Marek’s Disease Virus Infection of Natural Killer Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cells
2.2. Viruses
2.3. Growth Kinetics and Plaque Assays
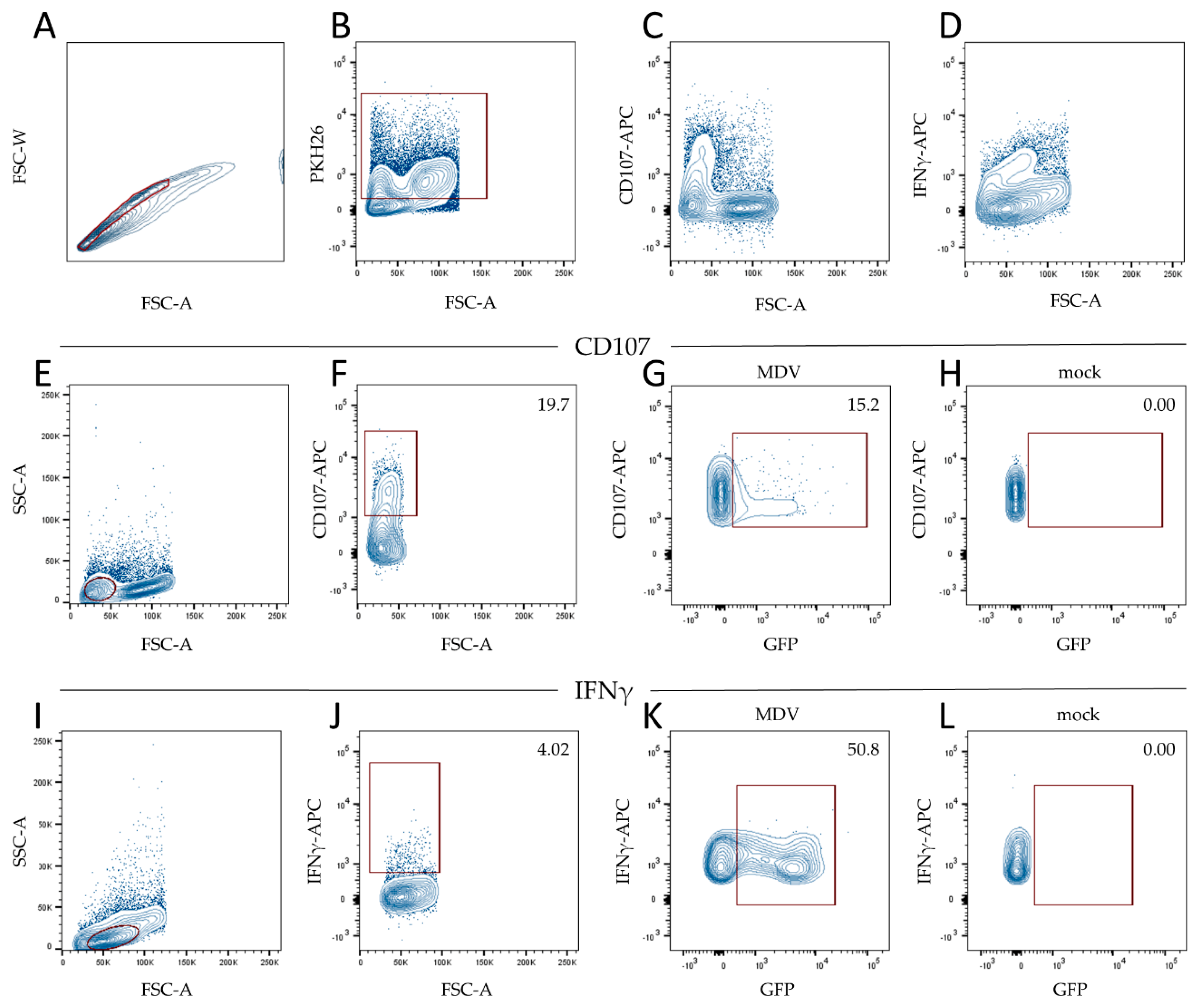
2.4. Infection of NK Cells
2.5. CD107 Assay
2.6. IFNγ Assay
2.7. Flow Cytometry
2.8. Statistical Analyses
3. Results and Discussion
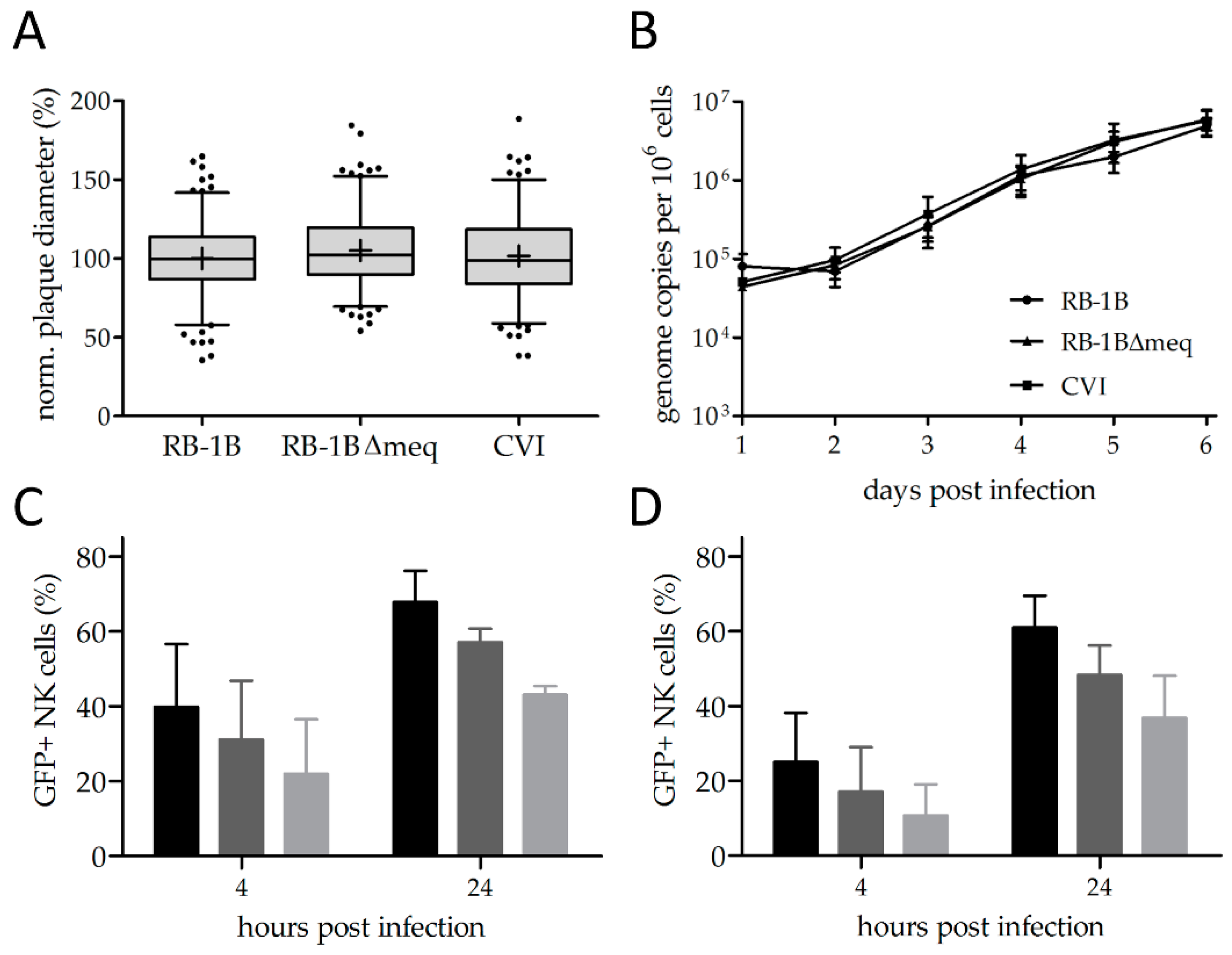
3.1. Reporter Viruses Replicate to Comparable Levels
3.2. MDV Can Readily Infect Chicken NK Cells
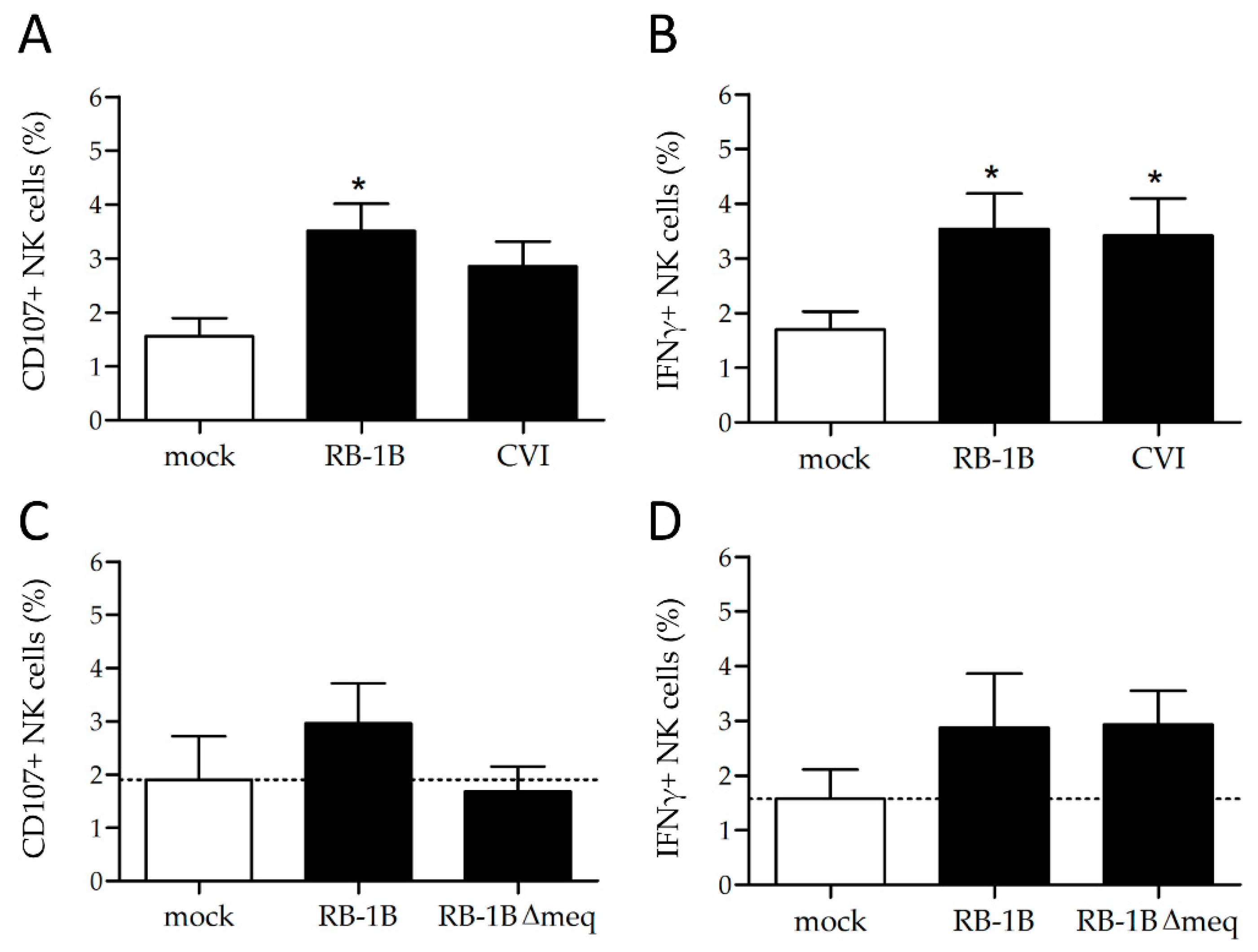
3.3. MDV Enhances NK Cell Degranulation and Increases IFNγ Production
3.4. Lower Rates of Activation in the Absence of the Major MDV Oncogene meq
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Schat, K.A.; Nair, V. Neoplastic Diseases. In Diseases of Poultry; Swayne, D.E., Ed.; John Wiley & Sons; Inc.: Hoboken, NJ, USA, 2017; pp. 513–673. [Google Scholar]
