthritis. Clin. Exp. Rheumatol. 2018, 36, 778–784. [Google Scholar]
- Erre, G.L.; Mameli, G.; Cossu, D.; Muzzeddu, B.; Piras, C.; Paccagnini, D.; Passiu, G.; Sechi, L.A. Increased Epstein-Barr Virus DNA Load and Antibodies Against EBNA1 and EA in Sardinian Patients with Rheumatoid Arthritis. Viral. Immunol. 2015, 28, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Mikuls, T.R.; Payne, J.B.; Reinhardt, R.A.; Thiele, G.M.; Maziarz, E.; Cannella, A.C.; Holers, V.M.; Kuhn, K.A.; O’Dell, J.R. Antibody responses to Porphyromonas gingivalis (P. gingivalis) in subjects with rheumatoid arthritis and periodontitis. Int. Immunopharmacol. 2009, 9, 38–42. [Google Scholar] [CrossRef] [PubMed]
- Ogrendik, M.; Kokino, S.; Ozdemir, F.; Bird, P.S.; Hamlet, S. Serum antibodies to oral anaerobic bacteria in patients with rheumatoid arthritis. MedGenMed 2005, 7, 2. [Google Scholar] [PubMed]
- Quirke, A.M.; Lugli, E.B.; Wegner, N.; Hamilton, B.C.; Charles, P.; Chowdhury, M.; Ytterberg, A.J.; Zubarev, R.A.; Potempa, J.; Culshaw, S.; et al. Heightened immune response to autocitrullinated Porphyromonas gingivalis peptidylarginine deiminase: A potential mechanism for breaching immunologic tolerance in rheumatoid arthritis. Ann. Rheum. Dis. 2014, 73, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Klatt, T.; Ouyang, Q.; Flad, T.; Koetter, I.; Bühring, H.J.; Kalbacher, H.; Pawelec, G.; Müller, C.A. Expansion of peripheral CD8+ CD28- T cells in response to Epstein-Barr virus in patients with rheumatoid arthritis. J. Rheumatol. 2005, 32, 239–251. [Google Scholar] [PubMed]
- Rickinson, A.B.; Moss, D.J. Human cytotoxic T lymphocyte responses to Epstein-Barr virus infection. Annu. Rev. Immunol. 1997, 15, 405–431. [Google Scholar] [CrossRef]
- Scotet, E.; David-Ameline, J.; Peyrat, M.A.; Moreau-Aubry, A.; Pinczon, D.; Lim, A.; Even, J.; Semana, G.; Berthelot, J.M.; Breathnach, R.; et al. T cell response to Epstein-Barr virus transactivators in chronic rheumatoid arthritis. J. Exp. Med. 1996, 184, 1791–1800. [Google Scholar] [CrossRef]
- Lünemann, J.D.; Frey, O.; Eidner, T.; Baier, M.; Roberts, S.; Sashihara, J.; Volkmer, R.; Cohen, J.I.; Hein, G.; Kamradt, T.; et al. Increased frequency of EBV-specific effector memory CD8+ T cells correlates with higher viral load in rheumatoid arthritis. J. Immunol. 2008, 181, 991–1000. [Google Scholar] [CrossRef]
- Trier, N.H.; Holm, B.E.; Heiden, J.; Slot, O.; Locht, H.; Lindegaard, H.; Svendsen, A.; Nielsen, C.T.; Jacobsen, S.; Theander, E.; et al. Antibodies to a strain-specific citrullinated Epstein-Barr virus peptide diagnoses rheumatoid arthritis. Sci. Rep. 2018, 8, 3684. [Google Scholar] [CrossRef]
- Sternbæk, L.; Draborg, A.H.; Østerlund, M.T.; Iversen, L.V.; Troelsen, L.; Theander, E.; Nielsen, C.T.; Jacobsen, S.; Houen, G. Increased antibody levels to stage-specific Epstein-Barr virus antigens in systemic autoimmune diseases reveal a common pathology. Scand. J. Clin. Lab. Investig. 2019, 79, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Motokawa, S.; Hasunuma, T.; Tajima, K.; Krieg, A.M.; Ito, S.; Iwasaki, K.; Nishioka, K. High prevalence of arthropathy in HTLV-I carriers on a Japanese island. Ann. Rheum. Dis. 1996, 55, 193–195. [Google Scholar] [CrossRef] [PubMed]
