The Adaptive Morphology of Bacillus subtilis Biofilms: A Defense Mechanism against Bacterial Starvation
Abstract
1. Introduction
2. Materials and Methods
2.1. Strain and Growth Media
2.2. Colony-Type Biofilm Formation
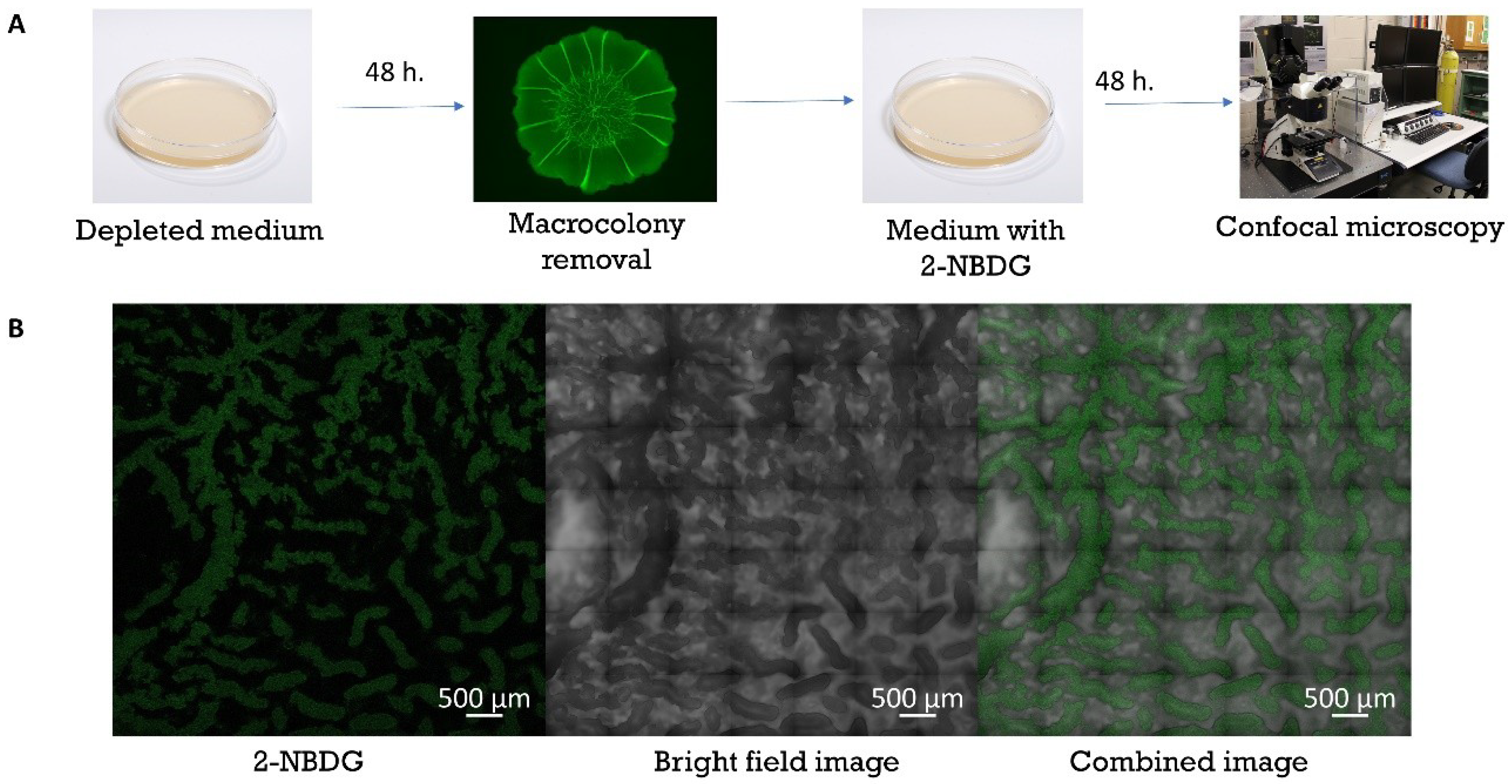
2.3. Native Uptake of Nutrients
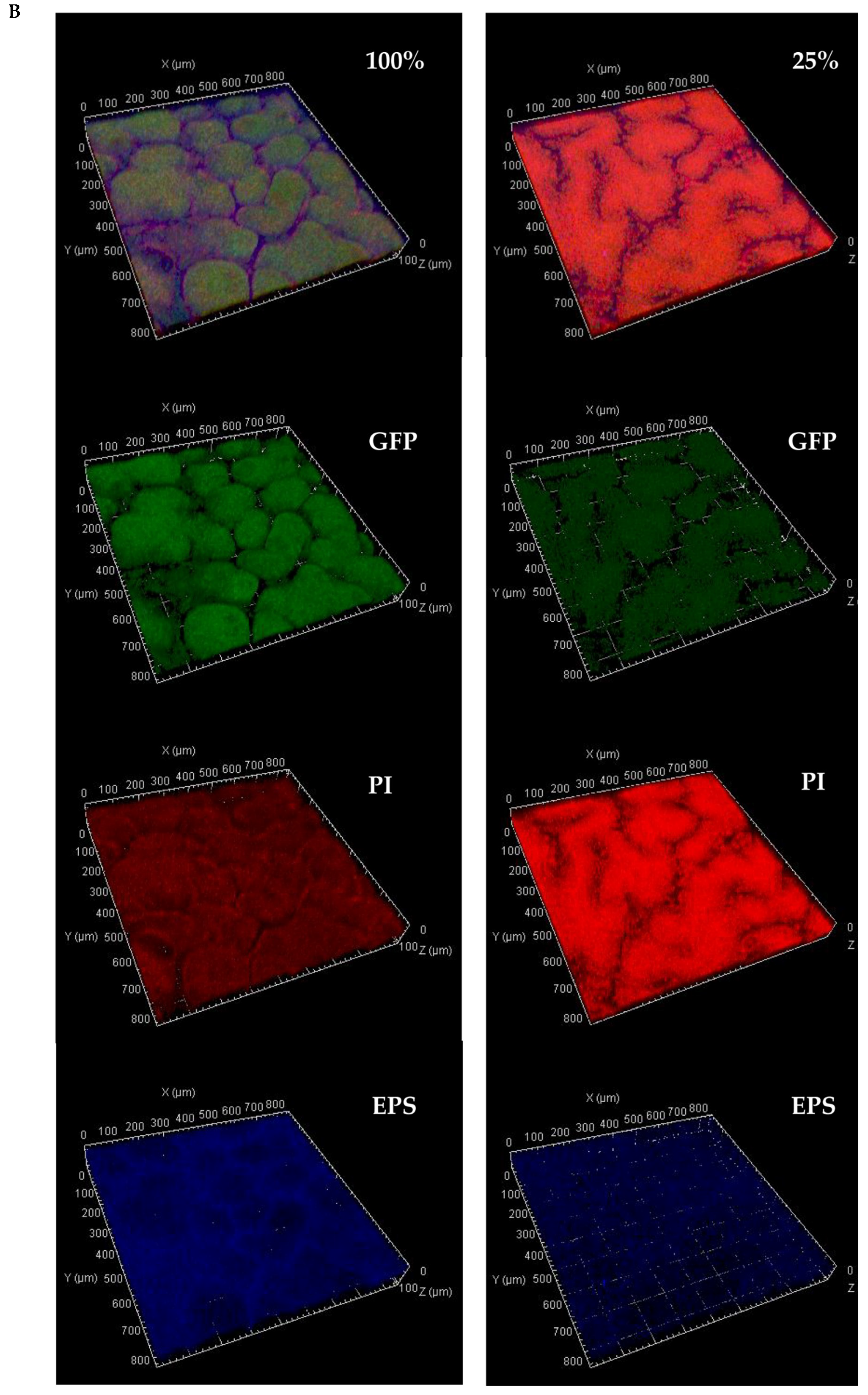
2.4. Multi-Stain Visualization of Colony-Type Biofilms
2.4.1. Confocal Microscopy
2.4.2. Multiphoton Microscopy
2.5. Image Processing
2.5.1. Two-Dimensional (2D) Analysis
2.5.2. Three-Dimensional (3D) Analysis
2.6. Statistical Analysis
3. Results
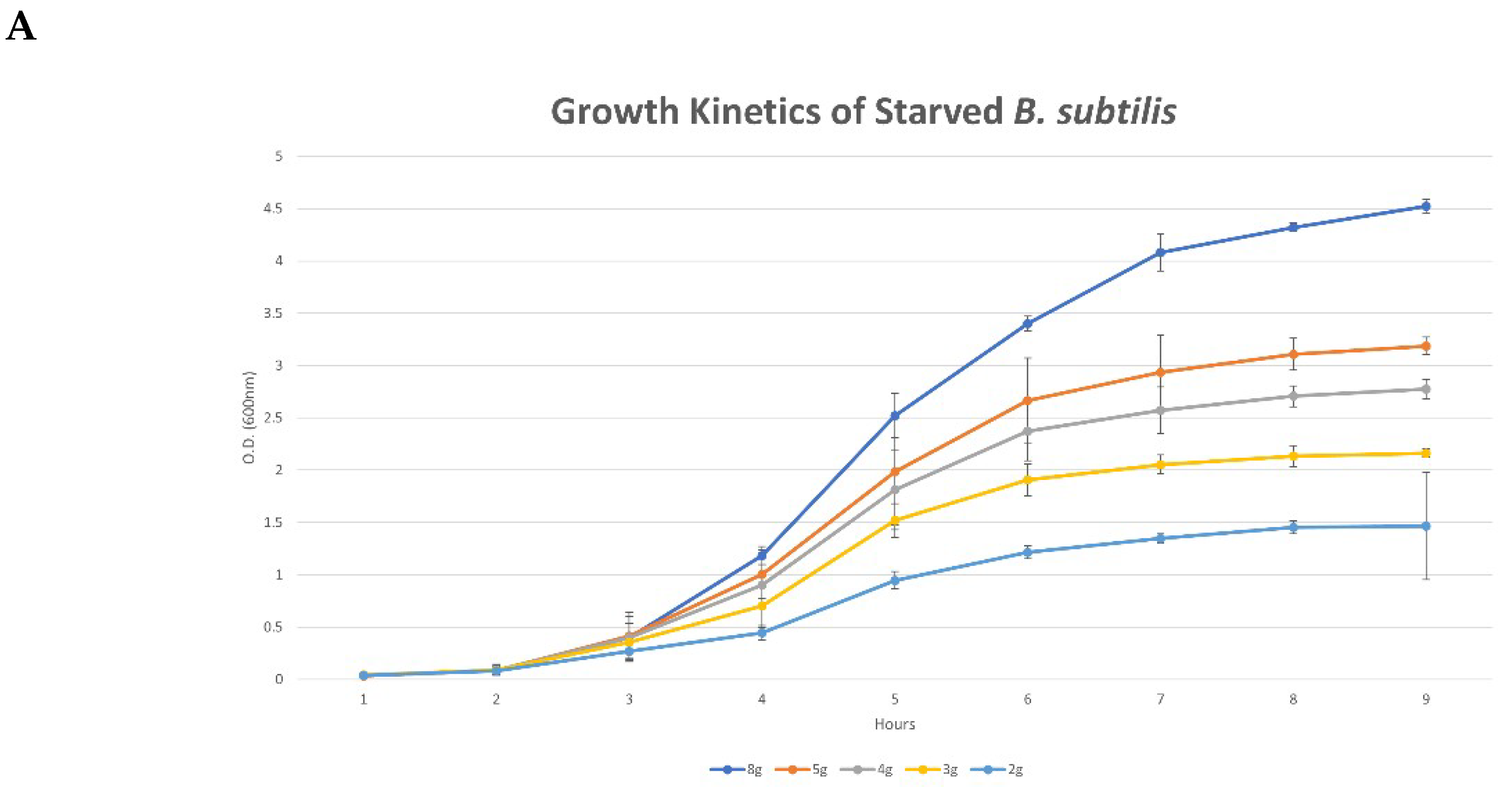
3.1. Characteristic Features of Bacterial Growth under Nutrient-Limiting Conditions
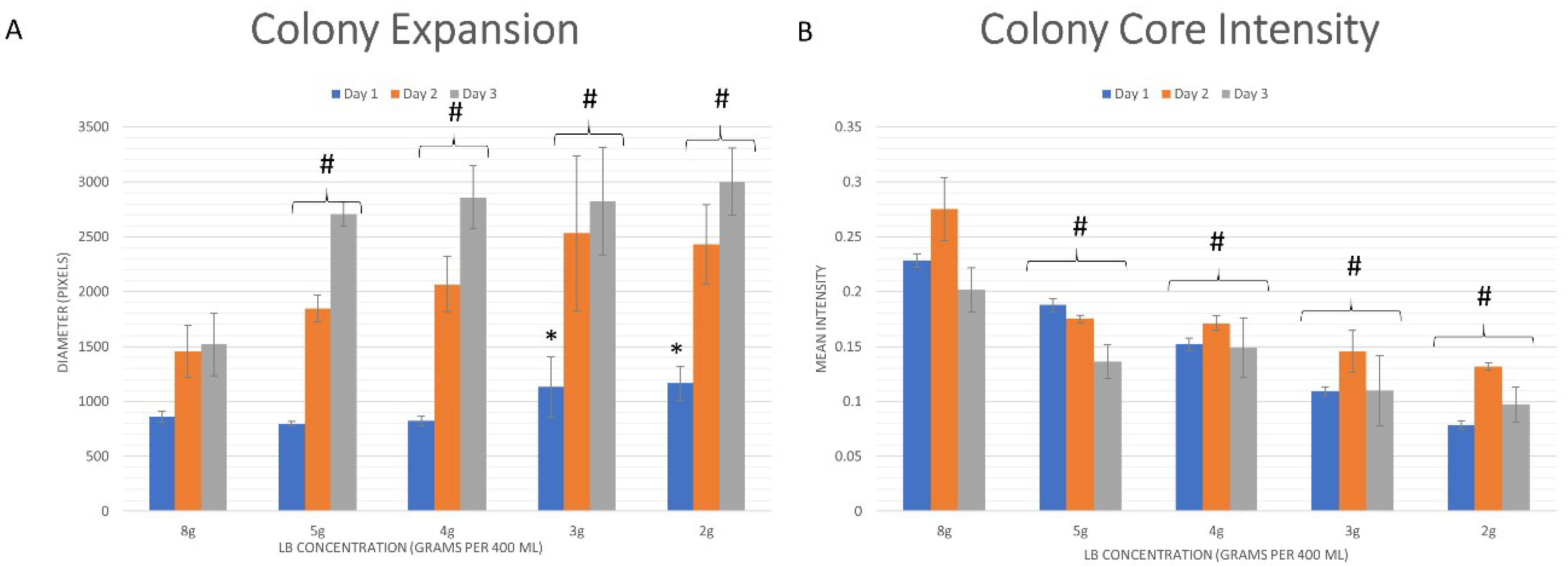
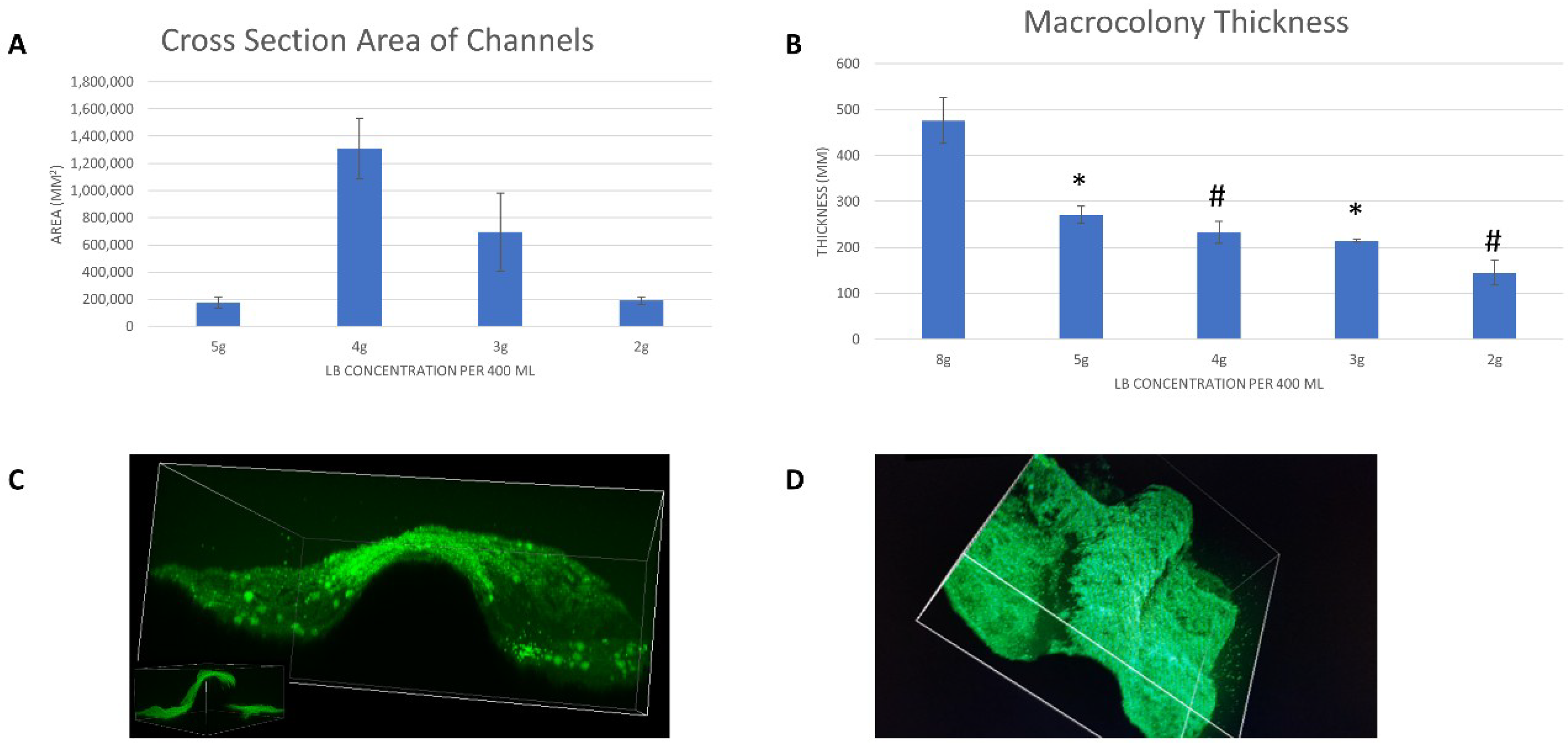
3.2. Quantitative Analysis
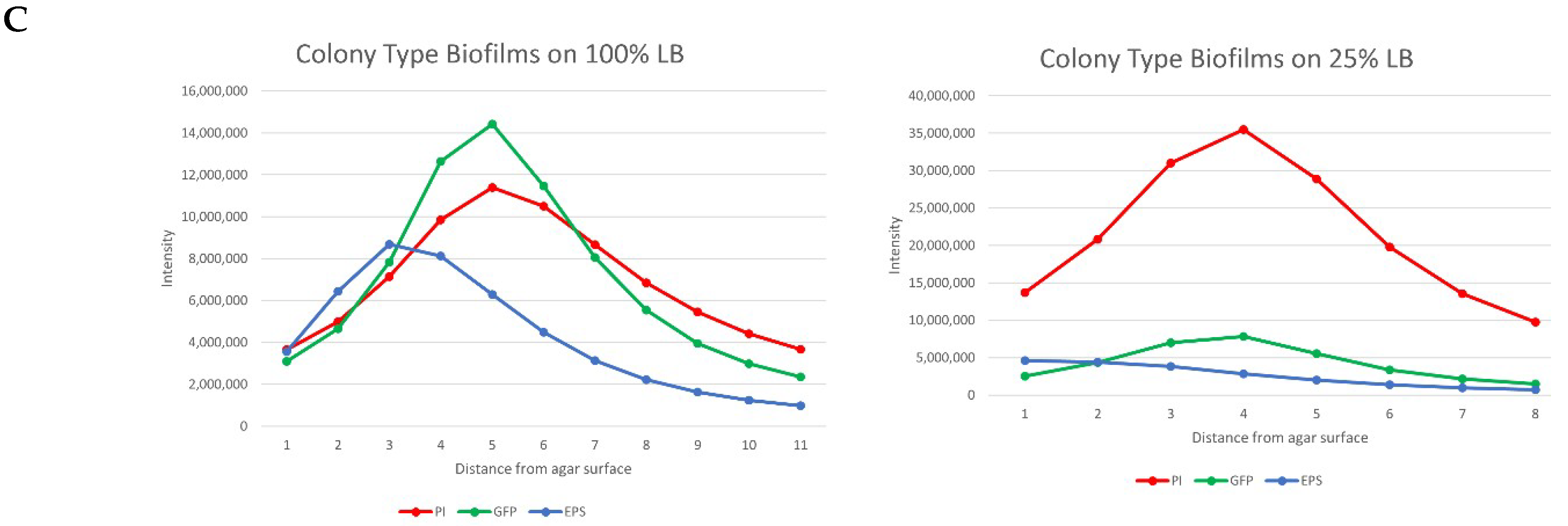
3.2.1. Whole Colony Analysis
3.2.2. Volumetric Analysis of Channels
3.3. Co-Localization of 2-NBDG within Channels
4. Discussion
4.1. Bacterial Kinetics
4.2. Biofilm Morphology
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Baker, R.M.; Singleton, F.L.; Hood, M.A. Effects of nutrient deprivation on Vibrio cholerae. Appl. Environ. Microbiol. 1983, 46, 930–940. [Google Scholar]
