Cave Drip Water-Related Samples as a Natural Environment for Aromatic Hydrocarbon-Degrading Bacteria
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling and DNA Extraction
2.2. Gas Chromatography Analysis
2.3. 16S rDNA Gene Sequencing
2.4. PCR Conditions and DGGE
2.5. Physicochemical Analysis
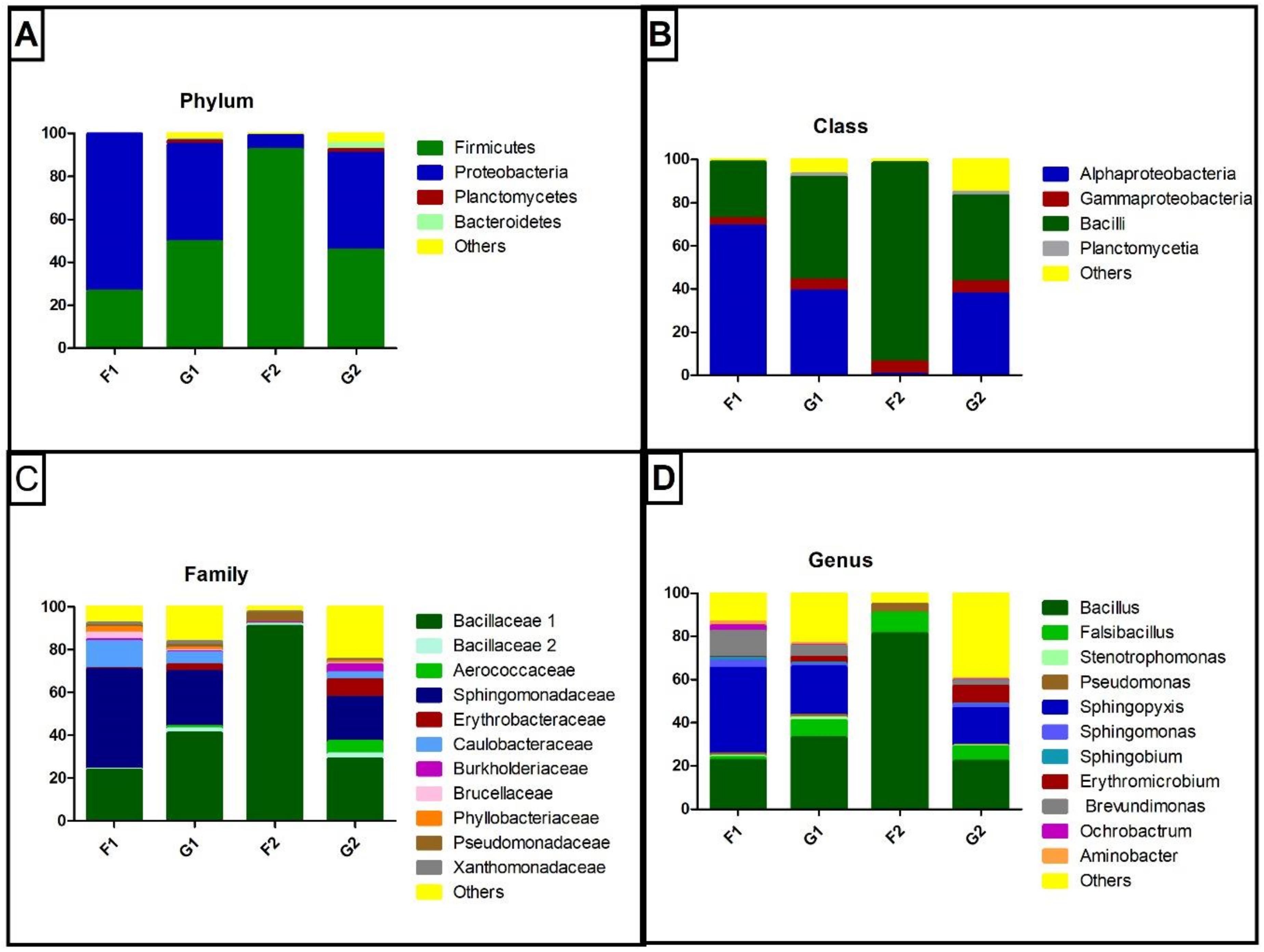
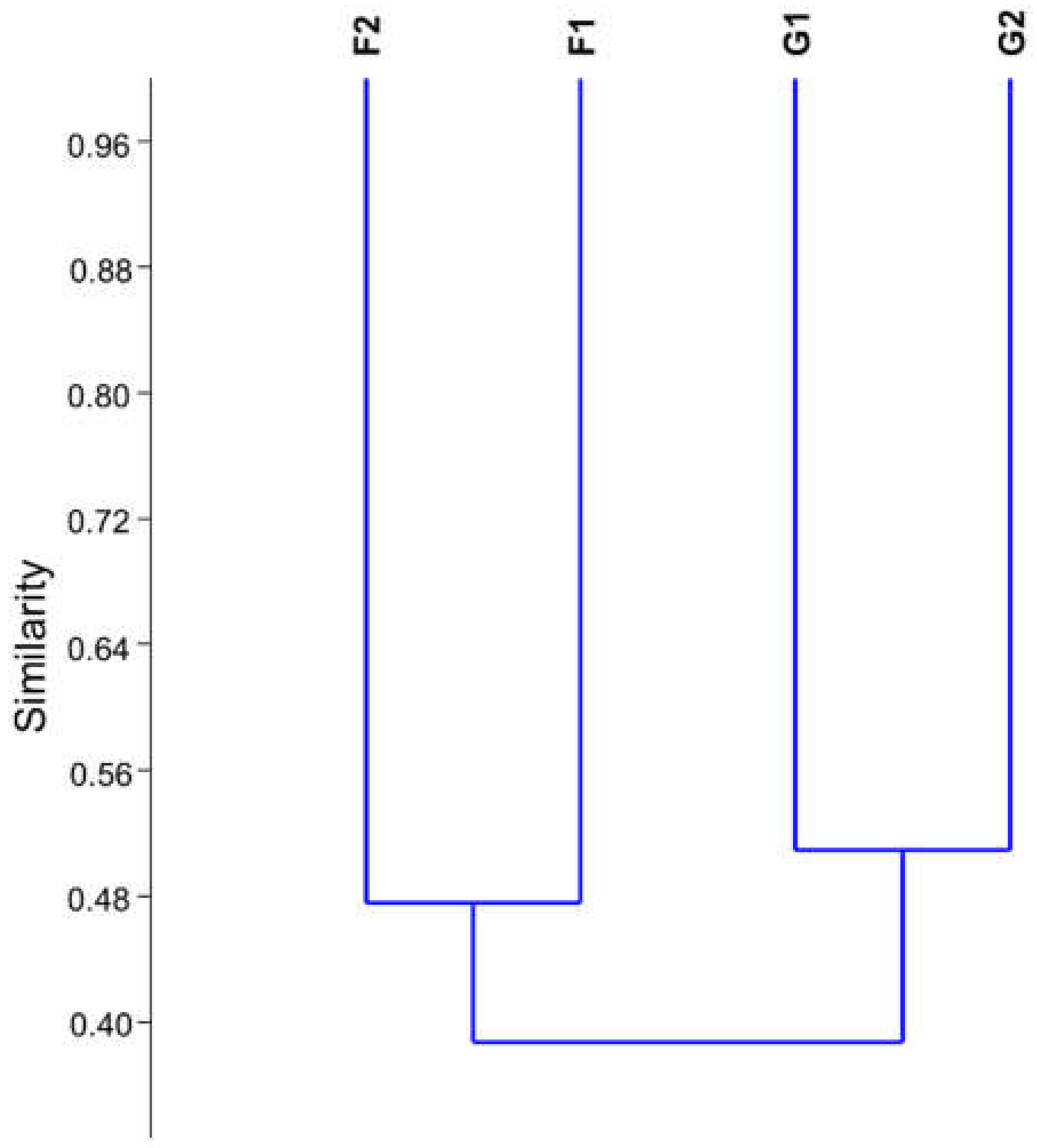
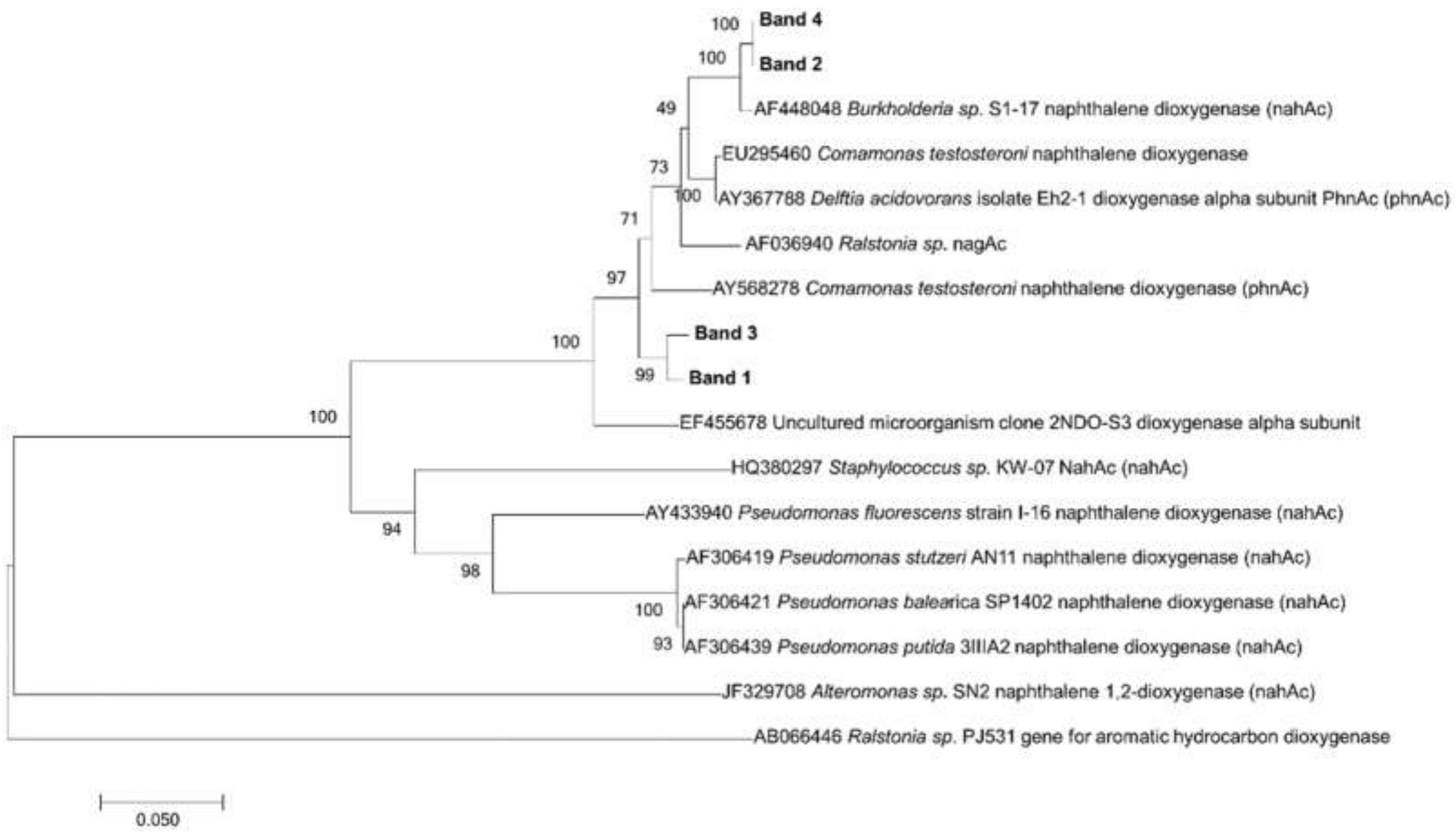
3. Results
3.1. Gas Chromatography Analysis
3.2. 16S rRNA Gene Sequencing
3.3. PCR and DGGE of ndo Genes
3.4. Physicochemical Analysis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Barton, H.A.; Jurado, V. What’s up down there? Microbial diversity in caves. Microbe 2007, 2, 132–138. [Google Scholar]
- Northup, D.E.; Lavoie, K.H. Geomicrobiology of caves: A review. Geomicrobiol. J. 2001, 18, 199–222. [Google Scholar]
- Poulson, T.L.; White, W.B. The cave environment. Science 1969, 165, 971–981. [Google Scholar] [CrossRef] [PubMed]
- Barton, H.A.; Taylor, N.M.; Kreate, M.P.; Springer, A.C.; Oehrle, S.A.; Bertog, J.L. The impact of host rock geochemistry on bacterial community structure in oligotrophic cave environments. Int. J. Speleol. 2007, 36, 93–104. [Google Scholar] [CrossRef]
- Simon, K.S.; Pipan, T.; Culver, D.C. A conceptual model of the flow and distribution of organic carbon in caves. J. Cave Karst Stud. 2007, 69, 279–284. [Google Scholar]
- Chen, Y.; Wu, L.; Boden, R.; Hillebrand, A.; Kumaresan, D.; Moussard, H.; Baciu, M.; Lu, Y.; Colin Murrell, J. Life without light: Microbial diversity and evidence of sulfur- and ammonium-based chemolithotrophy in Movile Cave. ISME J. 2009, 3, 1093–1104. [Google Scholar] [CrossRef] [PubMed]
- Porter, M.L.; Engel, A.S.; Kane, T.C.; Kinkle, B.K. Productivity-diversity relationships from chemolithoautotrophically based sulfidic karst systems. Int. J. Speleol. 2009, 38, 27–40. [Google Scholar] [CrossRef]
