Comparison of Phylogenetic Tree Topologies for Nitrogen Associated Genes Partially Reconstruct the Evolutionary History of Saccharomyces cerevisiae
Abstract
1. Introduction
2. Materials and Methods
2.1. Gene Selection and Genomic Information Obtention
2.2. Bioinformatic Analyses
2.2.1. Data Processing and Phylogenetic Inference
2.2.2. Comparison of the Phylogenetic Tree Topologies
3. Results and Discussion
3.1. Reconstructing the Population Structure of S. cerevisiae Using a Subset of Nitrogen Associated Genes
3.2. Comparison of Tree Topologies among Genes Revealed Similar Evolutionary Histories
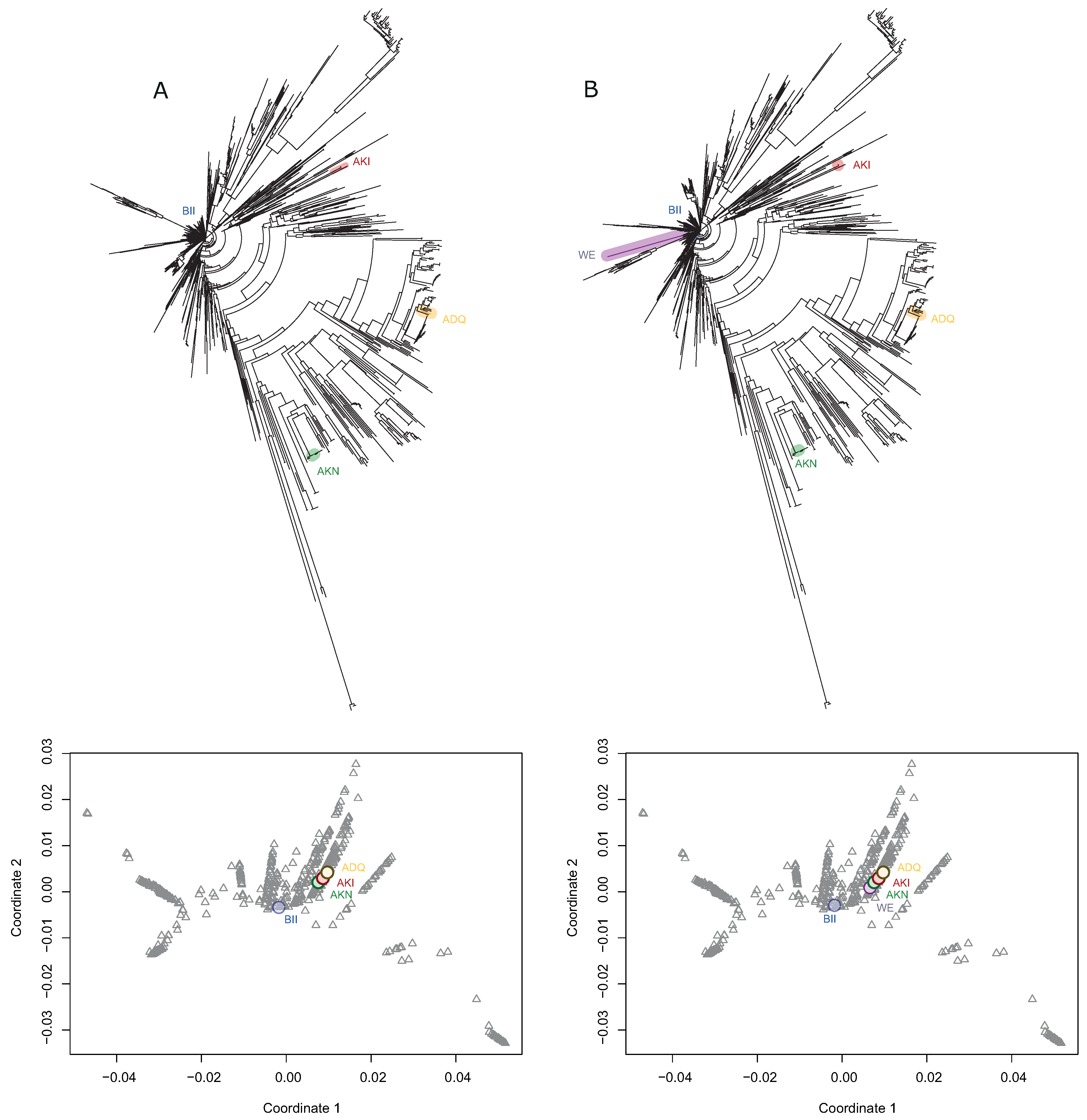
3.3. Representative Strains from Clean Lineages Reconstruct the Evolutionary History of the Species
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Querol, A.; Fernández-Espinar, M.; del Olmo, M.; Barrio, E. Adaptive evolution of wine yeast. Int. J. Food Microbiol. 2003, 86, 3–10. [Google Scholar] [CrossRef]
- Bisson, L. The Biotechnology of Wine Yeast. Food Biotechnol. 2004, 18, 63–96. [Google Scholar] [CrossRef]
- Goffeau, A.; Barrell, B.; Bussey, H.; Davis, R.; Dujon, B.; Feldmann, H.; Galibert, F.; Hoheisel, J.; Jacq, C.; Johnston, M.; et al. Life with 6000 Genes. Science 1996, 274, 546, 563–567. [Google Scholar] [CrossRef] [PubMed]
- Altshuler, D.; Durbin, R.; Abecasis, G.; Bentley, D.; Chakravarti, A.; Clark, A.; Collins, F.; De La Vega, F.; Donnelly, P.; Egholm, M.; et al. A map of human genome variation from population-scale sequencing. Nature 2010, 467, 1061–1073. [Google Scholar]
- Alonso-Blanco, C.; Andrade, J.; Becker, C.; Bemm, F.; Bergelson, J.; Borgwardt, K.M.; Cao, J.; Chae, E.; Dezwaan, T.M.; Ding, W.; et al. 1135 Genomes Reveal the Global Pattern of Polymorphism in Arabidopsis thaliana. Cell 2016, 166, 481–491. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Seshadri, R.; Varghese, N.; Eloe-Fadrosh, E.; Meier-Kolthoff, J.; Göker, M.; Coates, R.C.; Hadjithomas, M.; Pavlopoulos, G.; Paez Espino, D.; et al. 1003 reference genomes of bacterial and archaeal isolates expand coverage of the tree of life. Nat. Biotechnol. 2017, 35, 676–683. [Google Scholar] [CrossRef] [PubMed]
- Liti, G. The fascinating and secret wild life of the budding yeast S. cerevisiae. eLife 2015, 4, e05835. [Google Scholar] [CrossRef]
- Borneman, A.; Pretorius, I. Genomic Insights into the Saccharomyces sensu stricto Complex. Genetics 2015, 199, 281–291. [Google Scholar] [CrossRef]
- Libkind, D.; Hittinger, C.T.; Valerio, E.; Goncalves, C.; Dover, J.; Johnston, M.; Goncalves, P.; Sampaio, J.P. Microbe domestication and the identification of the wild genetic stock of lager-brewing yeast. Proc. Natl. Acad. Sci. USA 2011, 108, 14539–14544. [Google Scholar] [CrossRef]