- Côté, J.P.; French, S.; Gehrke, S.S.; MacNair, C.R.; Mangat, C.S.; Bharat, A.; Brown, E.D. The Genome-Wide Interaction Network of Nutrient Stress Genes in Escherichia coli. mBio 2016, 7. [Google Scholar] [CrossRef]
- Girard, L.P.; Ceri, H.; Gibb, A.P.; Olson, M.; Sepandj, F. MIC Versus MBEC to Determine the Antibiotic Sensitivity of Staphylococcus aureus in Peritoneal Dialysis Peritonitis. Perit. Dial. Int. 2010, 30, 652–656. [Google Scholar] [CrossRef]
- Sepandj, F.; Ceri, H.; Gibb, A.; Read, R.; Olson, M. Minimum inhibitory concentration (MIC) versus minimum biofilm eliminating concentration (MBEC) in evaluation of antibiotic sensitivity of gram-negative bacilli causing peritonitis. Perit. Dial. Int. 2004, 24, 65–67. [Google Scholar]
- Lee, K.W.K.; Periasamy, S.; Mukherjee, M.; Xie, C.; Kjelleberg, S.; Rice, S.A. Biofilm development and enhanced stress resistance of a model, mixed-species community biofilm. ISME J. 2014, 8, 894–907. [Google Scholar] [CrossRef]
- Branda, S.S.; Gonzalez-Pastor, J.E.; Ben-Yehuda, S.; Losick, R.; Kolter, R. Fruiting body formation by Bacillus subtilis. Proc. Natl. Acad. Sci. USA 2001, 98, 11621–11626. [Google Scholar] [CrossRef]