- Bastian, F.; Jurado, V.; Nováková, A.; Alabouvette, C.; Saiz-Jimenez, C. The microbiology of Lascaux Cave. Microbiology 2010, 156, 644–652. [Google Scholar] [CrossRef]
- Jurado, V.; Porca, E.; Cuezva, S.; Fernandez-cortes, A.; Sanchez-moral, S.; Saiz-jimenez, C. Fungal outbreak in a show cave. Sci. Total Environ. 2010, 408, 3632–3638. [Google Scholar] [CrossRef]
- Banks, E.D.; Taylor, N.M.; Gulley, J.; Lubbers, B.R.; Giarrizzo, J.G.; Bullen, H.A.; Hoehler, T.M.; Barton, H.A. Bacterial calcium carbonate precipitation in cave environments: A function of calcium homeostasis. Geomicrobiol. J. 2010, 27, 444–454. [Google Scholar] [CrossRef]
- Laiz, L.; Groth, I.; Gonzalez, I.; Saiz-Jimenez, C. Microbiological study of the dripping waters in Altamira cave (Santillana del Mar, Spain). J. Microbiol. Methods 1999, 36, 129–138. [Google Scholar] [CrossRef]
- Macalady, J.L.; Jones, D.S.; Lyon, E.H. Extremely acidic, pendulous cave wall biofilms from the Frasassi cave system, Italy. Environ. Microbiol. 2007, 9, 1402–1414. [Google Scholar] [CrossRef]
- Pašić, L.; Kovce, B.; Sket, B.; Herzog-Velikonja, B. Diversity of microbial communities colonizing the walls of a Karstic cave in Slovenia. FEMS Microbiol. Ecol. 2010, 71, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Yun, Y.; Xiang, X.; Wang, H.; Man, B.; Gong, L.; Liu, Q.; Dong, Q.; Wang, R. Five-year monitoring of bacterial communities in dripping water from the Heshang cave in central China: Implication for paleoclimate reconstruction and ecological functions. Geomicrobiol. J. 2016, 33, 1–11. [Google Scholar] [CrossRef]
- Bosle, J.M.; Mischel, S.A.; Schulze, A.L.; Scholz, D.; Hoffmann, T. Quantification of low molecular weight fatty acids in cave drip water and speleothems using HPLC-ESI-IT/MS-development and validation of a selective method. Anal. Bioanal. Chem. 2014, 406, 3167–3177. [Google Scholar] [CrossRef]