- Scannell, D.R.; Zill, O.A.; Rokas, A.; Payen, C.; Dunham, M.J.; Eisen, M.B.; Rine, J.; Johnston, M.; Hittinger, C.T. The Awesome Power of Yeast Evolutionary Genetics: New Genome Sequences and Strain Resources for the Saccharomyces sensu stricto Genus. G3(Bethesda) 2011, 1, 11–25. [Google Scholar] [CrossRef]
- Schwartz, D.C.; Cantor, C.R. Separation of yeast chromosome-sized DNAs by pulsed field gradient gel electrophoresis. Cell 1984, 37, 67–75. [Google Scholar] [CrossRef]
- Vezinhet, F.; Blondin, B.; Hallet, J.N. Chromosomal DNA patterns and mitochondrial DNA polymorphism as tools for identification of enological strains of Saccharomyces cerevisiae. Appl. Microbiol. Biotechnol. 1990, 32, 568–571. [Google Scholar] [CrossRef]
- Querol, A.; Barrio, E.; Huerta, T.; Ramon, D. Molecular monitoring of wine fermentations conducted by active dry yeast strains. Appl. Environ. Microbiol. 1992, 58, 2948–2953. [Google Scholar] [PubMed]
- Baleiras-Couto, M.M.; Eijsma, B.; Hofstra, H.; Huis in’t Veld, J.H.; van der Vossen, J. Evaluation of Molecular Typing Techniques to Assign Genetic Diversity among Saccharomyces cerevisiae Strains. Appl. Environ. Microbiol. 1996, 62, 41–46. [Google Scholar] [PubMed]
- Perez, M.A.; Gallego, F.J.; Hidalgo, P. Evaluation of molecular techniques for the genetic characterization of Saccharomyces cerevisiae strains. FEMS Microbiol. Lett. 2001, 205, 375–378. [Google Scholar] [CrossRef]
- Legras, J.L.; Merdinoglu, D.; Cornuet, J.M.; Karst, F. Bread, beer and wine: Saccharomyces cerevisiae diversity reflects human history. Mol. Ecol. 2007, 16, 2091–2102. [Google Scholar] [CrossRef] [PubMed]
- Martinez, C.; Gac, S.; Lavin, A.; Ganga, M. Genomic characterization of Saccharomyces cerevisiae strains isolated from wine-producing areas in South America. J. Appl. Microbiol. 2004, 96, 1161–1168. [Google Scholar] [CrossRef]
- Fay, J.; Benavides, J. Evidence for domesticated and wild populations of Sacchoromyces cerevisiae. PLoS Genet. 2005, 1, 66–71. [Google Scholar] [CrossRef]
- Liti, G.; Carter, D.M.; Moses, A.M.; Warringer, J.; Parts, L.; James, S.A.; Davey, R.P.; Roberts, I.N.; Burt, A.; Koufopanou, V.; et al. Population genomics of domestic and wild yeasts. Nature 2009, 458, 337–341. [Google Scholar] [CrossRef]
- Strope, P.K.; Skelly, D.A.; Kozmin, S.G.; Mahadevan, G.; Stone, E.A.; Magwene, P.M.; Dietrich, F.S.; McCusker, J.H. The 100-genomes strains, an S. cerevisiae resource that illuminates its natural phenotypic and genotypic variation and emergence as an opportunistic pathogen. Genome Res. 2015, 25, 762–774. [Google Scholar] [CrossRef]
- Peter, J.; De Chiara, M.; Friedrich, A.; Yue, J.X.; Pflieger, D.; Bergstrom, A.; Sigwalt, A.; Barre, B.; Freel, K.; Llored, A.; et al. Genome evolution across 1011 Saccharomyces cerevisiae isolates. Nature 2018, 556, 339–344. [Google Scholar] [CrossRef] [PubMed]