- Thompson, J.M.; Dodd, C.E.R.; Waites, W.M. Spoilage of bread by bacillus. Int. Biodeterior. Biodegrad. 1993, 32, 55–66. [Google Scholar] [CrossRef]
- Pavić, S.; Brett, M.; Petrić, I.; Laštre, D.; Smoljanović, M.; Atkinson, M.; Kovaćić, A.; Cetinić, E.; Ropac, D. An outbreak of food poisoning in a kindergarten caused by milk powder containing toxigenic Bacillus subtilis and Bacillus licheniformis. Archiv Fur Lebensm. 2005, 56, 20–22. [Google Scholar]
- Chen, Y.; Yan, F.; Chai, Y.; Liu, H.; Kolter, R.; Losick, R.; Guo, J.H. Biocontrol of tomato wilt disease by Bacillus subtilis isolates from natural environments depends on conserved genes mediating biofilm formation: Bacillus subtilis and plant biocontrol. Environ. Microbiol. 2013, 15, 848–864. [Google Scholar] [CrossRef]
- Yamane, K.; Ogawa, K.; Yoshida, M.; Hayashi, H.; Nakamura, T.; Yamanaka, T.; Tamaki, T.; Hojoh, H.; Leung, K.P.; Fukushima, H. Identification and Characterization of Clinically Isolated Biofilm-forming Gram-positive Rods from Teeth Associated with Persistent Apical Periodontitis. J. Endod. 2009, 35, 347–352. [Google Scholar] [CrossRef]