- Perrette, Y.; Poulenard, J.; Saber, A.I.; Fanget, B.; Guittonneau, S.; Ghaleb, B.; Garaudee, S. Polycyclic Aromatic Hydrocarbons in stalagmites: Occurrence and use for analyzing past environments. Chem. Geol. 2008, 251, 67–76. [Google Scholar] [CrossRef]
- Saiz-Jimenez, C.; Hermosin, B. Thermally assisted hydrolysis and methylation of dissolved organic matter in dripping waters from the Altamira Cave. J. Anal. Appl. Pyrolysis 1999, 49, 337–347. [Google Scholar] [CrossRef]
- Seo, J.-S.; Keum, Y.-S.; Li, Q.X. Bacterial Degradation of Aromatic Compounds. Int. J. Environ. Res. Public Health 2009, 6, 278–309. [Google Scholar] [CrossRef]
- Guggenberger, G.; Wolfgang, Z. Dissolved organic carbon in references forest floor leachates: Simple degradation products or humic substances. Sci. Total Environ. 1994, 152, 37–47. [Google Scholar] [CrossRef]
- Gomes, N.C.M.; Borges, L.R.; Paranhos, R.; Pinto, F.N.; Krögerrecklenfort, E.; Mendonça-Hagler, L.C.S.; Smalla, K. Diversity of ndo genes in mangrove sediments exposed to different sources of polycyclic aromatic hydrocarbon pollution. Appl. Environ. Microbiol. 2007, 73, 7392–7399. [Google Scholar] [CrossRef]
- Iker, B.C.; Kambesis, P.; Oehrle, S.A.; Groves, C.; Barton, H.A. Microbial atrazine breakdown in a karst groundwater system and its effect on ecosystem energetics. J. Environ. Qual. 2010, 39, 509–518. [Google Scholar] [CrossRef] [PubMed]
- Byl, T.D.; Metge, D.W.; Agymang, D.T.; Bradley, M.; Hileman, G.; Harvey, R.W. Adaptations of indigenous bacteria to fuel contamination in karst aquifers in south-central Kentucky. J. Cave Karst Stud. 2014, 76, 104–113. [Google Scholar] [CrossRef]
- Blase, R.C.; Patrick, E.L.; Mitchell, J.N.; Libardoni, M. Analysis of cave atmospheres by comprehensive two-dimensional gas chromatography (GC×GC) with flame ionization detection (FID). Anal. Chem. Res. 2015, 3, 54–62. [Google Scholar] [CrossRef]