- Cubillos, F.; Louis, E.; Liti, G. Generation of a large set of genetically tractable haploid and diploid Saccharomyces strains. FEMS Yeast Res. 2009, 9, 1217–1225. [Google Scholar] [CrossRef] [PubMed]
- Cubillos, F.; Billi, E.; Zörgö, E.; Parts, L.; Fargier, P.; Omholt, S.; Blomberg, A.; Warringer, J.; Louis, E.; Liti, G. Assessing the complex architecture of polygenic traits in diverged yeast populations. Mol. Ecol. 2011, 20, 1401–1413. [Google Scholar] [CrossRef]
- Liti, G.; Louis, E.J. Advances in quantitative trait analysis in yeast. PLoS Genet. 2012, 8, e1002912. [Google Scholar] [CrossRef] [PubMed]
- Cubillos, F.; Parts, L.; Salinas, F.; Bergström, A.; Scovacricchi, E.; Zia, A.; Illingworth, C.; Mustonen, V.; Ibstedt, S.; Warringer, J.; et al. High-Resolution Mapping of Complex Traits with a Four-Parent Advanced Intercross Yeast Population. Genetics 2013, 195, 1141–1155. [Google Scholar] [CrossRef] [PubMed]
- Kessi-Pérez, E.I.; Araos, S.; García, V.; Salinas, F.; Abarca, V.; Larrondo, L.F.; Martinez, C.; Cubillos, F.A. RIM15 antagonistic pleiotropy is responsible for differences in fermentation and stress response kinetics in budding yeast. FEMS Yeast Res. 2016, 16, fow021. [Google Scholar] [CrossRef][Green Version]
- Marullo, P.; Aigle, M.; Bely, M.; Masneuf-Pomarede, I.; Durrens, P.; Dubourdieu, D.; Yvert, G. Single QTL mapping and nucleotide-level resolution of a physiologic trait in wine Saccharomyces cerevisiae strains. FEMS Yeast Res. 2007, 7, 941–952. [Google Scholar] [CrossRef][Green Version]
- Parts, L.; Cubillos, F.A.; Warringer, J.; Jain, K.; Salinas, F.; Bumpstead, S.J.; Molin, M.; Zia, A.; Simpson, J.T.; Quail, M.A.; et al. Revealing the genetic structure of a trait by sequencing a population under selection. Genome Res. 2011, 21, 1131–1138. [Google Scholar] [CrossRef]
- Salinas, F.; Cubillos, F.A.; Soto, D.; Garcia, V.; Bergstrom, A.; Warringer, J.; Ganga, M.A.; Louis, E.J.; Liti, G.; Martinez, C. The genetic basis of natural variation in oenological traits in Saccharomyces cerevisiae. PLoS ONE 2012, 7, e49640. [Google Scholar] [CrossRef]
- Kessi-Pérez, E.I.; Salinas, F.; González, A.; Su, Y.; Guillamón, J.M.; Hall, M.N.; Larrondo, L.F.; Martínez, C. KAE1 Allelic Variants Affect TORC1 Activation and Fermentation Kinetics in Saccharomyces cerevisiae. Front. Microbiol. 2019, 10, 1686. [Google Scholar] [CrossRef]
- Ambroset, C.; Petit, M.; Christian, B.; Isabelle, S.; Delobel, P.; Guérin, C.; Chiapello, H.; Nicolas, P.; Bigey, F.; Dequin, S.; et al. Deciphering the Molecular Basis of Wine Yeast Fermentation Traits Using a Combined Genetic and Genomic Approach. G3 (Bethesda Md.) 2011, 1, 263–281. [Google Scholar] [CrossRef] [PubMed]