- Jain, K.; Parida, S.; Mangwani, N.; Dash, H.R.; Das, S. Isolation and characterization of biofilm-forming bacteria and associated extracellular polymeric substances from oral cavity. Ann. Microbiol. 2013, 63, 1553–1562. [Google Scholar] [CrossRef]
- Hong, H.A.; Khaneja, R.; Tam, N.M.; Cazzato, A.; Tan, S.; Urdaci, M.; Brisson, A.; Gasbarrini, A.; Barnes, I.; Cutting, S.M. Bacillus subtilis isolated from the human gastrointestinal tract. Res. Microbiol. 2009, 160, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Bridier, A.; Le Coq, D.; Dubois-Brissonnet, F.; Thomas, V.; Aymerich, S.; Briandet, R. The Spatial Architecture of Bacillus subtilis Biofilms Deciphered Using a Surface-Associated Model and In Situ Imaging. Driks A, editor. PLoS ONE 2011, 6, e16177. [Google Scholar] [CrossRef] [PubMed]
- Shemesh, M.; Chai, Y. A Combination of Glycerol and Manganese Promotes Biofilm Formation in Bacillus subtilis via Histidine Kinase KinD Signaling. J. Bacteriol. 2013, 195, 2747–2754. [Google Scholar] [CrossRef]
- Vlamakis, H.; Aguilar, C.; Losick, R.; Kolter, R. Control of cell fate by the formation of an architecturally complex bacterial community. Genes Dev. 2008, 22, 945–953. [Google Scholar] [CrossRef]
- Kjelleberg, S.; Hermansson, M. Starvation-induced effects on bacterial surface characteristics. Appl. Environ. Microbiol. 1984, 48, 497–503. [Google Scholar]
