Silver Nanoparticles against Foodborne Bacteria. Effects at Intestinal Level and Health Limitations
Abstract
1. Introduction
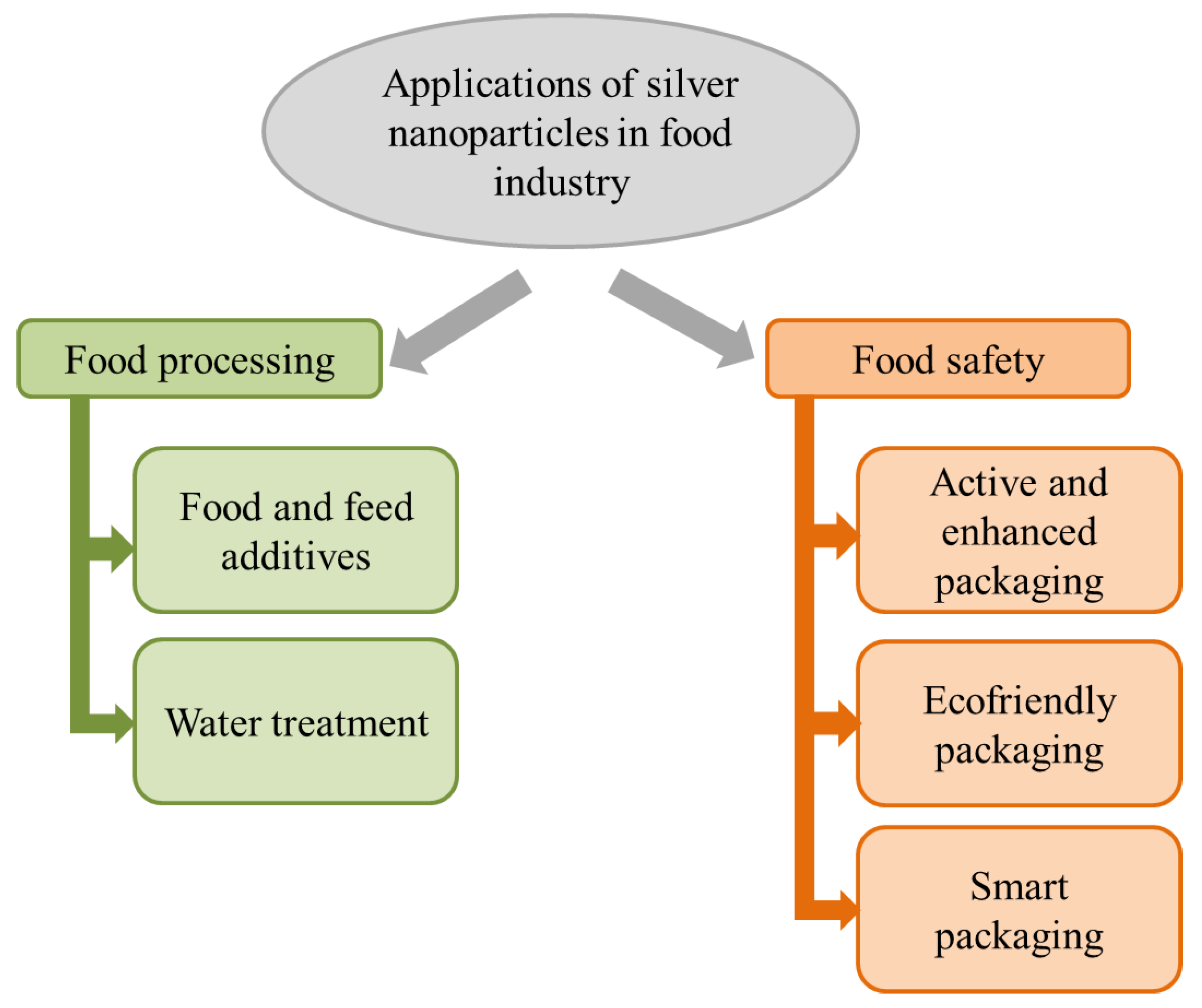
2. Applications of Antimicrobial Silver Nanoparticles in the Food Industry
2.1. Food Processing (Preservation)
2.2. Food Packaging (Safety)
2.3. Regulation about Silver Nanoparticles Use in Foods and Food Industry Packaging (Safety)
3. Impact of Dietary Exposure to Silver Nanoparticles in Health: Gut Nanotoxicology Effects
3.1. In Vitro Studies: Static and Dynamic Gut Simulators and Epithelium Cell Models
3.2. In Vivo Studies: Animal and Human Trials
4. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- WHO. Food Safety. 2019. Available online: https://www.who.int/news-room/fact-sheets/detail/food-safety (accessed on 1 October 2019).
- Bari, M.L.; Yeasmin, S. Chapter 8—Foodborne Diseases and Responsible Agents. In Food Safety and Preservation; Grumezescu, A.M., Holban, A.M., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 195–229. [Google Scholar]
- Malhotra, B.; Keshwani, A.; Kharkwal, H. Antimicrobial food packaging: Potential and pitfalls. Front. Microbiol. 2015, 6, 611. [Google Scholar] [CrossRef] [PubMed]
- Perez-Esteve, E.; Bernardos, A.; Martinez-Manez, R.; Barat, J.M. Nanotechnology in the development of novel functional foods or their package. An overview based in patent analysis. Recent Pat. Food Nutr. Agric. 2013, 5, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Monge, M.; Moreno-Arribas, M.V. Applications of Nanotechnology in Wine Production and Quality and Safety Control. In Wine: Safety, Consumer Preferences, and Health; Moreno-Arribas, M.V., Bartolomé, B., Eds.; Springer Life Sciences Publisher: New York, NY, USA, 2016; pp. 51–69. [Google Scholar]
- Rao, C.N.R.; Cheetham, A.K. Science and technology of nanomaterials: Current status and future prospects. J. Mater. Chem. 2001, 11, 2887–2894. [Google Scholar] [CrossRef]
- Edmundson, M.; Thanh, N.T.; Song, B. Nanoparticles based stem cell tracking in regenerative medicine. Theranostics 2013, 3, 573–582. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.P.; Silveira, A.P.; Bonatto, C.C.; Reis, I.G.; Milreu, P.V. Chapter 26—Silver Nanoparticles as Antimicrobial Agents: Past, Present, and Future. In Nanostructures for Antimicrobial Therapy; Ficai, A., Grumezescu, A.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 577–596. [Google Scholar]
- Rafique, M.; Sadaf, I.; Rafique, M.S.; Tahir, M.B. A review on green synthesis of silver nanoparticles and their applications. Artif. Cells Nanomed. Biotechnol. 2017, 45, 1272–1291. [Google Scholar] [CrossRef] [PubMed]
- Abbaszadegan, A.; Ghahramani, Y.; Gholami, A.; Hemmateenejad, B.; Dorostkar, S.; Nabavizadeh, M.; Sharghi, H. The Effect of Charge at the Surface of Silver Nanoparticles on Antimicrobial Activity against Gram-Positive and Gram-Negative Bacteria: A Preliminary Study. J. Nanomater. 2015, 2015, 8. [Google Scholar] [CrossRef]
- Jo, D.H.; Kim, J.H.; Lee, T.G.; Kim, J.H. Size, surface charge, and shape determine therapeutic effects of nanoparticles on brain and retinal diseases. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 1603–1611. [Google Scholar] [CrossRef]
- Gordienko, M.G.; Palchikova, V.V.; Kalenov, S.V.; Belov, A.A.; Lyasnikova, V.N.; Poberezhniy, D.Y.; Chibisova, A.V.; Sorokin, V.V.; Skladnev, D.A. Antimicrobial activity of silver salt and silver nanoparticles in different forms against microorganisms of different taxonomic groups. J. Hazard. Mater. 2019, 378, 120754. [Google Scholar] [CrossRef]
- Singh, J.; Dutta, T.; Kim, K.H.; Rawat, M.; Samddar, P.; Kumar, P. ‘Green’ synthesis of metals and their oxide nanoparticles: Applications for environmental remediation. J. Nanobiotechnol. 2018, 16, 84. [Google Scholar] [CrossRef]
- Ren, Y.Y.; Yang, H.; Wang, T.; Wang, C. Bio-synthesis of silver nanoparticles with antibacterial activity. Mater. Chem. Phys. 2019, 235, 121746. [Google Scholar] [CrossRef]
- Navarro Gallón, S.M.; Alpaslan, E.; Wang, M.; Larese-Casanova, P.; Londoño, M.E.; Atehortúa, L.; Pavón, J.J.; Webster, T.J. Characterization and study of the antibacterial mechanisms of silver nanoparticles prepared with microalgal exopolysaccharides. Mat. Sci. Eng. C Mater. 2019, 99, 685–695. [Google Scholar] [CrossRef] [PubMed]
- Pal, S.; Tak, Y.K.; Song, J.M. Does the antibacterial activity of silver nanoparticles depend on the shape of the nanoparticle? A study of the Gram-negative bacterium Escherichia coli. Appl. Environ. Microbiol. 2007, 73, 1712–1720. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, B.; Garmaroudi, F.S.; Hashemi, M.; Nezhad, H.R.; Nasrollahi, A.; Ardalan, S.; Ardalan, S. Comparison of the anti-bacterial activity on the nanosilver shapes: Nanoparticles, nanorods and nanoplates. Adv. Powder Technol. 2012, 23, 22–26. [Google Scholar] [CrossRef]
- Lu, W.; Yao, K.; Wang, J.; Yuan, J. Ionic liquids-water interfacial preparation of triangular Ag nanoplates and their shape-dependent antibacterial activity. J. Colloid Interf. Sci. 2015, 437, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Rong, K.; Li, J.; Yang, H.; Chen, R. Size-dependent antibacterial activities of silver nanoparticles against oral anaerobic pathogenic bacteria. J. Mater. Sci. Mater. Med. 2013, 24, 1465–1471. [Google Scholar] [CrossRef] [PubMed]
- Khurana, C.; Vala, A.K.; Andhariya, N.; Pandey, O.P.; Chudasama, B. Antibacterial activity of silver: The role of hydrodynamic particle size at nanoscale. J. Biomed. Mater. Res. A 2014, 102, 3361–3368. [Google Scholar] [CrossRef] [PubMed]
- Ivask, A.; Kurvet, I.; Kasemets, K.; Blinova, I.; Aruoja, V.; Suppi, S.; Vija, H.; Kakinen, A.; Titma, T.; Heinlaan, M.; et al. Size-dependent toxicity of silver nanoparticles to bacteria, yeast, algae, crustaceans and mammalian cells in vitro. PLoS ONE 2014, 9, e102108. [Google Scholar] [CrossRef]
- Qing, Y.; Cheng, L.; Li, R.; Liu, G.; Zhang, Y.; Tang, X.; Wang, J.; Liu, H.; Qin, Y. Potential antibacterial mechanism of silver nanoparticles and the optimization of orthopedic implants by advanced modification technologies. Int. J. Nanomed. 2018, 13, 3311–3327. [Google Scholar] [CrossRef]
- Duran, N.; Duran, M.; de Jesus, M.B.; Seabra, A.B.; Favaro, W.J.; Nakazato, G. Silver nanoparticles: A new view on mechanistic aspects on antimicrobial activity. Nanomed. Nanotechnol. 2016, 12, 789–799. [Google Scholar] [CrossRef]
- Gugala, N.; Lemire, J.; Chatfield-Reed, K.; Yan, Y.; Chua, G.; Turner, R.J. Using a chemical genetic screen to enhance our understanding of the antibacterial properties of silver. Genes 2016, 9, 344. [Google Scholar] [CrossRef]
