Blastocystis Colonization Is Associated with Increased Diversity and Altered Gut Bacterial Communities in Healthy Malian Children
Abstract
1. Introduction
2. Patients and Methods
2.1. Study Population
2.2. DNA Extraction
2.3. Real-Time PCR Assays
2.4. DNA Extraction and 16S Metabarcoding
2.5. Bioinformatics Analysis
2.6. Statistical Analysis
2.7. Ethical Considerations
3. Results
3.1. Characteristics of Study Subjects
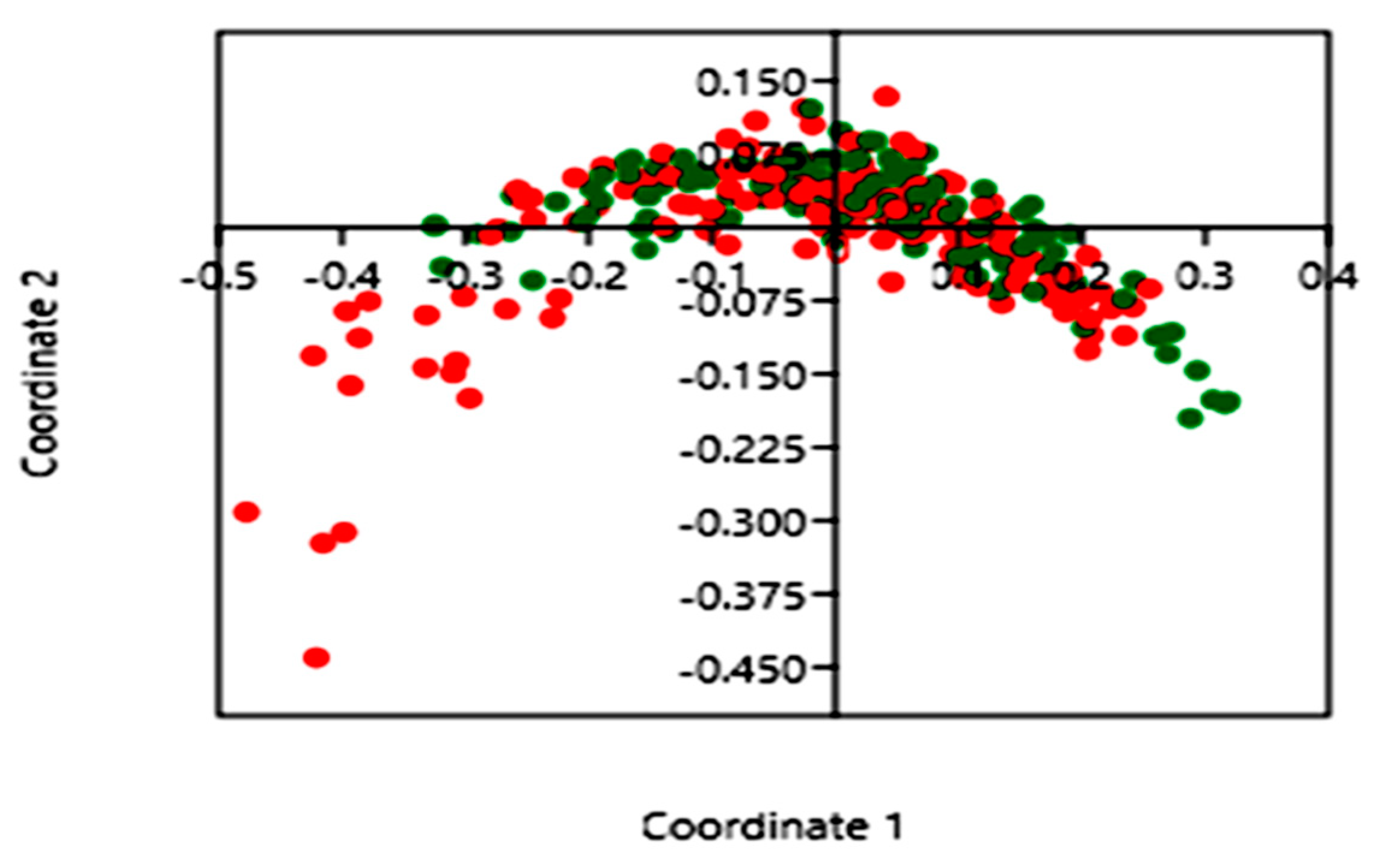
3.2. Diversity and Composition of the Microbiota in Stool Samples
3.3. Impact of Blastocystis on Gut Bacterial Communities
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lynch, K.M. Dauercystformation of Trichomonas intestinalis. J. Parasitol. 1916, 3, 28–33. [Google Scholar] [CrossRef]
- Silberman, J.D.; Sogin, M.L.; Leipe, D.D.; Clark, C.G. Human parasite finds taxonomic home. Nature 1996, 380, 398. [Google Scholar] [CrossRef] [PubMed]
- Tan, K.S.W. New Insights on Classification, Identification, and Clinical Relevance of Blastocystis spp. Clin. Microbiol. Rev. 2008, 21, 639–665. [Google Scholar] [CrossRef]
- Clark, C.G.; van der Giezen, M.; Alfellani, M.A.; Stensvold, C.R. Chapter One—Recent Developments in Blastocystis Research. In Advances in Parasitology; Rollinson, D., Ed.; Academic Press: New York, NY, USA, 2013; Volume 82, pp. 1–32. [Google Scholar]
- El Safadi, D.; Cian, A.; Nourrisson, C.; Pereira, B.; Morelle, C.; Bastien, P.; Bellanger, A.-P.; Botterel, F.; Candolfi, E.; Desoubeaux, G.; et al. Prevalence, risk factors for infection and subtype distribution of the intestinal parasite Blastocystis sp. from a large-scale multi-center study in France. BMC Infect. Dis. 2016, 16, 451. [Google Scholar] [CrossRef] [PubMed]
- El Safadi, D.; Gaayeb, L.; Meloni, D.; Cian, A.; Poirier, P.; Wawrzyniak, I.; Delbac, F.; Dabboussi, F.; Delhaes, L.; Seck, M.; et al. Children of Senegal River Basin show the highest prevalence of Blastocystis sp. ever observed worldwide. BMC Infect. Dis. 2014, 14, 164. [Google Scholar] [CrossRef]
- Bálint, A.; Dóczi, I.; Bereczki, L.; Gyulai, R.; Szűcs, M.; Farkas, K.; Urbán, E.; Nagy, F.; Szepes, Z.; Wittmann, T.; et al. Do not forget the stool examination!—Cutaneous and gastrointestinal manifestations of Blastocystis sp. infection. Parasitol. Res. 2014, 113, 1585–1590. [Google Scholar] [CrossRef]
- Chandramathi, S.; Suresh, K.; Sivanandam, S.; Kuppusamy, U.R. Stress Exacerbates Infectivity and Pathogenicity of Blastocystis hominis: In Vitro and In Vivo Evidences. PLoS ONE 2014, 9, e94567. [Google Scholar] [CrossRef]
- Chandramathi, S.; Suresh, K.; Shuba, S.; Mahmood, A.; Kuppusamy, U.R. High levels of oxidative stress in rats infected with Blastocystis hominis. Parasitology 2010, 137, 605–611. [Google Scholar] [CrossRef]
- Akgül, Ö.; Kart Yaşar, K.; Sapmaz, B.; Kırkoyun Uysal, H.; Yıldırmak, T.; Şimşek, F.; Karasakal, Ö.F.; Çalışkan, R.; Öner, Y.A. [Detection of intestinal parasites with conventional and molecular methods in follow-up HIV/AIDS cases]. Mikrobiyol. Bul. 2018, 52, 273–283. [Google Scholar] [CrossRef]