- Phaiboun, A.; Zhang, Y.; Park, B.; Kim, M. Survival Kinetics of Starving Bacteria Is Biphasic and Density-Dependent. Weitz JS, editor. PLoS Comput. Biol. 2015, 11, e1004198. [Google Scholar] [CrossRef]
- Pulschen, A.A.; Sastre, D.E.; Machinandiarena, F.; Crotta Asis, A.; Albanesi, D.; de Mendoza, D.; Gueiros-Filho, F.J. The stringent response plays a key role in B acillus subtilis survival of fatty acid starvation: Stringent response in fatty acid starved Bacillus subtilis. Mol. Microbiol. 2017, 103, 698–712. [Google Scholar] [CrossRef]
- Tam, L.T.; Eymann, C.; Antelmann, H.; Albrecht, D.; Hecker, M. Global Gene Expression Profiling of Bacillus subtilis in Response to Ammonium and Tryptophan Starvation as Revealed by Transcriptome and Proteome Analysis. J. Mol. Microbiol. Biotechnol. 2007, 12, 121–130. [Google Scholar] [CrossRef]
- Panahi, R.; Vasheghani-Farahani, E.; Shojaosadati, S.A.; Bambai, B. Induction of Bacillus subtilis expression system using environmental stresses and glucose starvation. Ann. Microbiol. 2014, 64, 879–882. [Google Scholar] [CrossRef]