- Dakal, T.C.; Kumar, A.; Majumdar, R.S.; Yadav, V. Mechanistic basis of antimicrobial actions of silver nanoparticles. Front. Microbiol. 2016, 7, 1831. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Patil, S.; Ahire, M.; Kitture, R.; Kale, S.; Pardesi, K.; Cameotra, S.S.; Bellare, J.; Dhavale, D.D.; Jabgunde, A.; et al. Synthesis of silver nanoparticles using Dioscorea bulbifera tuber extract and evaluation of its synergistic potential in combination with antimicrobial agents. Int. J. Nanomed. 2012, 7, 483–496. [Google Scholar]
- Yue, Z.G.; Wei, W.; Lv, P.P.; Yue, H.; Wang, L.Y.; Su, Z.G.; Ma, G.H. Surface charge affects cellular uptake and intracellular trafficking of chitosan-based nanoparticles. Biomacromolecules 2011, 12, 2440–2446. [Google Scholar] [CrossRef] [PubMed]
- Salas-Orozco, M.; Niño-Martínez, N.; Martínez-Castañón, G.; Torres Méndez, F.; Compean Jasso, M.E.; Ruiz, F. Mechanisms of resistance to silver nanoparticles in endodontic bacteria: A literature review. J. Nanomater. 2019, 2019, 11. [Google Scholar] [CrossRef]
- Zodrow, K.; Brunet, L.; Mahendra, S.; Li, D.; Zhang, A.; Li, Q.; Alvarez, P.J. Polysulfone ultrafiltration membranes impregnated with silver nanoparticles show improved biofouling resistance and virus removal. Water Res. 2009, 43, 715–723. [Google Scholar] [CrossRef] [PubMed]
- Dankovich, T.A.; Gray, D.G. Bactericidal paper impregnated with silver nanoparticles for point-of-use water treatment. Environ. Sci. Technol. 2011, 45, 1992–1998. [Google Scholar] [CrossRef] [PubMed]
- Kanmani, P.; Lim, S.T. Synthesis and structural characterization of silver nanoparticles using bacterial exopolysaccharide and its antimicrobial activity against food and multidrug resistant pathogens. Process Biochem. 2013, 48, 1099–1106. [Google Scholar] [CrossRef]
- Deus, D.; Kehrenberg, C.; Schaudien, D.; Klein, G.; Krischek, C. Effect of a nano-silver coating on the quality of fresh turkey meat during storage after modified atmosphere or vacuum packaging. Poultr. Sci. 2017, 96, 449–457. [Google Scholar] [CrossRef]
- Silvan, J.M.; Zorraquin-Pena, I.; Gonzalez de Llano, D.; Moreno-Arribas, M.V.; Martinez-Rodriguez, A.J. Antibacterial activity of glutathione-stabilized silver nanoparticles against Campylobacter multidrug-resistant strains. Front. Microbiol. 2018, 9, 458. [Google Scholar] [CrossRef]
- Wu, Z.; Huang, X.; Li, Y.C.; Xiao, H.; Wang, X. Novel chitosan films with laponite immobilized Ag nanoparticles for active food packaging. Carbohydr. Polym. 2018, 199, 210–218. [Google Scholar] [CrossRef]
- Chandhru, M.; Logesh, R.; Rani, S.K.; Ahmed, N.; Vasimalai, N. One-pot green route synthesis of silver nanoparticles from jack fruit seeds and their antibacterial activities with escherichia coli and salmonella bacteria. Biocatal. Agric. Biotechnol. 2019, 20, 101241. [Google Scholar] [CrossRef]
- Du, J.; Hu, Z.; Yu, Z.; Li, H.; Pan, J.; Zhao, D.; Bai, Y. Antibacterial activity of a novel Forsythia suspensa fruit mediated green silver nanoparticles against food-borne pathogens and mechanisms investigation. Mater. Sci. Eng. C 2019, 102, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Mathew, S.; Snigdha, S.; Mathew, J.; Radhakrishnan, E.K. Biodegradable and active nanocomposite pouches reinforced with silver nanoparticles for improved packaging of chicken sausages. Food Packag. Shelf Life 2019, 19, 155–166. [Google Scholar] [CrossRef]
- Yu, Z.; Wang, W.; Kong, F.; Lin, M.; Mustapha, A. Cellulose nanofibril/silver nanoparticle composite as an active food packaging system and its toxicity to human colon cells. Int. J. Biol. Macromol. 2019, 129, 887–894. [Google Scholar] [CrossRef]
- Singh, T.; Shukla, S.; Kumar, P.; Wahla, V.; Bajpai, V.K. Application of Nanotechnology in Food Science: Perception and Overview. Front. Microbiol. 2017, 8, 1501. [Google Scholar] [CrossRef]
- Fondevila, M.; Herrer, R.; Casallas, M.C.; Abecia, L.; Ducha, J.J. Silver nanoparticles as a potential antimicrobial additive for weaned pigs. Anim. Feed Sci. Tech. 2009, 150, 259–269. [Google Scholar] [CrossRef]
- Pineda, L.; Chwalibog, A.; Sawosz, E.; Lauridsen, C.; Engberg, R.; Elnif, J.; Hotowy, A.; Sawosz, F.; Gao, Y.; Ali, A.; et al. Effect of silver nanoparticles on growth performance, metabolism and microbial profile of broiler chickens. Arch. Anim. Nutr. 2012, 66, 416–429. [Google Scholar] [CrossRef]
- Elkloub, K.; El Moustafa, M.E.; Ghazalah, A.A.; Rehan, A. Effect of dietary nanosilver on broiler performance. Int. J. Poult. Sci. 2015, 14, 177–182. [Google Scholar] [CrossRef]
- Adegbeye, M.J.; Elghandour, M.M.M.Y.; Barbabosa-Pliego, A.; Monroy, J.C.; Mellado, M.; Ravi Kanth Reddy, P.; Salem, A.Z.M. Nanoparticles in Equine Nutrition: Mechanism of Action and Application as Feed Additives. J. Equine Vet. Sci. 2019, 78, 29–37. [Google Scholar] [CrossRef]
- Dalloul, R.A.; Lillehoj, H.S. Poultry coccidiosis: Recent advancements in control measures and vaccine development. Expert Rev. Vaccines 2006, 5, 143–163. [Google Scholar] [CrossRef]
- Chauke, N.; Siebrits, F.K. Evaluation of silver nanoparticles as a possible coccidiostat in broiler production. S. Afr. J. Anim. Sci. 2012, 42, 493–497. [Google Scholar] [CrossRef]
- Gherbawy, Y.A.; Shalaby, I.M.; El-Sadek, M.S.A.; Elhariry, H.M.; Abdelilah, B.A. The anti-fasciolasis properties of silver nanoparticles produced by Trichoderma harzianum and their improvement of the anti-fasciolasis drug triclabendazole. Int. J. Mol. Sci. 2013, 14, 21887–21898. [Google Scholar] [CrossRef] [PubMed]
- Izquierdo-Cañas, P.M.; García-Romero, E.; Huertas-Nebreda, B.; Gómez-Alonso, S. Colloidal silver complex as an alternative to sulphur dioxide in winemaking. Food Control 2012, 23, 73–81. [Google Scholar] [CrossRef]
- Garde-Cerdán, T.; López, R.; Garijo, P.; González-Arenzana, L.; Gutiérrez, A.R.; López-Alfaro, I.; Santamaría, P. Application of colloidal silver versus sulfur dioxide during vinification and storage of Tempranillo red wines. Aust. J. Grape Wine Res. 2014, 20, 51–61. [Google Scholar] [CrossRef]
- García-Ruiz, A.; Crespo, J.; López-de-Luzuriaga, J.M.; Olmos, M.E.; Monge, M.; Rodríguez-Álfaro, M.P.; Martín-Álvarez, P.J.; Bartolome, B.; Moreno-Arribas, M.V. Novel biocompatible silver nanoparticles for controlling the growth of lactic acid bacteria and acetic acid bacteria in wines. Food Control 2015, 50, 613–619. [Google Scholar] [CrossRef]
- Carbone, M.; Donia, D.T.; Sabbatella, G.; Antiochia, R. Silver nanoparticles in polymeric matrices for fresh food packaging. J. King Saud Univ. Sci. 2016, 28, 273–279. [Google Scholar] [CrossRef]
- Manso, S.; Cacho-Nerin, F.; Becerril, R.; Nerín, C. Combined analytical and microbiological tools to study the effect on Aspergillus flavus of cinnamon essential oil contained in food packaging. Food Control 2013, 30, 370–378. [Google Scholar] [CrossRef]