- Steyer, D.; Ambroset, C.; Brion, C.; Claudel, P.; Delobel, P.; Sanchez, I.; Erny, C.; Blondin, B.; Karst, F.; Legras, J.L. QTL mapping of the production of wine aroma compounds by yeast. BMC Genom. 2012, 13, 573. [Google Scholar] [CrossRef] [PubMed]
- Brice, C.; Sanchez, I.; Bigey, F.; Legras, J.L.; Blondin, B. A genetic approach of wine yeast fermentation capacity in nitrogen-starvation reveals the key role of nitrogen signaling. BMC Genom. 2014, 15, 495. [Google Scholar] [CrossRef] [PubMed]
- Ibstedt, S.; Stenberg, S.; Bagés, S.; Gjuvsland, A.; Salinas, F.; Kourtchenko, O.; Karloss, J.; Blomberg, A.; Omholt, S.; Liti, G.; et al. Concerted Evolution of Life Stage Performances Signals Recent Selection on Yeast Nitrogen Use. Mol. Biol. Evol. 2014, 32, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Jara, M.; Cubillos, F.A.; García, V.; Salinas, F.; Aguilera, O.; Liti, G.; Martínez, C. Mapping Genetic Variants Underlying Differences in the Central Nitrogen Metabolism in Fermenter Yeasts. PLoS ONE 2014, 9, e86533. [Google Scholar] [CrossRef] [PubMed]
- Alexandre, H.; Charpentier, C. Biochemical aspects of stuck and sluggish fermentation in grape must. J. Ind. Microbiol. Biotechnol. 1998, 20, 20–27. [Google Scholar] [CrossRef]
- Pretorius, I.S. Tailoring wine yeast for the new millennium: Novel approaches to the ancient art of winemaking. Yeast 2000, 16, 675–729. [Google Scholar] [CrossRef]
- Brice, C.; Cubillos, F.A.; Dequin, S.; Camarasa, C.; Martínez, C. Adaptability of the Saccharomyces cerevisiae yeasts to wine fermentation conditions relies on their strong ability to consume nitrogen. PLoS ONE 2018, 13, e0192383. [Google Scholar] [CrossRef]
- Cubillos, F.A.; Brice, C.; Molinet, J.; Tisné, S.; Abarca, V.; Tapia, S.M.; Oporto, C.; García, V.; Liti, G.; Martínez, C. Identification of Nitrogen Consumption Genetic Variants in Yeast Through QTL Mapping and Bulk Segregant RNA-Seq Analyses. G3 Genes Genomes Genet. 2017, 7, 1693–1705. [Google Scholar] [CrossRef]
- Salinas, F.; de Boer, C.G.; Abarca, V.; Garcia, V.; Cuevas, M.; Araos, S.; Larrondo, L.F.; Martinez, C.; Cubillos, F.A. Natural variation in non-coding regions underlying phenotypic diversity in budding yeast. Sci. Rep. 2016, 6, 21849. [Google Scholar] [CrossRef]
- Som, A. Causes, consequences and solutions of phylogenetic incongruence. Brief. Bioinform. 2015, 16, 536–548. [Google Scholar] [CrossRef] [PubMed]
- Hinchliff, C.; Smith, S.; Allman, J.; Burleigh, J.; Chaudhary, R.; Coghill, L.; Crandall, K.; Deng, J.B.; Drew, B.; Gazis, R.; et al. Synthesis of phylogeny and taxonomy into a comprehensive tree of life. Proc. Natl. Acad. Sci. USA 2015, 112, 12764–12769. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.A.; Brown, J.W. Constructing a broadly inclusive seed plant phylogeny. Am. J. Bot. 2018, 105, 302–314. [Google Scholar] [CrossRef] [PubMed]