- da Rocha Sobrinho, H.M.; Jarach, R.; da Silva, N.A.; Shio, M.T.; Jancar, S.; Timenetsky, J.; Oliveira, M.A.; Dorta, M.L.; Ribeiro-Dias, F. Mycoplasmal lipid-associated membrane proteins and Mycoplasma arthritidis mitogen recognition by serum antibodies from patients with rheumatoid arthritis. Rheumatol. Int. 2011, 31, 951–957. [Google Scholar] [CrossRef] [PubMed]
- Sawitzke, A.; Joyner, D.; Knudtson, K.; Mu, H.H.; Cole, B. Anti-MAM antibodies in rheumatic disease: Evidence for a MAM-like superantigen in rheumatoid arthritis? J. Rheumatol. 2000, 27, 358–364. [Google Scholar] [PubMed]
- Tzang, B.S.; Tsai, C.C.; Tsay, G.J.; Wang, M.; Sun, Y.S.; Hsu, T.C. Anti-human parvovirus B19 nonstructural protein antibodies in patients with rheumatoid arthritis. Clin. Chim. Acta 2009, 405, 76–82. [Google Scholar] [CrossRef]
- Shi, J.; Sun, X.; Zhao, Y.; Zhao, J.; Li, Z. Prevalence and significance of antibodies to citrullinated human papilloma virus-47 E2345-362 in rheumatoid arthritis. J. AutoImmun. 2008, 31, 131–135. [Google Scholar] [CrossRef]
- Mameli, G.; Erre, G.L.; Caggiu, E.; Mura, S.; Cossu, D.; Bo, M.; Cadoni, M.L.; Piras, A.; Mundula, N.; Colombo, E.; et al. Identification of a HERV-K env surface peptide highly recognized in Rheumatoid Arthritis (RA) patients: A cross-sectional case-control study. Clin. Exp. Immunol. 2017, 189, 127–131. [Google Scholar] [CrossRef]
- Chukkapalli, S.; Rivera-Kweh, M.; Gehlot, P.; Velsko, I.; Bhattacharyya, I.; Calise, S.J.; Satoh, M.; Chan, E.K.; Holoshitz, J.; Kesavalu, L. Periodontal bacterial colonization in synovial tissues exacerbates collagen-induced arthritis in B10.RIII mice. Arthritis Res. Ther. 2016, 18, 161. [Google Scholar] [CrossRef]
- Jung, H.; Jung, S.M.; Rim, Y.A.; Park, N.; Nam, Y.; Lee, J.; Park, S.-H.; Ju, J.H. Arthritic role of Porphyromonas gingivalis in collagen-induced arthritis mice. PLoS ONE 2017, 12, e0188698. [Google Scholar] [CrossRef]
- Yamakawa, M.; Ouhara, K.; Kajiya, M.; Munenaga, S.; Kittaka, M.; Yamasaki, S.; Takeda, K.; Takeshita, K.; Mizuno, N.; Fujita, T.; et al. Porphyromonas gingivalis infection exacerbates the onset of rheumatoid arthritis in SKG mice. Clin. Exp. Immunol. 2016, 186, 177–189. [Google Scholar] [CrossRef] [PubMed]
- Bartold, P.M.; Marino, V.; Cantley, M.; Haynes, D.R. Effect of Porphyromonas gingivalis-induced inflammation on the development of rheumatoid arthritis. J. Clin. Periodontol. 2010, 37, 405–411. [Google Scholar] [CrossRef]
- Cole, B.C.; Golightly-Rowland, L.; Ward, J.R. Arthritis of mice induced by Mycoplasma arthritidis. Humoral antibody and lymphocyte responses of CBA mice. Ann. Rheum. Dis. 1976, 35, 14–22. [Google Scholar] [CrossRef]
- Kuwana, Y.; Takei, M.; Yajima, M.; Imadome, K.-I.; Inomata, H.; Shiozaki, M.; Ikumi, N.; Nozaki, T.; Shiraiwa, H.; Kitamura, N.; et al. Epstein-Barr Virus Induces Erosive Arthritis in Humanized Mice. PLoS ONE 2011, 6, e26630. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, S.; Matsuda, G.; Imadome, K.-I. Humanized mouse models of epstein-barr virus infection and associated diseases. Pathogens 2013, 2, 153–176. [Google Scholar] [CrossRef] [PubMed]