- Wang, Y.; Qian, P.-Y. Conservative fragments in bacterial 16S rRNA genes and primer design for 16S ribosomal DNA amplicons in metagenomic studies. PLoS ONE 2009, 4, e7401. [Google Scholar] [CrossRef] [PubMed]
- Caporaso, J.G.; Lauber, C.L.; Walters, W.A.; Berg-Lyons, D.; Huntley, J.; Fierer, N.; Owens, S.M.; Betley, J.; Fraser, L.; Bauer, M.; et al. Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. ISME J. 2012, 6, 1621–1624. [Google Scholar] [CrossRef] [PubMed]
- Gohl, D.M.; Vangay, P.; Garbe, J.; MacLean, A.; Hauge, A.; Becker, A.; Gould, T.J.; Clayton, J.B.; Johnson, T.J.; Hunter, R.; et al. Systematic improvement of amplicon marker gene methods for increased accuracy in microbiome studies. Nat. Biotechnol. 2016, 34, 942–949. [Google Scholar] [CrossRef] [PubMed]
- Wright, E.S.; Yilmaz, L.S.; Noguera, D.R. DECIPHER, a search-based approach to chimera identification for 16S rRNA sequences. Appl. Environ. Microbiol. 2012, 78, 717–725. [Google Scholar] [CrossRef] [PubMed]
- Cole, J.R.; Wang, Q.; Fish, J.A.; Chai, B.; McGarrell, D.M.; Sun, Y.; Brown, C.T.; Porras-Alfaro, A.; Kuske, C.R.; Tiedje, J.M. Ribosomal Database Project: Data and tools for high throughput rRNA analysis. Nucleic Acids Res. 2014, 42, D633–D642. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.-H.; Ha, S.-M.; Kwon, S.; Lim, J.; Kim, Y.; Seo, H.; Chun, J. Introducing EzBioCloud: A taxonomically united database of 16S rRNA gene sequences and whole-genome assemblies. Int. J. Syst. Evol. Microbiol. 2017, 67, 1613–1617. [Google Scholar] [CrossRef] [PubMed]
- Ewing, B.; Green, P. Base-calling of automated sequencer traces using phred. II. Error probabilities. Genome Res. 1998, 8, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Ribeiro, A.C.; Guimarães, P.T.G.; Alvarez, V.H.V. Recomendações Para o uso de Corretivos e Fertilizantes em Minas Gerais, 5th ed.; Ribeiro, A.C., Guimarães, P.T.G., Alvarez, V.H.V., Eds.; Editora CFSEMG: Viçosa, Brazil, 1999. [Google Scholar]