- Fedosov, A.; Puillandre, N.; Herrmann, M.; Dgebuadze, P.; Bouchet, P. Phylogeny, systematics, and evolution of the family Costellariidae (Gastropoda: Neogastropoda). Zool. J. Linn. Soc. 2017, 179, 541–526. [Google Scholar]
- Moon, B.C. A new phylogeny of ichthyosaurs (Reptilia: Diapsida). J. Syst. Palaeontol. 2017, 17, 129–155. [Google Scholar] [CrossRef]
- Guzman, E.; Molina, J. The predictive utility of the plant phylogeny in identifying sources of cardiovascular drugs. Pharm. Biol. 2018, 56, 154–164. [Google Scholar] [CrossRef]
- Yang, Z.; Rannala, B. Molecular phylogenetics: Principles and practice. Nat. Rev. Genet. 2012, 13, 303–314. [Google Scholar] [CrossRef]
- Stamatakis, A. Phylogenetics: Applications, Software and Challenges. Cancer Genom. Proteom. 2005, 2, 301–305. [Google Scholar]
- Campbell, V.; Legendre, P.; Lapointe, F.J. The performance of the Congruence Among Distance Matrices (CADM) test in phylogenetic analysis. BMC Evol. Biol. 2011, 11, 64. [Google Scholar] [CrossRef]
- Garamszegi, L. Modern Phylogenetic Comparative Methods and Their Application in Evolutionary Biology: Concepts and Practice; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar] [CrossRef]
- Villalobos-Cid, M.; Dorn, M.; Ligabue-Braun, R.; Inostroza-Ponta, M. A Memetic Algorithm Based on an NSGA-II Scheme for Phylogenetic Tree Inference. IEEE Trans. Evol. Comput. 2019, 23, 776–787. [Google Scholar] [CrossRef]
- Molinet, J.; Cubillos, F.A.; Salinas, F.; Liti, G.; Martinez, C. Genetic variants of TORC1 signaling pathway affect nitrogen consumption in Saccharomyces cerevisiae during alcoholic fermentation. PLoS ONE 2019, 14, e0220515. [Google Scholar] [CrossRef] [PubMed]
- Bergström, A.; Simpson, J.; Salinas, F.; Barré, B.; Parts, L.; Zia, A.; Ba, A.; Moses, A.; Louis, E.; Mustonen, V.; et al. A High-Definition View of Functional Genetic Variation from Natural Yeast Genomes. Mol. Biol. Evol. 2014, 31, 872–888. [Google Scholar] [CrossRef] [PubMed]
- Knaus, B.J.; Grünwald, N.J. vcfr: A package to manipulate and visualize variant call format data in R. Mol. Ecol. Resour. 2017, 17, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Levasseur, C.; Lapointe, F.J. Total evidence, average consensus and matrix representation with parsimony: What a difference distances make. Evol. Bioinform. Online 2007, 2, 1–5. [Google Scholar] [CrossRef]
- McVay, J.D.; Carstens, B.C. Phylogenetic Model Choice: Justifying a Species Tree or Concatenation Analysis. J. Phylogenet. Evol. Biol. 2013, 1, 1–8. [Google Scholar]
- Schliep, K.P. Phangorn: Phylogenetic analysis in R. Bioinformatics 2010, 27, 592–593. [Google Scholar] [CrossRef]
- Gadagkar, S.; Rosenberg, M. Inferring Species Phylogenies From Multiple Genes: Concatenated Sequence Tree Versus Consensus Gene Tree. J. Exp. Zool. Part B Mol. Dev. Evol. 2005, 304, 64–74. [Google Scholar] [CrossRef]