- Gingichashvili, S.; Duanis-Assaf, D.; Shemesh, M.; Featherstone, J.D.B.; Feuerstein, O.; Steinberg, D. Bacillus subtilis Biofilm Development—A Computerized Study of Morphology and Kinetics. Front. Microbiol. 2017, 8, 2072. [Google Scholar] [CrossRef] [PubMed]
- Wilking, J.N.; Zaburdaev, V.; De Volder, M.; Losick, R.; Brenner, M.P.; Weitz, D.A. Liquid transport facilitated by channels in Bacillus subtilis biofilms. Proc. Natl. Acad. Sci. USA 2013, 110, 848–852. [Google Scholar] [CrossRef] [PubMed]
- Keren-Paz, A.; Brumfeld, V.; Oppenheimer-Shaanan, Y.; Kolodkin-Gal, I. Micro-CT X-ray imaging exposes structured diffusion barriers within biofilms. NPJ Biofilms Microbiomes 2018, 4. [Google Scholar] [CrossRef] [PubMed]
- Chai, Y.; Norman, T.; Kolter, R.; Losick, R. Evidence that metabolism and chromosome copy number control mutually exclusive cell fates in Bacillus subtilis. EMBO J. 2011, 30, 1402–1413. [Google Scholar] [CrossRef] [PubMed]
- Asally, M.; Kittisopikul, M.; Rué, P.; Du, Y.; Hu, Z.; Çağatay, T.; Robinson, A.B.; Lu, H.; Garcia-Ojalvo, J.; Süel, G.M. Localized cell death focuses mechanical forces during 3D patterning in a biofilm. Proc. Natl. Acad. Sci. USA 2012, 109, 18891–18896. [Google Scholar] [CrossRef] [PubMed]