- Brandao, G.C.; de Jesus, R.M.; da Silva, E.G.P.; Ferreira, S.L.C. Use of slurry sampling for the direct determination of zinc in yogurt by high resolution-continuum source flame atomic absorption spectrometry. Talanta 2010, 81, 1357–1359. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, C.; Huang, J.; Hu, C.; Xie, S. Seasonal variation of fatty acids from drip water in Heshang Cave, central China. Appl. Geochem. 2011, 26, 341–347. [Google Scholar] [CrossRef]
- Selifonov, S.A.; Grifoll, M.; Eaton, R.W.; Chapman, P.J. Oxidation of naphthenoaromatic and methyl-substituted aromatic compounds by naphthalene 1,2-dioxygenase. Appl. Environ. Microbiol. 1996, 62, 507–514. [Google Scholar] [PubMed]
- Annweiler, E.; Richnow, H.H.; Antranikian, G.; Hebenbrock, S.; Garms, C.; Franke, S.; Francke, W.; Michaelis, W. Naphthalene Degradation and Incorporation of Naphthalene-Derived Carbon into Biomass by the Thermophile Bacillus thermoleovorans. Appl. Environ. Microbiol. 2000, 66, 518–523. [Google Scholar] [CrossRef] [PubMed]
- Bosch, R.; García-Valdés, E.; Moore, E.R.B. Genetic characterization and evolutionary implications of a chromosomally encoded naphthalene-degradation upper pathway from Pseudomonas stutzeri AN10. Gene 1999, 236, 149–157. [Google Scholar] [CrossRef]
- Lin, C.; Gan, L.; Chen, Z.-L. Biodegradation of naphthalene by strain Bacillus fusiformis (BFN). J. Hazard. Mater. 2010, 182, 771–777. [Google Scholar] [CrossRef] [PubMed]
- Shi, T.; Fredrickson, J.K.; Balkwill, D.L. Biodegradation of polycyclic aromatic hydrocarbons by Sphingomonas strains isolated from the terrestrial subsurface. J. Ind. Microbiol. Biotechnol. 2001, 26, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.; Bai, X.; Lai, Q.; Xie, Y.; Chen, X.; Shao, Z. Draft genome sequence of Sphingobium sp. strain C100, a polycyclic aromatic hydrocarbon-degrading bacterium from the deep-sea sediment of the Arctic Ocean. Genome Announc. 2014, 2, e01210-13. [Google Scholar] [CrossRef] [PubMed]