- Cirioni, O.; Giacometti, A.; Drenaggi, D.; Ancarani, F.; Scalise, G. Prevalence and clinical relevance of Blastocystis hominis in diverse patient cohorts. Eur. J. Epidemiol. 1999, 15, 389–393. [Google Scholar] [CrossRef]
- Ghosh, K.; Ayyaril, M.; Nirmala, V. Acute GVHD involving the gastrointestinal tract and infestation with Blastocystis hominis in a patient with chronic myeloid leukaemia following allogeneic bone marrow transplantation. Bone Marrow Transplant. 1998, 22, 1115–1117. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rao, K.; Sekar, U.; Iraivan, K.T.; Abraham, G.; Soundararajan, P. Blastocystis hominis—An emerging cause of diarrhoea in renal transplant recipients. J. Assoc. Physicians India 2003, 51, 719–721. [Google Scholar] [PubMed]
- Shariati, A.; Fallah, F.; Pormohammad, A.; Taghipour, A.; Safari, H.; Chirani, A. salami; Sabour, S.; Alizadeh-Sani, M.; Azimi, T. The possible role of bacteria, viruses, and parasites in initiation and exacerbation of irritable bowel syndrome. J. Cell. Physiol. 2019, 234, 8550–8569. [Google Scholar] [CrossRef] [PubMed]
- Khademvatan, S.; Masjedizadeh, R.; Rahim, F.; Mahbodfar, H.; Salehi, R.; Yousefi-Razin, E.; Foroutan, M. Blastocystis and irritable bowel syndrome: Frequency and subtypes from Iranian patients. Parasitol. Int. 2017, 66, 142–145. [Google Scholar] [CrossRef] [PubMed]
- Scanlan, P.D.; Stensvold, C.R.; Rajilić-Stojanović, M.; Heilig, H.G.H.J.; De Vos, W.M.; O’Toole, P.W.; Cotter, P.D. The microbial eukaryote Blastocystis is a prevalent and diverse member of the healthy human gut microbiota. FEMS Microbiol. Ecol. 2014, 90, 326–330. [Google Scholar] [CrossRef]
- Scanlan, P.D.; Marchesi, J.R. Micro-eukaryotic diversity of the human distal gut microbiota: Qualitative assessment using culture-dependent and-independent analysis of faeces. ISME J. 2008, 2, 1183. [Google Scholar] [CrossRef]
- Petersen, A.M.; Stensvold, C.R.; Mirsepasi, H.; Engberg, J.; Friis-Møller, A.; Porsbo, L.J.; Hammerum, A.M.; Nordgaard-Lassen, I.; Nielsen, H.V.; Krogfelt, K.A. Active ulcerative colitis associated with low prevalence of Blastocystis and Dientamoeba fragilis infection. Scand. J. Gastroenterol. 2013, 48, 638–639. [Google Scholar] [CrossRef]
- Krogsgaard, L.R.; Engsbro, A.L.; Stensvold, C.R.; Nielsen, H.V.; Bytzer, P. The Prevalence of Intestinal Parasites Is Not Greater Among Individuals with Irritable Bowel Syndrome: A Population-based Case-control Study. Clin. Gastroenterol. Hepatol. 2015, 13, 507–513.e2. [Google Scholar] [CrossRef]
- Rossen, N.G.; Bart, A.; Verhaar, N.; van Nood, E.; Kootte, R.; de Groot, P.F.; D’Haens, G.R.; Ponsioen, C.Y.; van Gool, T. Low prevalence of Blastocystis sp. in active ulcerative colitis patients. Eur. J. Clin. Microbiol. Infect. Dis. 2015, 34, 1039–1044. [Google Scholar] [CrossRef]