- Medina-Jaramillo, C.; Ochoa-Yepes, O.; Bernal, C.; Famá, L. Active and smart biodegradable packaging based on starch and natural extracts. Carbohydr. Polym. 2017, 176, 187–194. [Google Scholar] [CrossRef]
- Moreno, M.A.; Orqueda, M.E.; Gómez-Mascaraque, L.G.; Isla, M.I.; López-Rubio, A. Crosslinked electrospun zein-based food packaging coatings containing bioactive chilto fruit extracts. Food Hydrocoll. 2019, 95, 496–505. [Google Scholar] [CrossRef]
- Duncan, T.V. Applications of nanotechnology in food packaging and food safety: Barrier materials, antimicrobials and sensors. J. Colloid Interface Sci. 2011, 363, 1–24. [Google Scholar] [CrossRef]
- Tavakoli, H.; Rastegar, H.; Taherian, M.; Samadi, M.; Rostami, H. The effect of nano-silver packaging in increasing the shelf life of nuts: An in vitro model. Ital. J. Food Saf. 2017, 6, 6874. [Google Scholar] [CrossRef] [PubMed]
- Inbaraj, B.S.; Chen, B.H. Nanomaterial-based sensors for detection of foodborne bacterial pathogens and toxins as well as pork adulteration in meat products. J. Food Drug Anal. 2016, 24, 15–28. [Google Scholar] [CrossRef] [PubMed]
- Abbaspour, A.; Norouz-Sarvestani, F.; Noori, A.; Soltani, N. Aptamer-conjugated silver nanoparticles for electrochemical dual-aptamer-based sandwich detection of staphylococcus aureus. Biosens. Bioelectron. 2015, 68, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Roh, S.G.; Robby, A.I.; Phuong, P.T.M.; In, I.; Park, S.Y. Photoluminescence-tunable fluorescent carbon dots-deposited silver nanoparticle for detection and killing of bacteria. Mater. Sci. Eng. C 2019, 97, 613–623. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Cui, Q.; Yao, C.; Li, S.; Zhang, P.; Sun, H.; Lv, F.; Liu, L.; Li, L.; Wang, S. Conjugated Polyelectrolyte-Silver Nanostructure Pair for Detection and Killing of Bacteria. Adv. Mater. Technol. 2017, 2, 1700033. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA). Scientific opinion on the re-evaluation of silver (E 174) as food additive. EFSA J. 2016, 14, 4664. [Google Scholar]
- European Food Safety Authority (EFSA). Guidance on risk assessment of the application of nanoscience and nanotechnologies in the food and feed chain: Part 1, human and animal health. EFSA J. 2018, 16, 5327. [Google Scholar]
- Ávalos Fúnez, A.; Haza, A.; Morales, P. Nanotecnología en la industria alimentaria I: Aplicaciones. Rev. Complut. Cienc. Vet. 2016, 10, 1–17. [Google Scholar] [CrossRef][Green Version]
- Food and Drug Administration (FDA). Considering Whether an FDA-Regulated Product Involves the Application of Nanotechnology; FDA: Washington, DC, USA, 2014.
- Richa, S.; Dimple, S.C. Regulatory Approval of Silver Nanoparticles. Appl. Clin. Res. Clin. Trials Regul. Aff. 2018, 5, 74–79. [Google Scholar]
- Echegoyen, Y.; Nerín, C. Nanoparticle release from nano-silver antimicrobial food containers. Food Chem. Toxicol. 2013, 62, 16–22. [Google Scholar] [CrossRef]
- Cushen, M.; Kerry, J.; Morris, M.; Cruz-Romero, M.; Cummins, E. Evaluation and Simulation of Silver and Copper Nanoparticle Migration from Polyethylene Nanocomposites to Food and an Associated Exposure Assessment. J. Agric. Food Chem. 2014, 62, 1403–1411. [Google Scholar] [CrossRef] [PubMed]
- Gallocchio, F.; Cibin, V.; Biancotto, G.; Roccato, A.; Muzzolon, O.; Carmen, L.; Simone, B.; Manodori, L.; Fabrizi, A.; Patuzzi, I.; et al. Testing nano-silver food packaging to evaluate silver migration and food spoilage bacteria on chicken meat. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2016, 33, 1063–1071. [Google Scholar] [CrossRef] [PubMed]
- Mercier-Bonin, M.; Despax, B.; Raynaud, P.; Houdeau, E.; Thomas, M. Mucus and microbiota as emerging players in gut nanotoxicology: The example of dietary silver and titanium dioxide nanoparticles. Crit. Rev. Food Sci. Nutr. 2018, 8, 1023–1032. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Tang, M.; Xue, Y. Review of the effects of silver nanoparticle exposure on gut bacteria. J. Appl. Toxicol. 2019, 39, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Walczak, A.P.; Fokkink, R.; Peters, R.; Tromp, P.; Herrera Rivera, Z.E.; Rietjens, I.M.; Hendriksen, P.J.; Bouwmeester, H. Behaviour of silver nanoparticles and silver ions in an in vitro human gastrointestinal digestion model. Nanotoxicology 2013, 7, 1198–1210. [Google Scholar] [CrossRef]
- Bouwmeester, H.; van der Zande, M.; Jepson, M.A. Effects of food-borne nanomaterials on gastrointestinal tissues and microbiota. WIREs Nanomed. Nanobiotechnol. 2018, 10, e1481. [Google Scholar] [CrossRef]
- Bove, P.; Malvindi, M.A.; Kote, S.S.; Bertorelli, R.; Summa, M.; Sabella, S. Dissolution test for risk assessment of nanoparticles: A pilot study. Nanoscale 2017, 9, 6315–6326. [Google Scholar] [CrossRef]
- Gil-Sánchez, I.; Monge, M.; Miralles, B.; Armentia, G.; Cueva, C.; Crespo, J.; de Luzuriaga, J.M.L.; Olmos, M.E.; Bartolomé, B.; de Llano, D.G.; et al. Some new findings on the potential use of biocompatible silver nanoparticles in winemaking. Innov. Food Sci. Emerg. 2019, 51, 64–72. [Google Scholar] [CrossRef]
- Cueva, C.; Gil-Sánchez, I.; Ayuda-Durán, B.; González-Manzano, S.; González-Paramás, A.M.; Santos-Buelga, C.; Moreno-Arribas, M. An integrated view of the effects of wine polyphenols and their relevant metabolites on gut and host health. Molecules 2017, 22, 99. [Google Scholar] [CrossRef]
- Akter, M.; Sikder, M.T.; Rahman, M.M.; Ullah, A.K.M.A.; Hossain, K.F.B.; Banik, S.; Hosokawa, T.; Saito, T.; Kurasaki, M. A systematic review on silver nanoparticles-induced cytotoxicity: Physicochemical properties and perspectives. J. Adv. Res. 2018, 9, 1–16. [Google Scholar] [CrossRef]
- Verkhovskii, R.; Kozlova, A.; Atkin, V.; Kamyshinsky, R.; Shulgina, T.; Nechaeva, O. Physical properties and cytotoxicity of silver nanoparticles under different polymeric stabilizers. Heliyon 2019, 5, e01305. [Google Scholar] [CrossRef] [PubMed]
- Fahmy, H.M.; Mosleh, A.M.; Elghany, A.A.; Shams-Eldin, E.; Abu Serea, E.S.; Ali, S.A.; Shalan, A.E. Coated silver nanoparticles: Synthesis, cytotoxicity, and optical properties. RSC Adv. 2019, 9, 20118–20136. [Google Scholar] [CrossRef]
- Inkielewicz-Stepniak, I.; Santos-Martinez, M.J.; Medina, C.; Radomski, M.W. Pharmacological and toxicological effects of co-exposure of human gingival fibroblasts to silver nanoparticles and sodium fluoride. Int. J. Nanomed. 2014, 9, 1677–1687. [Google Scholar]
- Niska, K.; Knap, N.; Kedzia, A.; Jaskiewicz, M.; Kamysz, W.; Inkielewicz-Stepniak, I. Capping Agent-Dependent Toxicity and Antimicrobial Activity of Silver Nanoparticles: An In Vitro Study. Concerns about Potential Application in Dental Practice. Int. J. Med. Sci. 2016, 13, 772–782. [Google Scholar] [CrossRef]