- Mallick, S.; Chakraborty, J.; Dutta, T.K. Role of oxygenases in guiding diverse metabolic pathways in the bacterial degradation of low-molecular-weight polycyclic aromatic hydrocarbons: A review. Crit. Rev. Microbiol. 2011, 37, 64–90. [Google Scholar] [CrossRef] [PubMed]
- McDonald, I.R.; Kämpfer, P.; Topp, E.; Warner, K.L.; Cox, M.J.; Hancock, T.L.C.; Miller, L.G.; Larkin, M.J.; Ducrocq, V.; Coulter, C.; et al. Aminobacter ciceronei sp. nov. and Aminobacter lissarensis sp. nov., isolated from various terrestrial environments. Int. J. Syst. Evol. Microbiol. 2005, 55, 1827–1832. [Google Scholar] [CrossRef]
- Arulazhagan, P.; Vasudevan, N. Biodegradation of polycyclic aromatic hydrocarbons by a halotolerant bacterial strain Ochrobactrum sp. VA1. Mar. Pollut. Bull. 2011, 62, 388–394. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Guo, L.; Wang, S.; Lu, Y. Comparative impact of cadmium on two phenanthrene-degrading bacteria isolated from cadmium and phenanthrene co-contaminated soil in China. J. Hazard. Mater. 2010, 174, 818–823. [Google Scholar] [CrossRef] [PubMed]
- Herrick, J.B.; Stuart-Keil, K.G.; Ghiorse, W.C.; Madsen, E.L. Natural horizontal transfer of a naphthalene dioxygenase gene between bacteria native to a coal tar-contaminated field site. Appl. Environ. Microbiol. 1997, 63, 2330–2337. [Google Scholar] [PubMed]
- Gan, H.Y.; Gan, H.M.; Tarasco, A.M.; Busairi, N.I.; Barton, H.A.; Hudson, A.O.; Savka, M.A. Whole-genome sequences of five oligotrophic bacteria isolated from deep within Lechuguilla cave, New Mexico. Genome Announc. 2014, 2, e01133-14. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Wang, H.; Zhao, R.; Qiu, X.; Gong, L. Bacteria isolated from dripping water in the oligotrophic Heshang cave in central China. J. Earth Sci. 2010, 21, 325–328. [Google Scholar] [CrossRef]