- Röhner, E.; Detert, J.; Kolar, P.; Hocke, A.; N’Guessan, P.; Matziolis, G.; Kanitz, V.; Bernimoulin, J.P.; Kielbassa, A.; Burmester, G.R.; et al. Induced apoptosis of chondrocytes by Porphyromonas gingivalis as a possible pathway for cartilage loss in rheumatoid arthritis. Calcif. Tissue Int. 2010, 87, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Choi, I.A.; Kim, J.H.; Kim, K.H.; Lee, E.Y.; Lee, E.B.; Lee, Y.M.; Song, Y.W. Association between anti-Porphyromonas gingivalis or anti-α-enolase antibody and severity of periodontitis or rheumatoid arthritis (RA) disease activity in RA. BMC Musculoskelet. Disord. 2015, 16, 190. [Google Scholar] [CrossRef] [PubMed]
- Rojas, M.; Restrepo-Jiménez, P.; Monsalve, D.M.; Pacheco, Y.; Acosta-Ampudia, Y.; Ramírez-Santana, C.; Leung, P.S.C.; Ansari, A.A.; Gershwin, M.E.; Anaya, J.M. Molecular mimicry and autoimmunity. J. AutoImmun. 2018, 95, 100–123. [Google Scholar] [CrossRef]
- Lundberg, K.; Kinloch, A.; Fisher, B.A.; Wegner, N.; Wait, R.; Charles, P.; Mikuls, T.R.; Venables, P.J. Antibodies to citrullinated alpha-enolase peptide 1 are specific for rheumatoid arthritis and cross-react with bacterial enolase. Arthritis Rheum. 2008, 58, 3009–3019. [Google Scholar] [CrossRef] [PubMed]
- Bo, M.; Erre, G.L.; Niegowska, M.; Piras, M.; Taras, L.; Longu, M.G.; Passiu, G.; Sechi, L.A. Interferon regulatory factor 5 is a potential target of autoimmune response triggered by Epstein-barr virus and Mycobacterium avium subsp. paratuberculosis in rheumatoid arthritis: Investigating a mechanism of molecular mimicry. Clin. Exp. Rheumatol. 2018, 36, 376–381. [Google Scholar]
- Ebringer, A.; Rashid, T.; Wilson, C. Rheumatoid arthritis, Proteus, anti-CCP antibodies and Karl Popper. AutoImmun. Rev. 2010, 9, 216–223. [Google Scholar] [CrossRef]
- Ebringer, A.; Rashid, T. Rheumatoid arthritis is an autoimmune disease triggered by Proteus urinary tract infection. Clin. Dev. Immunol. 2006, 13, 41–48. [Google Scholar] [CrossRef]
- Wilson, C.; Ebringer, A.; Ahmadi, K.; Wrigglesworth, J.; Tiwana, H.; Fielder, M.; Binder, A.; Ettelaie, C.; Cunningham, P.; Joannou, C.; et al. Shared amino acid sequences between major histocompatibility complex class II glycoproteins, type XI collagen and Proteus mirabilis in rheumatoid arthritis. Ann. Rheum. Dis. 1995, 54, 216–220. [Google Scholar] [CrossRef]
- Ebringer, A.; Rashid, T. Rheumatoid arthritis is caused by a Proteus urinary tract infection. Apmis 2014, 122, 363–368. [Google Scholar] [CrossRef]
- Tiwana, H.; Wilson, C.; Alvarez, A.; Abuknesha, R.; Bansal, S.; Ebringer, A. Cross-reactivity between the rheumatoid arthritis-associated motif EQKRAA and structurally related sequences found in Proteus mirabilis. Infect. Immun. 1999, 67, 2769–2775. [Google Scholar] [CrossRef] [PubMed]
- Wilson, C.; Tiwana, H.; Ebringer, A. Molecular mimicry between HLA-DR alleles associated with rheumatoid arthritis and Proteus mirabilis as the Aetiological basis for autoimmunity. Microbes Infect. 2000, 2, 1489–1496. [Google Scholar] [CrossRef]
- Hou, Y.; Lin, H.; Zhu, L.; Liu, Z.; Hu, F.; Shi, J.; Yang, T.; Shi, X.; Zhu, M.; Godley, B.F.; et al. Lipopolysaccharide increases the incidence of collagen-induced arthritis in mice through induction of protease HTRA-1 expression. Arthritis Rheum. 2013, 65, 2835–2846. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, W.; Buhrmann, C.; Mobasheri, A.; Lueders, C.; Shakibaei, M. Bacterial lipopolysaccharides form procollagen-endotoxin complexes that trigger cartilage inflammation and degeneration: Implications for the development of rheumatoid arthritis. Arthritis Res. Ther. 2013, 15, R111. [Google Scholar] [CrossRef]