- Hernandez-Sierra, J.F.; Galicia-Cruz, O.; Angelica, S.A.; Ruiz, F.; Pierdant-Perez, M.; Pozos-Guillen, A.J. In vitro cytotoxicity of silver nanoparticles on human periodontal fibroblasts. J. Clin. Pediatr. Dent. 2011, 36, 37–41. [Google Scholar] [CrossRef]
- Tang, X.; Li, L.; Meng, X.; Liu, T.; Hu, Q.; Miao, L. Cytotoxicity of Silver Nanoparticles on Human Periodontal Ligament Fibroblasts. Nanosci. Nanotechnol. Lett. 2017, 9, 1015–1022. [Google Scholar] [CrossRef]
- Panpaliya, N.P.; Dahake, P.T.; Kale, Y.J.; Dadpe, M.V.; Kendre, S.B.; Siddiqi, A.G.; Maggavi, U.R. In vitro evaluation of antimicrobial property of silver nanoparticles and chlorhexidine against five different oral pathogenic bacteria. Saudi Dent. J. 2019, 31, 76–83. [Google Scholar] [CrossRef]
- Vargas-Reus, M.A.; Memarzadeh, K.; Huang, J.; Ren, G.G.; Allaker, R.P. Antimicrobial activity of nanoparticulate metal oxides against peri-implantitis pathogens. Int. J. Antimicrob. Agents 2012, 40, 135–139. [Google Scholar] [CrossRef]
- Lichtenstein, D.; Ebmeyer, J.; Knappe, P.; Juling, S.; Bohmert, L.; Selve, S.; Niemann, B.; Braeuning, A.; Thunemann, A.F.; Lampen, A. Impact of food components during in vitro digestion of silver nanoparticles on cellular uptake and cytotoxicity in intestinal cells. Biol. Chem. 2015, 396, 1255–1264. [Google Scholar] [CrossRef]
- Pinďáková, L.; Kašpárková, V.; Kejlová, K.; Dvořáková, M.; Krsek, D.; Jírová, D.; Kašparová, L. Behaviour of silver nanoparticles in simulated saliva and gastrointestinal fluids. Int. J. Pharm. 2017, 527, 12–20. [Google Scholar] [CrossRef]
- Gurunathan, S.; Qasim, M.; Park, C.; Yoo, H.; Kim, J.H.; Hong, K. Cytotoxic Potential and Molecular Pathway Analysis of Silver Nanoparticles in Human Colon Cancer Cells HCT116. Int. J. Mol. Sci. 2018, 19, 2269. [Google Scholar] [CrossRef] [PubMed]
- Vila, L.; García-Rodríguez, A.; Cortés, C.; Marcos, R.; Hernández, A. Assessing the effects of silver nanoparticles on monolayers of differentiated Caco-2 cells, as a model of intestinal barrier. Food Chem. Toxicol. 2018, 116, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Abdelkhaliq, A.; van der Zande, M.; Undas, A.K.; Peters, R.J.B.; Bouwmeester, H. Impact of in vitro digestion on gastrointestinal fate and uptake of silver nanoparticles with different surface modifications. Nanotoxicology 2019, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Trickler, W.J.; Lantz, S.M.; Murdock, R.C.; Schrand, A.M.; Robinson, B.L.; Newport, G.D.; Schlager, J.J.; Oldenburg, S.J.; Paule, M.G.; Slikker, W., Jr.; et al. Silver nanoparticle induced blood-brain barrier inflammation and increased permeability in primary rat brain microvessel endothelial cells. Toxicol. Sci. 2010, 118, 160–170. [Google Scholar] [CrossRef] [PubMed]
- Çiftçi, H.; Türk, M.; Tamer, U.; Karahan, S.; Menemen, Y. Silver nanoparticles: Cytotoxic, apoptotic, and necrotic effects on MCF-7 cells. Turk. J. Biol. 2013, 37, 573–581. [Google Scholar] [CrossRef]
- Jiao, Z.H.; Li, M.; Feng, Y.X.; Shi, J.C.; Zhang, J.; Shao, B. Hormesis effects of silver nanoparticles at non-cytotoxic doses to human hepatoma cells. PLoS ONE 2014, 9, e102564. [Google Scholar] [CrossRef] [PubMed]
- Khorrami, S.; Zarrabi, A.; Khaleghi, M.; Danaei, M.; Mozafari, M.R. Selective cytotoxicity of green synthesized silver nanoparticles against the MCF-7 tumor cell line and their enhanced antioxidant and antimicrobial properties. Int. J. Nanomed. 2018, 13, 8013–8024. [Google Scholar] [CrossRef]
- Chernousova, S.; Epple, M. Silver as antibacterial agent: Ion, nanoparticle, and metal. Angew. Chem. Int. Ed. 2013, 52, 1636–1653. [Google Scholar] [CrossRef]
- Yuan, Y.G.; Zhang, S.; Hwang, J.Y.; Kong, I.K. Silver Nanoparticles Potentiates Cytotoxicity and Apoptotic Potential of Camptothecin in Human Cervical Cancer Cells. Oxid. Med. Cell. Longev. 2018, 2018, 6121328. [Google Scholar] [CrossRef]
- Sharma, H.S.; Ali, S.F.; Hussain, S.M.; Schlager, J.J.; Sharma, A. Influence of engineered nanoparticles from metals on the blood-brain barrier permeability, cerebral blood flow, brain edema and neurotoxicity. An experimental study in the rat and mice using biochemical and morphological approaches. J. Nanosci. Nanotechnol. 2009, 9, 5055–5072. [Google Scholar] [CrossRef]
- Mwilu, S.K.; El Badawy, A.M.; Bradham, K.; Nelson, C.; Thomas, D.; Scheckel, K.G.; Tolaymat, T.; Ma, L.; Rogers, K.R. Changes in silver nanoparticles exposed to human synthetic stomach fluid: Effects of particle size and surface chemistry. Sci. Total Environ. 2013, 447, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Marchioni, M.; Jouneau, P.H.; Chevallet, M.; Michaud-Soret, I.; Deniaud, A. Silver nanoparticle fate in mammals: Bridging in vitro and in vivo studies. Coord. Chem. Rev. 2018, 364, 118–136. [Google Scholar] [CrossRef]
- Cueva, C.; Gil-Sánchez, I.; Tamargo, A.; Miralles, B.; Crespo, J.; Bartolomé, B.; Moreno-Arribas, M.V. Gastrointestinal digestion of food-use silver nanoparticles in the dynamic SIMulator of the GastroIntestinal tract (simgi®). Impact on human gut microbiota. Food Chem. Toxicol. 2019, 132, 110657. [Google Scholar] [CrossRef] [PubMed]
- Das, P.; McDonald, J.; Petrof, E.; Allen-Vercoe, E.; Walker, V. Nanosilver-mediated change in human intestinal microbiota. J. Nanomed. Nanotechnol. 2014, 5, 1. [Google Scholar]
- Cattò, C.; Garuglieri, E.; Borruso, L.; Erba, D.; Casiraghi, M.C.; Cappitelli, F.; Villa, F.; Zecchin, S.; Zanchi, R. Impacts of dietary silver nanoparticles and probiotic administration on the microbiota of an in-vitro gut model. Environ. Pollut. 2019, 245, 754–763. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.F.; Wang, J.; Patterson, T.A.; Saini, U.T.; Robinson, B.L.; Newport, G.D.; Murdock, R.C.; Schlager, J.J.; Hussain, S.M.; Ali, S.F. Expression of genes related to oxidative stress in the mouse brain after exposure to silver-25 nanoparticles. Toxicol. Lett. 2009, 187, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Jeong, G.N.; Jo, U.B.; Ryu, H.Y.; Kim, Y.S.; Song, K.S.; Yu, I.J. Histochemical study of intestinal mucins after administration of silver nanoparticles in Sprague-Dawley rats. Arch. Toxicol. 2010, 84, 63–69. [Google Scholar] [CrossRef]
- Kim, Y.S.; Song, M.Y.; Park, J.D.; Song, K.S.; Ryu, H.R.; Chung, Y.H.; Chang, H.K.; Lee, J.H.; Oh, K.H.; Kelman, B.J.; et al. Subchronic oral toxicity of silver nanoparticles. Part. Fibre Toxicol. 2010, 7, 20. [Google Scholar] [CrossRef]
- Shahare, B.; Yashpal, M. Toxic effects of repeated oral exposure of silver nanoparticles on small intestine mucosa of mice. Toxicol. Mech. Method 2013, 23, 161–167. [Google Scholar] [CrossRef]
- Skalska, J.; Frontczak-Baniewicz, M.; Strużyńska, L. Synaptic degeneration in rat brain after prolonged oral exposure to silver nanoparticles. NeuroToxicology 2015, 46, 145–154. [Google Scholar] [CrossRef]