- Lyra, A.; Lahtinen, S. Dysbiosis of the Intestinal Microbiota in IBS. In Current Concepts in Colonic Disorders; InTech: Vienna, Austria, 2012. [Google Scholar]
- Manichanh, C.; Borruel, N.; Casellas, F.; Guarner, F. The gut microbiota in IBD. Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 599. [Google Scholar] [CrossRef]
- Nieves-Ramírez, M.E.; Partida-Rodríguez, O.; Laforest-Lapointe, I.; Reynolds, L.A.; Brown, E.M.; Valdez-Salazar, A.; Morán-Silva, P.; Rojas-Velázquez, L.; Morien, E.; Parfrey, L.W.; et al. Asymptomatic Intestinal Colonization with Protist Blastocystis Is Strongly Associated with Distinct Microbiome Ecological Patterns. mSystems 2018, 3, e00007-18. [Google Scholar]
- Ras, R.; Huynh, K.; Desoky, E.; Badawy, A.; Widmer, G. Perturbation of the intestinal microbiota of mice infected with Cryptosporidium parvum. Int. J. Parasitol. 2015, 45, 567–573. [Google Scholar] [CrossRef] [PubMed]
- Beatty, J.K.; Akierman, S.V.; Motta, J.-P.; Muise, S.; Workentine, M.L.; Harrison, J.J.; Bhargava, A.; Beck, P.L.; Rioux, K.P.; McKnight, G.W.; et al. Giardia duodenalis induces pathogenic dysbiosis of human intestinal microbiota biofilms. Int. J. Parasitol. 2017, 47, 311–326. [Google Scholar] [CrossRef] [PubMed]
- Gilchrist, C.A.; Petri, S.E.; Schneider, B.N.; Reichman, D.J.; Jiang, N.; Begum, S.; Watanabe, K.; Jansen, C.S.; Elliott, K.P.; Burgess, S.L.; et al. Role of the Gut Microbiota of Children in Diarrhea Due to the Protozoan Parasite Entamoeba histolytica. J. Infect. Dis. 2016, 213, 1579–1585. [Google Scholar] [CrossRef]
- Burgess, S.L.; Petri, W.A. The Intestinal Bacterial Microbiome and E. histolytica Infection. Curr. Trop. Med. Rep. 2016, 3, 71–74. [Google Scholar] [CrossRef]
- Menu, E.; Mary, C.; Toga, I.; Raoult, D.; Ranque, S.; Bittar, F. Evaluation of two DNA extraction methods for the PCR-based detection of eukaryotic enteric pathogens in fecal samples. BMC Res. Notes 2018, 11, 206. [Google Scholar] [CrossRef]
- Dridi, B.; Henry, M.; Khéchine, A.E.; Raoult, D.; Drancourt, M. High Prevalence of Methanobrevibacter smithii and Methanosphaera stadtmanae Detected in the Human Gut Using an Improved DNA Detection Protocol. PLoS ONE 2009, 4, e7063. [Google Scholar] [CrossRef]
- Sow, D.; Parola, P.; Sylla, K.; Ndiaye, M.; Delaunay, P.; Halfon, P.; Camiade, S.; Dieng, T.; Tine, R.C.K.; Faye, B.; et al. Performance of Real-Time Polymerase Chain Reaction Assays for the Detection of 20 Gastrointestinal Parasites in Clinical Samples from Senegal. Am. J. Trop. Med. Hyg. 2017, 97, 173–182. [Google Scholar] [CrossRef]
- Angelakis, E.; Bachar, D.; Henrissat, B.; Armougom, F.; Audoly, G.; Lagier, J.-C.; Robert, C.; Raoult, D. Glycans affect DNA extraction and induce substantial differences in gut metagenomic studies. Sci. Rep. 2016, 6, 26276. [Google Scholar] [CrossRef]
- XEGEN—The Specialist in High Performance and High Throughput NGS Data Analysis and Functional Annotation. Available online: http://xegen.eu/ (accessed on 17 November 2019).