- Nakayama, M.; Niki, Y.; Kawasaki, T.; Takeda, Y.; Horiuchi, K.; Sasaki, A.; Okada, Y.; Umezawa, K.; Ikegami, H.; Toyama, Y.; et al. Enhanced susceptibility to lipopolysaccharide-induced arthritis and endotoxin shock in interleukin-32 alpha transgenic mice through induction of tumor necrosis factor alpha. Arthritis Res. Ther. 2012, 14, R120. [Google Scholar] [CrossRef]
- Yücel, G.; Zhao, Z.; El-Battrawy, I.; Lan, H.; Lang, S.; Li, X.; Buljubasic, F.; Zimmermann, W.-H.; Cyganek, L.; Utikal, J.; et al. Lipopolysaccharides induced inflammatory responses and electrophysiological dysfunctions in human-induced pluripotent stem cell derived cardiomyocytes. Sci. Rep. 2017, 7, 2935. [Google Scholar] [CrossRef]
- Frost, R.A.; Nystrom, G.J.; Lang, C.H. Lipopolysaccharide regulates proinflammatory cytokine expression in mouse myoblasts and skeletal muscle. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2002, 283, R698–R709. [Google Scholar] [CrossRef] [PubMed]
- Barksby, H.E.; Nile, C.J.; Jaedicke, K.M.; Taylor, J.J.; Preshaw, P.M. Differential expression of immunoregulatory genes in monocytes in response to Porphyromonas gingivalis and Escherichia coli lipopolysaccharide. Clin. Exp. Immunol. 2009, 156, 479–487. [Google Scholar] [CrossRef] [PubMed]
- Nile, C.J.; Barksby, E.; Jitprasertwong, P.; Preshaw, P.M.; Taylor, J.J. Expression and regulation of interleukin-33 in human monocytes. Immunology 2010, 130, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Burns, E.; Bachrach, G.; Shapira, L.; Nussbaum, G. Cutting Edge: TLR2 is required for the innate response to Porphyromonas gingivalis: Activation leads to bacterial persistence and TLR2 deficiency attenuates induced alveolar bone resorption. J. Immunol. 2006, 177, 8296–8300. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Bi, L.; Yu, X.; Kawai, T.; Taubman, M.A.; Shen, B.; Han, X. Porphyromonas gingivalis exacerbates ligature-induced, RANKL-dependent alveolar bone resorption via differential regulation of Toll-like receptor 2 (TLR2) and TLR4. Infect. Immun. 2014, 82, 4127–4134. [Google Scholar] [CrossRef]
- Yang, S.; Tamai, R.; Akashi, S.; Takeuchi, O.; Akira, S.; Sugawara, S.; Takada, H. Synergistic effect of muramyldipeptide with lipopolysaccharide or lipoteichoic acid to induce inflammatory cytokines in human monocytic cells in culture. Infect. Immun. 2001, 69, 2045–2053. [Google Scholar] [CrossRef]
- Yang, S.; Takahashi, N.; Yamashita, T.; Sato, N.; Takahashi, M.; Mogi, M.; Uematsu, T.; Kobayashi, Y.; Nakamichi, Y.; Takeda, K.; et al. Muramyl Dipeptide Enhances Osteoclast Formation Induced by Lipopolysaccharide, IL-1α, and TNF-α through Nucleotide-Binding Oligomerization Domain 2-Mediated Signaling in Osteoblasts. J. Immunol. 2005, 175, 1956–1964. [Google Scholar] [CrossRef]
- Shehab, M.; Sherri, N.; Hussein, H.; Salloum, N.; Rahal, E.A. Endosomal Toll-Like Receptors Mediate Enhancement of Interleukin-17A Production Triggered by Epstein-Barr Virus DNA in Mice. J. Virol. 2019, 93, e00987-19. [Google Scholar] [CrossRef]
- Salloum, N.; Hussein, H.M.; Jammaz, R.; Jiche, S.; Uthman, I.W.; Abdelnoor, A.M.; Rahal, E.A. Epstein-Barr virus DNA modulates regulatory T-cell programming in addition to enhancing interleukin-17A production via Toll-like receptor 9. PLoS ONE 2018, 13, e0200546. [Google Scholar] [CrossRef]