- Xu, L.; Shao, A.; Zhao, Y.; Wang, Z.; Zhang, C.; Sun, Y.; Deng, J.; Chou, L.L. Neurotoxicity of Silver Nanoparticles in Rat Brain After Intragastric Exposure. J. Nanosci. Nanotechnol. 2015, 15, 4215–4223. [Google Scholar] [CrossRef]
- Wilding, L.A.; Bassis, C.M.; Walacavage, K.; Hashway, S.; Leroueil, P.R.; Morishita, M.; Maynard, A.D.; Philbert, M.A.; Bergin, I.L. Repeated dose (28-day) administration of silver nanoparticles of varied size and coating does not significantly alter the indigenous murine gut microbiome. Nanotoxicology 2016, 10, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Williams, K.; Milner, J.; Boudreau, M.D.; Gokulan, K.; Cerniglia, C.E.; Khare, S. Effects of subchronic exposure of silver nanoparticles on intestinal microbiota and gut-associated immune responses in the ileum of Sprague-Dawley rats. Nanotoxicology 2015, 9, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Hendrickson, O.D.; Klochkov, S.G.; Novikova, O.V.; Bravova, I.M.; Shevtsova, E.F.; Safenkova, I.V.; Zherdev, A.V.; Bachurin, S.O.; Dzantiev, B.B. Toxicity of nanosilver in intragastric studies: Biodistribution and metabolic effects. Toxicol. Lett. 2016, 241, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Siczek, K.; Zatorski, H.; Chmielowiec-Korzeniowska, A.; Pulit-Prociak, J.; Smiech, M.; Kordek, R.; Tymczyna, L.; Banach, M.; Fichna, J. Synthesis and evaluation of anti-inflammatory properties of silver nanoparticle suspensions in experimental colitis in mice. Chem. Biol. Drug Des. 2017, 89, 538–547. [Google Scholar] [CrossRef] [PubMed]
- van den Brule, S.; Ambroise, J.; Lecloux, H.; Levard, C.; Soulas, R.; de Temmerman, P.; Palmai-Pallag, M.; Marbaix, E.; Lison, D. Dietary silver nanoparticles can disturb the gut microbiota in mice. Part. Fibre Toxicol. 2016, 13, 38. [Google Scholar] [CrossRef]
- Bacchetta, C.; Ale, A.; Simoniello, M.F.; Gervasio, S.; Davico, C.; Rossi, A.S.; Desimone, M.F.; Poletta, G.; López, G.; Monserrat, J.M.; et al. Genotoxicity and oxidative stress in fish after a short-term exposure to silver nanoparticles. Ecol. Indic. 2017, 76, 230–239. [Google Scholar] [CrossRef]
- Merrifield, D.; Shaw, B.; Harper, G.; Saoud, I.; Davies, S.; Handy, R.; Henry, T. Ingestion of metal-nanoparticle contaminated food disrupts endogenous microbiota in zebrafish (Danio rerio). Environ. Pollut. 2012, 174, 157–163. [Google Scholar] [CrossRef]
- Liu, H.; Wang, X.; Wu, Y.; Hou, J.; Zhang, S.; Zhou, N.; Wang, X. Toxicity responses of different organs of zebrafish (Danio rerio) to silver nanoparticles with different particle sizes and surface coatings. Environ. Pollut. 2019, 246, 414–422. [Google Scholar] [CrossRef]
- Liu, X.; Dumitrescu, E.; Kumar, A.; Austin, D.; Goia, D.; Wallace, K.N.; Andreescu, S. Differential lethal and sublethal effects in embryonic zebrafish exposed to different sizes of silver nanoparticles. Environ. Pollut. 2019, 248, 627–634. [Google Scholar] [CrossRef]
- Schultz, C.L.; Wamucho, A.; Tsyusko, O.V.; Unrine, J.M.; Crossley, A.; Svendsen, C.; Spurgeon, D.J. Multigenerational exposure to silver ions and silver nanoparticles reveals heightened sensitivity and epigenetic memory in Caenorhabditis elegans. Proc. Biol. Sci. 2016, 283, 20152911. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Xu, G.; Xu, S.; Chen, S.; Xu, A.; Wu, L. Effect of ionic strength on bioaccumulation and toxicity of silver nanoparticles in Caenorhabditis elegans. Ecotoxicol. Environ. Saf. 2018, 165, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.; Kwak, J.I.; An, Y.J. The effects of silver nanomaterial shape and size on toxicity to Caenorhabditis elegans in soil media. Chemosphere 2019, 215, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Wamucho, A.; Unrine, J.M.; Kieran, T.J.; Glenn, T.C.; Schultz, C.L.; Farman, M.; Svendsen, C.; Spurgeon, D.J.; Tsyusko, O.V. Genomic mutations after multigenerational exposure of Caenorhabditis elegans to pristine and sulfidized silver nanoparticles. Environ. Pollut. 2019, 254, 113078. [Google Scholar] [CrossRef]
- Panacek, A.; Prucek, R.; Safarova, D.; Dittrich, M.; Richtrova, J.; Benickova, K.; Zboril, R.; Kvitek, L. Acute and chronic toxicity effects of silver nanoparticles (NPs) on Drosophila melanogaster. Environ. Sci. Technol. 2011, 45, 4974–4979. [Google Scholar] [CrossRef]
- Araj, S.E.A.; Salem, N.M.; Ghabeish, I.H.; Awwad, A.M. Toxicity of Nanoparticles against Drosophila melanogaster (Diptera: Drosophilidae). J. Nanomater. 2015, 2015, 9. [Google Scholar] [CrossRef]
- Raj, A.; Shah, P.; Agrawal, N. Dose-dependent effect of silver nanoparticles (AgNPs) on fertility and survival of Drosophila: An in-vivo study. PLoS ONE 2017, 12, e0178051. [Google Scholar] [CrossRef]
- Alaraby, M.; Romero, S.; Hernández, A.; Marcos, R. Toxic and Genotoxic Effects of Silver Nanoparticles in Drosophila. Environ. Mol. Mutagen. 2019, 60, 277–285. [Google Scholar] [CrossRef]
- Munger, M.A.; Radwanski, P.; Hadlock, G.C.; Stoddard, G.; Shaaban, A.; Falconer, J.; Grainger, D.W.; Deering-Rice, C.E. In vivo human time-exposure study of orally dosed commercial silver nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2014, 10, 1–9. [Google Scholar] [CrossRef]
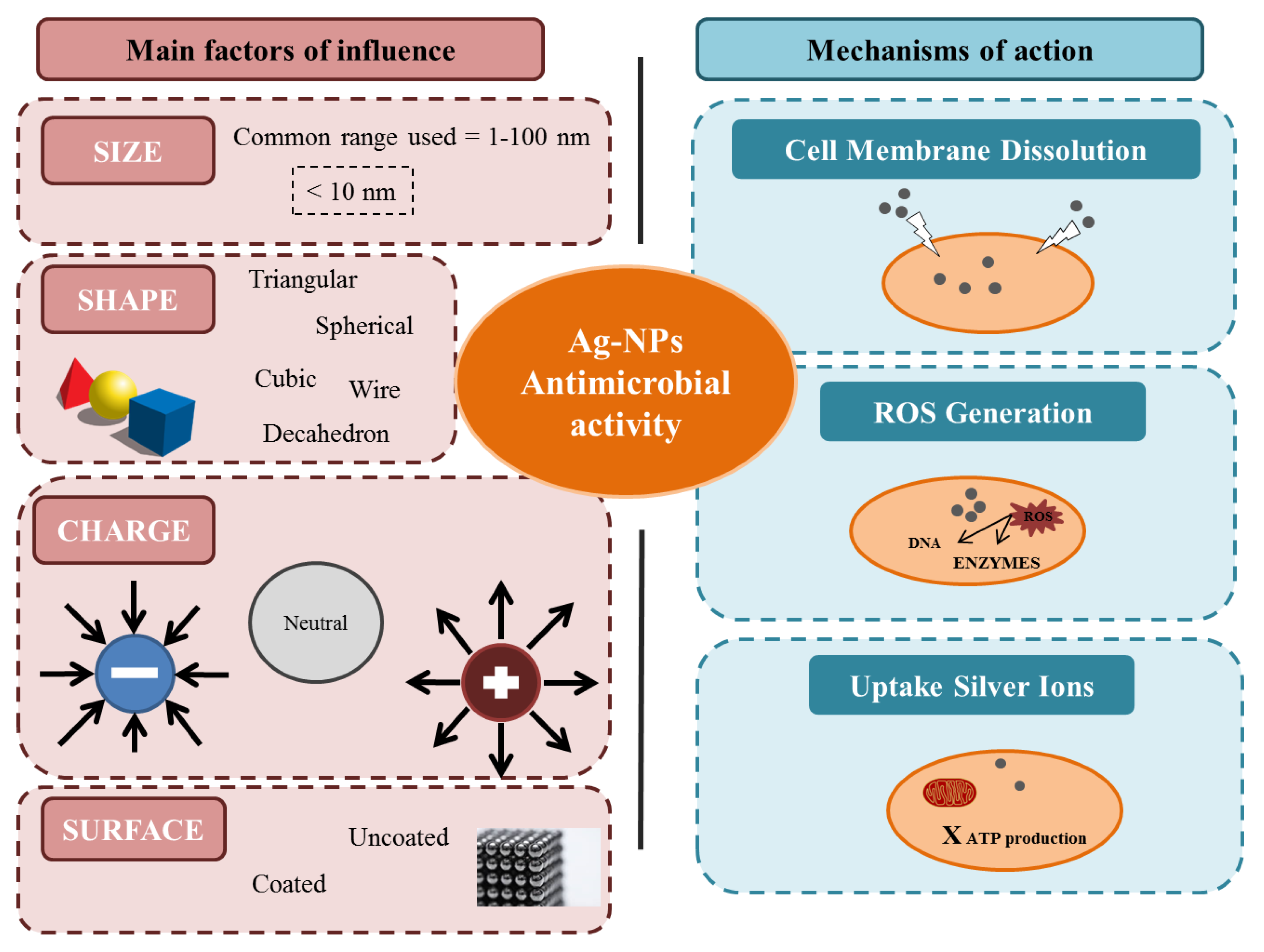
| Ag-NPs Size | Ag-NPs Concentration | Gram (-) Pathogens | Gram (+) Pathogens | Yeast/Fungus | Main Results | Reference |
|---|---|---|---|---|---|---|
| - | 0.034 μg Ag/mL | Escherichia coli K12 | - | - | 2 log reduction of E. coli after membrane filtration. | [29] |
| ≈ 7 nm and 27.5 nm | 0.26–26.5 mg Ag/dry g paper | Escherichia coli | Enterococcus faecalis | - | After filtration, the paper with a higher content of Ag-NPs almost completely deactivated bacterial growth. Reductions of 7 and 3 log were produced for E. coli and E. faecalis, respectively. | [30] |
| 75 nm (spherical) and 8–20 nm (triangular) | - | Escherichia coli, Pseudomonas aeruginosa, Salmonella typhi, Acinetobacter baumannii, Enterobacter cloacae, Haemophilus influenzae, Klebsiella pneumoniae, Neisseria mucosa, Proteus mirabilis, Serratia odorifera, Vibrio parahaemolyticus and Paenibacillus koreensis | Staphylococcus aureus, Bacillus subtilis and Paenibacillus koreensis | - | The highest antimicrobial activity of the Ag-NPs was against E. coli and P. aeruginosa. For S. typhi and B. subtilis this activity was moderate and low for S. aureus. | [26] |
| 14.6 nm | 0.2, 0.5, 1, 1.5, 2 mg/mL | Escherichia coli, Pseudomonas aeruginosa and Klebsiella pneumoniae | Lactobacillus rhamnosus GG, Bacillus cereus and Listeria monocytogenes | Aspergillus and Penicillium | Inhibition of bacterial growth was dose dependent. P. aeruginosa was the bacteria most sensitive to Ag-NPs, followed by E. coli. On the contrary L. monocytogenes was the most resistant. | [31] |