- Robinson, D.; Foulds, L. Comparison of phylogenetic trees. Math. Biosci. 1981, 53, 131–147. [Google Scholar] [CrossRef]
- Jombart, T.; Kendall, M.; Almagro-Garcia, J.; Colijn, C. treespace: Statistical exploration of landscapes of phylogenetic trees. Mol. Ecol. Resour. 2017, 17, 1385–1392. [Google Scholar] [CrossRef]
- Kessi-Pérez, E.I.; Salinas, F.; Molinet, J.; González, A.; Muñiz, S.; Guillamón, J.M.; Hall, M.N.; Larrondo, L.F.; Martínez, C. Indirect monitoring of TORC1 signalling pathway reveals molecular diversity among different yeast strains. Yeast 2019, 36, 65–74. [Google Scholar] [CrossRef]
- De Leeuw, J.; Mair, P. Multidimensional Scaling using majorization: SMACOF in R. J. Stat. Softw. 2009, 31, 1. [Google Scholar] [CrossRef]
- Ramazzotti, M.; Berná, L.; Stefanini, I.; Cavalieri, D. A computational pipeline to discover highly phylogenetically informative genes in sequenced genomes: Application to Saccharomyces cerevisiae natural strains. Nucleic Acids Res. 2012, 40, 3834–3848. [Google Scholar] [CrossRef] [PubMed]


| Strain/Origin | N | TE | REF | OUT |
|---|---|---|---|---|
| Others | 1001 | 27 | 100 | 0 |
| Chile | 6 | 0 | 100 | 0 |
| Representatives of clean lineages | 4 | 100 | 100 | 0 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Villalobos-Cid, M.; Salinas, F.; Kessi-Pérez, E.I.; De Chiara, M.; Liti, G.; Inostroza-Ponta, M.; Martínez, C. Comparison of Phylogenetic Tree Topologies for Nitrogen Associated Genes Partially Reconstruct the Evolutionary History of Saccharomyces cerevisiae. Microorganisms 2020, 8, 32. https://doi.org/10.3390/microorganisms8010032
Villalobos-Cid M, Salinas F, Kessi-Pérez EI, De Chiara M, Liti G, Inostroza-Ponta M, Martínez C. Comparison of Phylogenetic Tree Topologies for Nitrogen Associated Genes Partially Reconstruct the Evolutionary History of Saccharomyces cerevisiae. Microorganisms. 2020; 8(1):32. https://doi.org/10.3390/microorganisms8010032
Chicago/Turabian StyleVillalobos-Cid, Manuel, Francisco Salinas, Eduardo I. Kessi-Pérez, Matteo De Chiara, Gianni Liti, Mario Inostroza-Ponta, and Claudio Martínez. 2020. "Comparison of Phylogenetic Tree Topologies for Nitrogen Associated Genes Partially Reconstruct the Evolutionary History of Saccharomyces cerevisiae" Microorganisms 8, no. 1: 32. https://doi.org/10.3390/microorganisms8010032
APA StyleVillalobos-Cid, M., Salinas, F., Kessi-Pérez, E. I., De Chiara, M., Liti, G., Inostroza-Ponta, M., & Martínez, C. (2020). Comparison of Phylogenetic Tree Topologies for Nitrogen Associated Genes Partially Reconstruct the Evolutionary History of Saccharomyces cerevisiae. Microorganisms, 8(1), 32. https://doi.org/10.3390/microorganisms8010032