- Hsiao, F.C.; Lin, M.; Tai, A.; Chen, G.; Huber, B.T. Cutting edge: Epstein-Barr virus transactivates the HERV-K18 superantigen by docking to the human complement receptor 2 (CD21) on primary B cells. J. Immunol. 2006, 177, 2056–2060. [Google Scholar] [CrossRef]
- Ford, D.K.; da Roza, D.M.; Schulzer, M.; Reid, G.D.; Denegri, J.F. Persistent synovial lymphocyte responses to cytomegalovirus antigen in some patients with rheumatoid arthritis. Arthritis Rheum. 1987, 30, 700–704. [Google Scholar] [CrossRef] [PubMed]
- Kinloch, A.J.; Alzabin, S.; Brintnell, W.; Wilson, E.; Barra, L.; Wegner, N.; Bell, D.A.; Cairns, E.; Venables, P.J. Immunization with Porphyromonas gingivalis enolase induces autoimmunity to mammalian α-enolase and arthritis in DR4-IE-transgenic mice. Arthritis Rheum. 2011, 63, 3818–3823. [Google Scholar] [CrossRef] [PubMed]
- Wegner, N.; Wait, R.; Sroka, A.; Eick, S.; Nguyen, K.A.; Lundberg, K.; Kinloch, A.; Culshaw, S.; Potempa, J.; Venables, P.J. Peptidylarginine deiminase from Porphyromonas gingivalis citrullinates human fibrinogen and α-enolase: Implications for autoimmunity in rheumatoid arthritis. Arthritis Rheum. 2010, 62, 2662–2672. [Google Scholar] [CrossRef] [PubMed]
- Maresz, K.J.; Hellvard, A.; Sroka, A.; Adamowicz, K.; Bielecka, E.; Koziel, J.; Gawron, K.; Mizgalska, D.; Marcinska, K.A.; Benedyk, M.; et al. Porphyromonas gingivalis facilitates the development and progression of destructive arthritis through its unique bacterial peptidylarginine deiminase (PAD). PloS Pathog. 2013, 9, e1003627. [Google Scholar] [CrossRef] [PubMed]
- Courbon, G.; Rinaudo-Gaujous, M.; Blasco-Baque, V.; Auger, I.; Caire, R.; Mijola, L.; Vico, L.; Paul, S.; Marotte, H. Porphyromonas gingivalis experimentally induces periodontis and an anti-CCP2-associated arthritis in the rat. Ann. Rheum. Dis. 2019, 78, 594–599. [Google Scholar] [CrossRef]
| Presence of microbial contents in RA patients tissues and serum | Mycobacteria, P. gingivalis, EBV, Mycoplasma, Bordetalla, Haemophilus, Acinetobacter, Parvovirus, CMV, Bacterial cell wall |
| Presence of immune response to infection in RA patients tissues and serum | Mycobacteria, P. gingivalis, EBV, HTLV, Mycoplasma, Parvovirus B19, Papilloma virus, HERV |
Induction of Arthritis by Infections in Animal Models | Mycobacteria, P. gingivalis, Mycoplasma, EBV |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bo, M.; Jasemi, S.; Uras, G.; Erre, G.L.; Passiu, G.; Sechi, L.A. Role of Infections in the Pathogenesis of Rheumatoid Arthritis: Focus on Mycobacteria. Microorganisms 2020, 8, 1459. https://doi.org/10.3390/microorganisms8101459
Bo M, Jasemi S, Uras G, Erre GL, Passiu G, Sechi LA. Role of Infections in the Pathogenesis of Rheumatoid Arthritis: Focus on Mycobacteria. Microorganisms. 2020; 8(10):1459. https://doi.org/10.3390/microorganisms8101459
Chicago/Turabian StyleBo, Marco, Seyedesomaye Jasemi, Giuseppe Uras, Gian Luca Erre, Giuseppe Passiu, and Leonardo A. Sechi. 2020. "Role of Infections in the Pathogenesis of Rheumatoid Arthritis: Focus on Mycobacteria" Microorganisms 8, no. 10: 1459. https://doi.org/10.3390/microorganisms8101459
APA StyleBo, M., Jasemi, S., Uras, G., Erre, G. L., Passiu, G., & Sechi, L. A. (2020). Role of Infections in the Pathogenesis of Rheumatoid Arthritis: Focus on Mycobacteria. Microorganisms, 8(10), 1459. https://doi.org/10.3390/microorganisms8101459