| 10, 20, 40, 60 and 80 nm | 8 µg Ag/mL (10 nm), 11 µg Ag/mL (20 nm), 5 µg Ag/mL (40, 60 and 80 nm) | Escherichia coli and Pseudomonas fluorescens | - | Saccharomyces cerevisiae | Nanoparticles of a size equal to or less than 10 nm were more bioavailable when interacting with the cells. It was also shown that the toxicity of Ag-NPs decreased with increasing size. | [21] |
| 8 nm (59 and 83 nm hydrodynamic size) | 0–400 µg Ag/mL | Proteus vulgaris and Shigella sonnei | Staphylococcus aureus, Bacillus megaterium | - | The smaller size of Ag-NPs produced a greater growth inhibition. For both sizes the MIC values for the bacteria were between 75–400 ug/mL. | [20] |
| - | 4.5 μg Ag/g film | Pseudomonas and Enterobacteriaceae | - | - | No significant differences were observed in the use of the film with nanoparticles compared to the conventional film. | [32] |
| 10–50 nm | 197 µg Ag/mL | Campylobacter jejuni (collection strain and isolates of patients and food chain) | - | - | The concentrations between 9.85 and 39.4 μg/mL were bactericidal after 24 h of incubation. In addition, the lower concentrations (1.23 and 4.92 μg/mL) significantly inhibited the growth of the collection strain. | [33] |
| - | - | Escherichia coli | Staphylococcus aureus | Aspergillus niger and Penicillium citrinum | The antimicrobial activity of the chitosan, laponite and Ag-NPs hybrid film turned out to be slightly less than the chitosan film because laponite decreases the release of silver. There was also a greater inhibition of gram-positive bacteria compared to gram-negative bacteria. | [34] |
| 20–30 nm | 2.37, 4.75, 9.5 and 19 μg Ag/mL | Escherichia coli and Salmonella typhimurium | - | - | The concentration of 4.75 μg/mL Ag-NPs completely inhibited the growth of the two bacteria and the concentration of 9.5 μg/mL was sufficient to kill them. | [35] |
| 47.3 nm | 0–100 μg Ag/mL | Escherichia coli O157:H7, Vibrio parahaemolyticu, Pseudomonas aeruginosa and Salmonella typhimurium | Listeria monocytogenes and Staphylococcus aureus | - | Ag-NPs exerted a strong antimicrobial activity against all the pathogens tested. MIC of V. parahaemolyticus and S. aureus were 6.25 μg/mL and 50 μg/mL, respectively, and MBCs of V. parahaemolyticus and S. aureus were 12.5 μg/mL and 100 μg/mL, respectively. | [36] |
| 6–25 nm (chemical synthesis) 80–120 nm and 40–100 nm (synthesized with Fusarium nivale and Penicillium glabrum) | 170 µg Ag/mL | Pseudomonas aeruginosa PA01 4/4–15 | Bacillus cereus B 504T UNIQEM, Staphylococcus aureus 209p | Fusarium oxysporum | Chemically synthesized AG-NPs inhibited microbial growth at 6 h of exposure, while with microbiologically synthesized nanoparticles it occurred at 24 h. S. aureus was the most resistant microorganism to both types of Ag-NPs. | [12] |
| 5–15 nm | 0.5, 1.0, 2.5, 5.0, 7.5, 10.0, 20.0 and 30.0 μg Ag/mL | Escherichia coli | Staphylococcus aureus and methicillin-resistant Staphylococcus aureus | - | The nanoparticles produced a total inhibition of E. coli growth at the concentration of 7.5 μg/mL. On the contrary, a concentration of >30 μg/mL is required for the complete inhibition of S. aureus and the resistant strain. | [15] |
| 10–20 nm | 8.34 × 10−7, 3.61 × 10−6, 5.79 × 10−5 and 4.63 × 10−4 mol/L | Escherichia coli | Staphylococcus aureus | - | Ag-NPs exerted a higher antimicrobial activity than the AgNO3 solution. This activity was concentration dependent and greater than other studies in which they use green synthesis due to their small size and spherical shape. | [14] |
| - | - | Salmonella typhimurium | Staphylococcus aureus | - | The film that generated Ag-NPs in situ exerted a clear antimicrobial activity against both pathogens. A lower microbial growth was also observed when using this material to store chicken sausages for 4 days at 4 °C compared to the traditional film. | [37] |
| 8–15 nm | 30, 75, 150, and 300 μg Ag/mL | Escherichia coli O157:H7 | Listeria monocytogenes | - | The material containing Ag-NPs exerted a greater antimicrobial activity against E. coli than against L. monocytogenes due to the greater wall thickness of the gram-positive bacteria. | [38] |
| Cell Line | Ag-NPs Size | Main Results | Reference |
|---|---|---|---|
| Periodontal fibroblasts extracted from volunteers | <10 nm, 15–20 nm, and 80–100 nm | Small-sized Ag-NPs (<20 nm) increased cytotoxicity in cells in a dose and time dependent manner. | [80] |
| Human gingival fibroblast (CRL-2014) | 2 nm | Ag-NPs increased oxidative stress, inflammation and cell apoptosis. | [78] |
| Human gingival fibroblasts (HGF-1) | 10 nm | All the nanoparticles tested were less toxic and exerted a greater antimicrobial action than the silver nitrate solution. | [79] |
| Human periodontal fibroblasts (HPLF) | - | Ag-NPs at low concentration did not alter morphology or cell proliferation, while at high concentration they significantly altered morphology, inhibited proliferation, and stopped cell cycle. | [81] |
| Human colon epithelial cells (Caco-2) | - | There were no significant differences in cell viability between digested and undigested nanoparticles up to a concentration of 40 μg/mL. There was a viability reduction (65%) when adding a food matrix. | [84] |
| EpiIntestinal, EpiOral and EpiGinvival tissues | 16 nm in average with sporadic occurrence of particles with a size of around 80 nm | Ag-NPs did not affect the viability of EpiOral and EpiGingival tissues. In addition, the release of IL-1 decreased significantly in EpiOral tissue. On the other hand, exposure of the EpiIntestinal tissue to gastric fluids with or without AG-NPs produced a slight decrease in viability. | [85] |
| Human colon epithelial cells (HT-28 and HCT-116) | 6 nm | After 24 h of exposure with Ag-NPS, a decrease in dose-dependent cell viability was observed (2–10 µg/mL). A cytotoxicity of approximately 50% was reached at a concentration of 4 µg/mL. | [86] |
| Human colon epithelial cells (HT-29 and Caco-2) and colon regular cells (CCD-18) | 10–50 nm | Cytotoxicity occurred in the cells at a concentration of Ag-NPs between 9.85 and 39.4 μg/mL. | [33] |
| Human colon epithelial cells (Caco-2) | ≈7.74 nm | In this work, there was no significant decrease in cell viability after 24 h at a concentration of 100 μg/mL. | [87] |
| Human colon epithelial cells (Caco-2/HT-29-MTX) | 51–52 nm | Cellular uptake decreased when using digested versus undigested Ag-NPs and the nanoparticles coated with lipolic acid dissolved to a greater extent than those coated with citrate. | [88] |
| Human colon epithelial cells (Caco-2) | 5–25 nm for PEG-AgNPs 20; 4–6 nm and 10–50 nm for GSH-AgNPs | A significant decrease in cell viability was observed by exposing cells to digested nanoparticles (both coatings), but not to undigested nanoparticles. | [74] |