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef]
- Audebert, C.; Even, G.; Cian, A.; The Blastocystis Investigation Group; Loywick, A.; Merlin, S.; Viscogliosi, E.; Chabé, M. Colonization with the enteric protozoa Blastocystis is associated with increased diversity of human gut bacterial microbiota. Sci. Rep. 2016, 6, 25255. [Google Scholar] [CrossRef] [PubMed]
- Yildiz, S.; Doğan, İ.; Doğruman-Al, F.; Nalbantoğlu, U.; Üstek, D.; Sarzhanov, F.; Yildirim, S. Association of Enteric Protist Blastocystis spp. and Gut Microbiota with Hepatic Encephalopathy. J. Gastrointest. Liver Dis. JGLD 2016, 25, 489–497. [Google Scholar]
- O’Brien Andersen, L.; Karim, A.B.; Roager, H.M.; Vigsnæs, L.K.; Krogfelt, K.A.; Licht, T.R.; Stensvold, C.R. Associations between common intestinal parasites and bacteria in humans as revealed by qPCR. Eur. J. Clin. Microbiol. Infect. Dis. Off. Publ. Eur. Soc. Clin. Microbiol. 2016, 35, 1427–1431. [Google Scholar] [CrossRef] [PubMed]
- Forsell, J.; Bengtsson-Palme, J.; Angelin, M.; Johansson, A.; Evengård, B.; Granlund, M. The relation between Blastocystis and the intestinal microbiota in Swedish travellers. BMC Microbiol. 2017, 17, 231. [Google Scholar] [CrossRef] [PubMed]
- Nagel, R.; Traub, R.J.; Allcock, R.J.N.; Kwan, M.M.S.; Bielefeldt-Ohmann, H. Comparison of faecal microbiota in Blastocystis-positive and Blastocystis-negative irritable bowel syndrome patients. Microbiome 2016, 4, 47. [Google Scholar] [CrossRef]
- Iebba, V.; Santangelo, F.; Totino, V.; Pantanella, F.; Monsia, A.; Di Cristanziano, V.; Di Cave, D.; Schippa, S.; Berrilli, F.; D’Alfonso, R. Gut microbiota related to Giardia duodenalis, Entamoeba spp. and Blastocystis hominis infections in humans from Côte d’Ivoire. J. Infect. Dev. Ctries. 2016, 10, 1035–1041. [Google Scholar] [CrossRef]
- Stensvold, C.R.; van der Giezen, M. Associations between Gut Microbiota and Common Luminal Intestinal Parasites. Trends Parasitol. 2018, 34, 369–377. [Google Scholar] [CrossRef]
- Andersen, L.O.; Bonde, I.; Nielsen, H.B.; Stensvold, C.R. A retrospective metagenomics approach to studying Blastocystis. FEMS Microbiol. Ecol. 2015, 91. [Google Scholar] [CrossRef]
- Pham, T.A.N.; Lawley, T.D. Emerging insights on intestinal dysbiosis during bacterial infections. Curr. Opin. Microbiol. 2014, 17, 67–74. [Google Scholar] [CrossRef]
- Sokol, H.; Seksik, P.; Furet, J.P.; Firmesse, O.; Nion-Larmurier, I.; Beaugerie, L.; Cosnes, J.; Corthier, G.; Marteau, P.; Doré, J. Low counts of Faecalibacterium prausnitzii in colitis microbiota. Inflamm. Bowel Dis. 2009, 15, 1183–1189. [Google Scholar] [CrossRef]
- Tedelind, S.; Westberg, F.; Kjerrulf, M.; Vidal, A. Anti-inflammatory properties of the short-chain fatty acids acetate and propionate: A study with relevance to inflammatory bowel disease. World J. Gastroenterol. WJG 2007, 13, 2826–2832. [Google Scholar] [CrossRef] [PubMed]