- Cuezva, S.; Sanchez-moral, S.; Saiz-jimenez, C.; Cañaveras, J.C. Microbial communities and associated mineral fabrics in Altamira cave, Spain. Int. J. Speleol. 2009, 38, 83–92. [Google Scholar]
- Northup, D.E.; Barns, S.M.; Yu, L.E.; Spilde, M.N.; Schelble, R.T.; Dano, K.E.; Crossey, L.J.; Connolly, C.A.; Boston, P.J.; Natvig, D.O.; et al. Diverse microbial communities inhabiting ferromanganese deposits in Lechuguilla and Spider Caves. Environ. Microbiol. 2003, 5, 1071–1086. [Google Scholar] [CrossRef]
- Baker, A.; Genty, D. Environmental pressures on conserving cave speleothems: Effects of changing surface land use and increased cave tourism. J. Environ. Manag. 1998, 53, 165–175. [Google Scholar] [CrossRef]
| Sample | Aromatic Compound (ng/g or ng/mL) | |
|---|---|---|
| Naphthalene | Acenaphthene | |
| F1 | 3.65 ± 0.96 | 1.65 ± 0.67 |
| F2 | 2.71 ± 0.43 | 1.13 ± 0.48 |
| G1 | 1.96 ± 0.32 | - |
| G2 | 1.47 ± 0.62 | - |
| G3 | 0.53 ± 0.15 | - |
| Sample | pH | Temp °C | OM g/L | P mg/L | K mmole/L | Ca mmole/L | Mg mmole/L | H + Al mmole/L | SB | CEC | V% |
|---|---|---|---|---|---|---|---|---|---|---|---|
| GPB2 | 4.5 | 28.0 | 36 | 252 | 1.0 | 724 | 96 | 171 | 820.8 | 992.2 | 83 |
| GBP3 | 6.5 | 30.0 | 15 | 4464 | 14.5 | 1070 | 168 | 17 | 1252.0 | 1269.0 | 99 |
| FFMP2 | 6.8 | 23.0 | 81 | 311 | 9.5 | 430 | 45 | 9 | 484.5 | 493.9 | 98 |
| FFMP3 | 7.0 | 23.5 | 57 | 533 | 4.1 | 520 | 24 | 9 | 548.1 | 556.6 | 98 |
| Sample | pH | Drip/s | Cu mg/L | Ca mg/L | Mn mg/L | Fe mg/L | Zn mg/L | Mg mg/L |
|---|---|---|---|---|---|---|---|---|
| GBP1 | 6.1 | 1.1 | 0.015 | 7.353 | <0.001 | <0.001 | <0.001 | 51.657 |
| FFMP1 | 6.7 | 0.6 | 0.026 | 11.250 | <0.001 | <0.001 | <0.001 | 69.598 |