| Rat brain microvessel endothelial cells (rBMEC) | 25, 50 and 80 nm | Ag-NPs were more cytotoxic at lower concentrations for a size of 25 and 40 nm. On the contrary, for a size of 80 nm greater concentrations were needed. | [89] |
| Human breast epithelial cells (MCF-7) | 20–80 nm | Ag-NPs caused apoptosis and necrosis in a dose-dependent manner to a concentration of 80 μg/mL. At higher concentrations, the apoptotic effect decreased while the necrotic effect became prominent. | [90] |
| Human liver epithelial cells (HepG2) | 10 and 100 nm | Ag-NPs at low doses increased cell proliferation. | [91] |
| Human breast epithelial cells (MCF-7) | 31.4 nm | Ag-NPs at a concentration of 60 µg/mL exhibited a cytotoxicity of 70% against the cell line. It was also observed that AgNP were much less cytotoxic when tested against a non-cancerous cell line. | [92] |
| Human dermal fibroblast (NHDF) | 20–45 nm | Except for the sodium oleate and sodium dodecyl sulfate solutions, the rest prevented the aggregation of the nanoparticles, stabilized them and did not produce a significant cytotoxic effect on the cells. | [76] |
| Static/Dynamic | Particle Size | Main Results | Reference |
|---|---|---|---|
| Static | Ag-NPs 10–50 nm | The range of MIC and MBC for oral bacteria was between 100 and 250 µg/mL. Of the four oral bacteria tested, the most sensitive to silver nanoparticles were Porphyromonas gingivalis and Fusobacterium nucleatum. | [83] |
| Static | Ag-NPs 5, 15 and 55 nm | In this work it was observed that for the smaller nanoparticles the MIC was between 25 and 50 µg/mL. Oral aerobic bacteria were more susceptible than anaerobic bacteria. | [19] |
| Static | Ag-NPs 30–50 nm | A MIC between 15 and 90 µg/mL was reported for the exposure of Ag-NPs against 5 oral pathogens, much lower than for chlorhexidine. | [82] |
| Static | Ag-NPs 60 nm | AG-NPs of a size of 60 nm digested under physiological conditions can reach the wall of the intestine. It was also observed that after ingestion of Ag + ions nanoparticles ended up forming. | [71] |
| Static | Ag-NPs 10 and 75 nm | After the intake of Ag-NPs, these nanoparticles can be aggregated and chemically modified in the stomach depending on the size and surface chemistries. | [96] |
| Static | Ag-NPs 10 nm | There was a reduction in the production of capric and stearic fatty acids after exposure of the human feces sample to Ag-NPs, while palmitic acid increased. The presence of Bacteroidetes was also drastically reduced. | [99] |
| Static | 16 nm in average with sporadic occurrence of particles with a size of around 80 nm | The size and morphology of the Ag-NPs changed due to the action of different gastric fluids and digestive enzymes. The study showed that nanoparticles agglomerate and partially react to form AgCl during exposure to fluids. | [85] |
| Static | Ag-NPs 14 nm | A decrease in Bacteroidetes and an increase in Firmicutes was observed, resulting in an alteration of the Firmicutes/Bacteroidetes ratio. Exposure with Ag-NPs for 24 h also altered the Faecalibacterium prausnitzii and Clostridium coccoides/Eubacterium rectal taxa. | [100] |
| Static | 5–25 nm for PEG-AgNPs 20; 4–6 nm and 10–50 nm for GSH-AgNPs | AgNPs agglomerated less and were less toxic in colon cells than PEG-AgNPs 20. | [74] |
| Dynamic | Ag-NPs 15 and 40 nm | It was observed that 90% of the silver nanoparticles had dissolved as they passed through the stomach and the resulting ions joined the digestive matrices. | [73] |
| Dynamic SIMulator of the GastroIntestinal tract (simgi®) | 3–5 nm and 10–25 nm for PEG-AgNPs 20; 4–6 nm and 10–50 nm for GSH-AgNPs | Ingestion of Ag-NPs did not alter the microbial composition of the intestine or the metabolic activity of the bacteria. It was also observed how during the digestion the nanoparticle size was predominantly 3–5 nm, although small populations of agglomerates of these small nanoparticles were found. | [98] |
| Model | Study Design | Main Results | Reference |
|---|---|---|---|
| C57BL/6N mice | Ag-NPs 29,3 nm Dose: 100 mg/kg, 500 mg/kg or 1000 mg/kg | The production of significant alterations of selective genes in the caudate, frontal cortex and hippocampus of mice was observed after exposure to the nanoparticles. The data concluded that nanoparticles can produce neurotoxicity by generating oxidative stress. | [101] |
| Sprague–Dawley rats | Ag-NPs 60 nm; 28 days Four groups (10 rats in each group): vehicle control, low-dose group (30 mg/kg), middle-dose group (300 mg/kg), and high-dose group (1000 mg/kg) | A dose-dependent increased accumulation of Ag-NPs was observed in the lamina propria in both the small and large intestine, and also in the tip of the upper villi in the ileum and protruding surface of the fold in the colon. Rats that consumed nanoparticles also released more anormal mucus in the crypt lumen and ileal lumen and there was also detachment of cells at the tip of the villi. | [102] |
| F344 rats | Ag-NPs 56 nm; 13 weeks Four groups (10 rats in each group): vehicle control, low-dose (30 mg/kg), middle-dose (125 mg/kg), and high-dose (500 mg/kg). | Significant dose-dependent changes were found in alkaline phosphatase and cholesterol, indicating that exposure to more than 125 mg/kg of silver nanoparticles may result in slight liver damage. Histopathologic examination revealed a higher incidence of bile-duct hyperplasia, with or without necrosis. There was also a dose-dependent accumulation of silver in all tissues examined. | [103] |
| Mice | Ag-NPs 3–20 nm; 21 days Daily dose: 5, 10, 15 y 20 mg/kg | Mice treated with a dose of 10 mg/kg showed great weight loss. It was found that Ag-NPs damaged the microvilli of epithelial cells and intestinal glands. This may be the cause of weight loss due to intestinal malabsorption. | [104] |
| Wistar rats | Ag-NPs 10 nm; 14 days Daily dose: 0.02 mg/kg | Ag-NPs intake produced a synaptic degeneration and potential neuronal cell death due to alterations in synaptic structures and reduced levels of proteins associated with these structures | [105] |
| Sprague–Dawley rats | Ag-NPs 3–10 nm (98.7%), 10–30 nm (1.3%); 14 days Daily dose: 1 mg/kg or 10 mg/kg Three groups (6 rats in each group): control group, low-dose group (1 mg/kg), high-dose group (10 mg/kg) | After ingestion of Ag-NPs, neuron shrinkage, cytoplasmic or foot inflammation of the astrocytes and extravascular lymphocytes occurred. This led to the conclusion that Ag-NPs can induce neuronal degeneration and swelling of astrocytes even with oral exposure at low doses. | [106] |
| C57BL/6NCrl mice | Ag-NPs 110 nm and 20 nm (PVP), 110 nm and 20 nm (Citrate); 28 days Daily dose: 10 mg/kg | None of the nanoparticles tested caused alterations in the structure or diversity of the intestinal microbiota of the mice. | [107] |