- Trejo, M.; Douarche, C.; Bailleux, V.; Poulard, C.; Mariot, S.; Regeard, C.; Raspaud, E. Elasticity and wrinkled morphology of Bacillus subtilis pellicles. Proc. Natl. Acad. Sci. USA 2013, 110, 2011–2016. [Google Scholar] [CrossRef]
- Stewart, P.S.; Franklin, M.J. Physiological heterogeneity in biofilms. Nat. Rev. Microbiol. 2008, 6, 199–210. [Google Scholar] [CrossRef]
- Molina-Santiago, C.; Pearson, J.R.; Navarro, Y.; Berlanga-Clavero, M.V.; Caraballo-Rodriguez, A.M.; Petras, D.; García-Martín, M.L.; Lamon, G.; Haberstein, B.; Cazorla, F.M.; et al. The extracellular matrix protects Bacillus subtilis colonies from Pseudomonas invasion and modulates plant co-colonization. Nat. Commun. 2019, 10, 1919. [Google Scholar] [CrossRef]
- Zhang, W.; Seminara, A.; Suaris, M.; Brenner, M.P.; Weitz, D.A.; Angelini, T.E. Nutrient depletion in Bacillus subtilis biofilms triggers matrix production. New J. Phys. 2014, 16, 015028. [Google Scholar] [CrossRef]
- Seminara, A.; Angelini, T.E.; Wilking, J.N.; Vlamakis, H.; Ebrahim, S.; Kolter, R.; Weitz, D.A.; Brenner, M.P. Osmotic spreading of Bacillus subtilis biofilms driven by an extracellular matrix. Proc. Natl. Acad. Sci. USA 2012, 109, 1116–1121. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gingichashvili, S.; Duanis-Assaf, D.; Shemesh, M.; Featherstone, J.D.B.; Feuerstein, O.; Steinberg, D. The Adaptive Morphology of Bacillus subtilis Biofilms: A Defense Mechanism against Bacterial Starvation. Microorganisms 2020, 8, 62. https://doi.org/10.3390/microorganisms8010062
Gingichashvili S, Duanis-Assaf D, Shemesh M, Featherstone JDB, Feuerstein O, Steinberg D. The Adaptive Morphology of Bacillus subtilis Biofilms: A Defense Mechanism against Bacterial Starvation. Microorganisms. 2020; 8(1):62. https://doi.org/10.3390/microorganisms8010062
Chicago/Turabian StyleGingichashvili, Sarah, Danielle Duanis-Assaf, Moshe Shemesh, John D. B. Featherstone, Osnat Feuerstein, and Doron Steinberg. 2020. "The Adaptive Morphology of Bacillus subtilis Biofilms: A Defense Mechanism against Bacterial Starvation" Microorganisms 8, no. 1: 62. https://doi.org/10.3390/microorganisms8010062
APA StyleGingichashvili, S., Duanis-Assaf, D., Shemesh, M., Featherstone, J. D. B., Feuerstein, O., & Steinberg, D. (2020). The Adaptive Morphology of Bacillus subtilis Biofilms: A Defense Mechanism against Bacterial Starvation. Microorganisms, 8(1), 62. https://doi.org/10.3390/microorganisms8010062