| Sample | C | O | Fe | Mg | Al | Si | P | K | Ca | S | Co | Na | Ru | Mo |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GBP2 | 31.7 ± 9.7 | 52.9 ± 4.9 | 5 ± 3.4 | 1.6 ± 0.9 | 0.66 ± 0.05 | 1.3 ± 0.5 | 2.1 ± 1.0 | <0.01 | 1.7 ± 0.8 | 2.5 ± 0.8 | 0.9 ± 0.9 | 1.5 ± 0.3 | <0.01 | <0.01 |
| GBP3 | 11.7 ± 2.4 | 58.1 ± 3.0 | 0.4 ± 0.3 | 0.91 ± 0.84 | 7.2 ± 3.4 | 10.2 ± 5.5 | 8.6 ± 7.1 | 1.6 ± 1.1 | 0.19 ± 0.11 | <0.01 | <0.01 | <0.01 | 0.21 ± 0.12 | <0.01 |
| FFMP2 | 1.5 ± 1.5 | 48.5 ± 3.1 | 0.8 ± 0.2 | 1.25 ± 0.1 | 12.1 ± 2.5 | 32.3 ± 2.9 | 2.0 ± 1.1 | 1.1 ± 0.4 | 1.5 ± 0.6 | <0.01 | 0.02 ± 0.02 | <0.01 | <0.01 | <0.01 |
| FFMP3 | 9.0 ± 3.3 | 48.4 ± 1.4 | 16.6 ± 0.4 | 4.1 ± 1.3 | 5.8 ± 0.7 | 13.1 ± 5.6 | 1.5 ± 0.6 | 0.05 ± 0.04 | 0.97 ± 0.4 | <0.01 | <0.01 | <0.01 | <0.01 | 0.15 ± 0.2 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marques, E.L.S.; Silva, G.S.; Dias, J.C.T.; Gross, E.; Costa, M.S.; Rezende, R.P. Cave Drip Water-Related Samples as a Natural Environment for Aromatic Hydrocarbon-Degrading Bacteria. Microorganisms 2019, 7, 33. https://doi.org/10.3390/microorganisms7020033
Marques ELS, Silva GS, Dias JCT, Gross E, Costa MS, Rezende RP. Cave Drip Water-Related Samples as a Natural Environment for Aromatic Hydrocarbon-Degrading Bacteria. Microorganisms. 2019; 7(2):33. https://doi.org/10.3390/microorganisms7020033
Chicago/Turabian StyleMarques, Eric L. S., Gislaine S. Silva, João C. T. Dias, Eduardo Gross, Moara S. Costa, and Rachel P. Rezende. 2019. "Cave Drip Water-Related Samples as a Natural Environment for Aromatic Hydrocarbon-Degrading Bacteria" Microorganisms 7, no. 2: 33. https://doi.org/10.3390/microorganisms7020033
APA StyleMarques, E. L. S., Silva, G. S., Dias, J. C. T., Gross, E., Costa, M. S., & Rezende, R. P. (2019). Cave Drip Water-Related Samples as a Natural Environment for Aromatic Hydrocarbon-Degrading Bacteria. Microorganisms, 7(2), 33. https://doi.org/10.3390/microorganisms7020033