| Sprague–Dawley rats | Ag-NPs 10, 75 and 100 nm; 13 weeks Daily dose: 9, 18 and 36 mg/kg twice a day | It was possible to observe how the nanoparticles produced changes in the intestinal microbiota of the rats. There was an increase in Gram-negative bacteria. Exposure to smaller Ag-NPs resulted in a decrease in Lactobacillus spp. and the Firmicutes phyla. | [108] |
| Sprague–Dawley rats | Ag-NPs 12 nm; single exposure and multiple exposures over 30 days Daily doses: 2000 and 250 mg/kg for single and multiple administrations, respectively. | Single and multiple administrations resulted in silver accumulation in the liver, kidneys, spleen, stomach, and small intestine. But, concentrations of silver detected in tissues were far smaller than the administered doses (<99%), indicating its efficient excretion from the organism. | [109] |
| BALB/C mice | Ag-NPs 294 nm (NanoAg1) and 122 nm (NanoAg 2); 3 days Daily dose: 100 µL suspension | The administration of NanoAg1 increased the number of Clostridium perfringens and Escherichia coli and decreased that of Lactobacillus spp., But the results were not significant. NanoAg2 acted in reverse. It could also be seen how nanoparticle suspensions reversed a severe colonic lesion in mice. | [110] |
| Mice | Ag-NPs 55.17 nm; 28 days Doses: 0 (control), 11.4, 114 and 1140 μg Ag-NP/kg | In this work, an increase in the Firmicutes/Bacteroidetes ratio was observed, similar to that described in studies of obesity and inflammatory diseases. | [111] |
| Fish (Piaractus mesopotamicus) | Ag-NPs 50 nm; 24 h Dose: 0 (control), 2.5, 10, and 25 μg Ag-NPs/L | More silver accumulated in the brain than in gills and liver at all concentrations. There was also an increase in oxidative stress, as well as damage to the enterocytes in fish exposed to higher concentrations. | [112] |
| Zebrafish | Ag-NPs 58.6 nm; 14 days Dose: 500 mg/kg twice a day | Despite not finding lesions in the integrity of the intestinal epithelium, in this study it was observed that Ag-NPs decreased to a non-detectable level to beneficial bacterial populations of fish. | [113] |
| Zebrafish | Ag-NPs 10, 40 and 100 nm; 4 days Dose: 1, 5, 10, 50, 100, 150 y 200 ppm | It was observed that the salts and cations of the medium decreased the dissolution of the silver, thus limiting its action. Ag-NPs with a size of 10 and 100 nm caused developmental defects in the muscles and intestine of the embryo, while those of 40 nm produced lethal effects. | [114] |
| Zebrafish | Ag-NPs 20 and 100 nm; 96 h Dose: 0.61, 1.07, 0.67, and 1.28 mg/L | The coating of the nanoparticles increased the survival rate of the fish compared to the control. It was also observed that the smaller Ag-NPs were more lethal than the 100 nm. More nanoparticles accumulated in the intestines than in the gills. | [115] |
| Caenorhabditis elegans | Ag-NPs 79 nm | The effect of silver nanoparticles for 10 generations of the nematode was studied. From the second a pronounced sensitization to the nanomaterial was observed. | [116] |
| Caenorhabditis elegans | Ag-NPs 25 and 75 nm; 12 h Dose: 5 mg/L | Exposure of E. coli to the nanoparticles and of the nematode to E. coli induced reproductive toxicity, as well as neurotoxicity. | [117] |
| Caenorhabditis elegans | Ag-NPs <100; 40 h Dose: 0, 1, 3, and 5 mg/kg | Different silver nanomaterials induce growth inhibition and reproductive toxicity when the soil is found at a concentration of ≥5 mg/kg. | [118] |
| Caenorhabditis elegans | Ag-NPs ≈ 69 nm | Factors that increased sensitivity and reproductive toxicity from the second generation could not be verified. Therefore, long-term risk cannot be assessed and other inheritance mechanisms, such as epigenetics, may be at play in multigenerational reproductive toxicity. | [119] |
| Drosophila melanogaster | Dose: 10–100 µg Ag/mL (accute intake) and 5 µg Ag/mL (chronic exposure) | After the acute intake, a significant toxic effect was observed at the concentration of 20 µg/mL and 50% of the flies could not complete their development cycle. In the case of the chronic exposure in 8 generations, a decrease in fertility was observed in the first three generations, after which it returned to normal. | [120] |
| Drosophila melanogaster | Ag-NPs 5–22 nm Dose: 10, 50, 100, 200 g/mL | All nanoparticles tested (synthesized from different natural extracts) significantly reduced the number of hatched larvae. In addition, those synthesized from mulberry, fig and olive produced a high mortality of larvae and adults. | [121] |
| Drosophila melanogaster | Ag-NPs 20–100 nm; 3, 10 and 30 days Dose: 5, 25, 50 and 250 µg Ag/mL | The effect of Ag-NPs depends on the dose and the stage of development of the flies. In general it alters the ability to lay eggs, decrease the size of the ovary and decrease survival and longevity. | [122] |
| Drosophila melanogaster | Ag-NPs 3.44 nm; 10 days Dose: 0.016, 0.08, 0.4, 1 y 2 mM | The 10 nM dose was completely toxic. Despite this, depigmentation was observed at all concentrations. Significant levels of intracellular ROS and DNA damage were also observed. | [123] |
| Humans | Volunteers: 60 Ag-NPs 5–10 nm (10 ppm) or 25–40 nm (32 ppm) Study 1: 10 ppm with 3, 7, and 14 day time periods Study 2: 32 ppm for 14 days Daily dose: 100 μg/day for 10 ppm, and 480 μg/day for 32 ppm | No significant changes were observed in metabolism, hematology, urine, physical findings, sputum morphology or changes in images. Nor were statistically significant changes detected in the markers of hydrogen peroxide production or peroxiredoxin protein expression. Instead, silver could be detected in human serum. | [124] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zorraquín-Peña, I.; Cueva, C.; Bartolomé, B.; Moreno-Arribas, M.V. Silver Nanoparticles against Foodborne Bacteria. Effects at Intestinal Level and Health Limitations. Microorganisms 2020, 8, 132. https://doi.org/10.3390/microorganisms8010132
Zorraquín-Peña I, Cueva C, Bartolomé B, Moreno-Arribas MV. Silver Nanoparticles against Foodborne Bacteria. Effects at Intestinal Level and Health Limitations. Microorganisms. 2020; 8(1):132. https://doi.org/10.3390/microorganisms8010132
Chicago/Turabian StyleZorraquín-Peña, Irene, Carolina Cueva, Begoña Bartolomé, and M. Victoria Moreno-Arribas. 2020. "Silver Nanoparticles against Foodborne Bacteria. Effects at Intestinal Level and Health Limitations" Microorganisms 8, no. 1: 132. https://doi.org/10.3390/microorganisms8010132
APA StyleZorraquín-Peña, I., Cueva, C., Bartolomé, B., & Moreno-Arribas, M. V. (2020). Silver Nanoparticles against Foodborne Bacteria. Effects at Intestinal Level and Health Limitations. Microorganisms, 8(1), 132. https://doi.org/10.3390/microorganisms8010132