- Brestoff, J.R.; Artis, D. Commensal bacteria at the interface of host metabolism and the immune system. Nat. Immunol. 2013, 14, 676–684. [Google Scholar] [CrossRef] [PubMed]
- Hamer, H.M.; Jonkers, D.; Venema, K.; Vanhoutvin, S.; Troost, F.J.; Brummer, R.-J. Review article: The role of butyrate on colonic function. Aliment. Pharmacol. Ther. 2008, 27, 104–119. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; McKenzie, C.; Potamitis, M.; Thorburn, A.N.; Mackay, C.R.; Macia, L. The role of short-chain fatty acids in health and disease. Adv. Immunol. 2014, 121, 91–119. [Google Scholar] [PubMed]

| Blastocystis Status of the Children | Colonized | Noncolonized | p Value | ||
|---|---|---|---|---|---|
| Bacterial community richness | Observed OTUs | Mean (standard deviation) | 3008 (1496) | 2476 (1079) | 0.0023 |
| Median (interquartile range) | 2622 (1951–3630) | 2378 (1839–3138) | |||
| Chao-1 index | Mean (standard deviation) | 9966 (6104) | 8021 (4321) | 0.007 | |
| Median (interquartile range) | 8391 (5573–12300) | 6824 (4833–10375) | |||
| Bacterial diversity | Shannon index | Mean (standard deviation) | 7.536 (0.4698) | 7.299 (0.5710) | 0.0002 |
| Median (interquartile range) | 7.511 (7.238–7.072) | 7.324 (7.833–7.683) | |||
| Simpson index | Mean (standard deviation) | 0.9991 (0.0005267) | 0.9987 (0.001515) | <10−4 | |
| Median (interquartile range) | 0.9992 (0.9988–0.9995) | 0.9990 (0.9985–0.9994) | |||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kodio, A.; Coulibaly, D.; Koné, A.K.; Konaté, S.; Doumbo, S.; Guindo, A.; Bittar, F.; Gouriet, F.; Raoult, D.; Thera, M.A.; et al. Blastocystis Colonization Is Associated with Increased Diversity and Altered Gut Bacterial Communities in Healthy Malian Children. Microorganisms 2019, 7, 649. https://doi.org/10.3390/microorganisms7120649
Kodio A, Coulibaly D, Koné AK, Konaté S, Doumbo S, Guindo A, Bittar F, Gouriet F, Raoult D, Thera MA, et al. Blastocystis Colonization Is Associated with Increased Diversity and Altered Gut Bacterial Communities in Healthy Malian Children. Microorganisms. 2019; 7(12):649. https://doi.org/10.3390/microorganisms7120649
Chicago/Turabian StyleKodio, Aly, Drissa Coulibaly, Abdoulaye Kassoum Koné, Salimata Konaté, Safiatou Doumbo, Abdoulaye Guindo, Fadi Bittar, Frédérique Gouriet, Didier Raoult, Mahamadou Aly Thera, and et al. 2019. "Blastocystis Colonization Is Associated with Increased Diversity and Altered Gut Bacterial Communities in Healthy Malian Children" Microorganisms 7, no. 12: 649. https://doi.org/10.3390/microorganisms7120649
APA StyleKodio, A., Coulibaly, D., Koné, A. K., Konaté, S., Doumbo, S., Guindo, A., Bittar, F., Gouriet, F., Raoult, D., Thera, M. A., & Ranque, S. (2019). Blastocystis Colonization Is Associated with Increased Diversity and Altered Gut Bacterial Communities in Healthy Malian Children. Microorganisms, 7(12), 649. https://doi.org/10.3390/microorganisms7120649