- Baigent, S.J.; Ross, L.J.; Davison, T.F. A flow cytometric method for identifying Marek’s disease virus pp38 expression in lymphocyte subpopulations. Avian Pathol. 1996, 25, 255–267. [Google Scholar] [CrossRef]
- Baaten, B.J.; Staines, K.A.; Smith, L.P.; Skinner, H.; Davison, T.F.; Butter, C. Early replication in pulmonary B cells after infection with Marek’s disease herpesvirus by the respiratory route. Viral Immunol. 2009, 22, 431–444. [Google Scholar] [CrossRef] [PubMed]
- Bertzbach, L.D.; Laparidou, M.; Hartle, S.; Etches, R.J.; Kaspers, B.; Schusser, B.; Kaufer, B.B. Unraveling the role of B cells in the pathogenesis of an oncogenic avian herpesvirus. Proc. Natl. Acad. Sci. USA 2018, 115, 11603–11607. [Google Scholar] [CrossRef] [PubMed]
- Morgan, R.W.; Xie, Q.; Cantello, J.L.; Miles, A.M.; Bernberg, E.L.; Kent, J.; Anderson, A. Marek’s disease virus latency. Curr. Top. Microbiol. Immunol. 2001, 255, 223–243. [Google Scholar] [PubMed]
- Barrow, A.D.; Burgess, S.C.; Baigent, S.J.; Howes, K.; Nair, V.K. Infection of macrophages by a lymphotropic herpesvirus: A new tropism for Marek’s disease virus. J. Gen. Virol. 2003, 84, 2635–2645. [Google Scholar] [CrossRef]
- Chakraborty, P.; Vervelde, L.; Dalziel, R.G.; Wasson, P.S.; Nair, V.; Dutia, B.M.; Kaiser, P. Marek’s disease virus infection of phagocytes: A de novo in vitro infection model. J. Gen. Virol. 2017, 98, 1080–1088. [Google Scholar] [CrossRef]
- Lion, A.; Esnault, E.; Kut, E.; Guillory, V.; Trapp-Fragnet, L.; Soubies, S.M.; Chanteloup, N.; Niepceron, A.; Guabiraba, R.; Marc, D.; et al. Chicken endothelial cells are highly responsive to viral innate immune stimuli and are susceptible to infections with various avian pathogens. Avian Pathol. 2018, 48, 1–41. [Google Scholar] [CrossRef]
- Haq, K.; Schat, K.A.; Sharif, S. Immunity to Marek’s disease: Where are we now? Dev. Comp. Immunol. 2013, 41, 439–446. [Google Scholar] [CrossRef]
- Sharma, J.M. Natural killer cell activity in chickens exposed to Marek’s disease virus: Inhibition of activity in susceptible chickens and enhancement of activity in resistant and vaccinated chickens. Avian Dis. 1981, 25, 882–893. [Google Scholar] [CrossRef]
- Newman, K.C.; Riley, E.M. Whatever turns you on: Accessory-cell-dependent activation of NK cells by pathogens. Nat. Rev. Immunol. 2007, 7, 279–291. [Google Scholar] [CrossRef]
- Jost, S.; Altfeld, M. Control of human viral infections by natural killer cells. Annu. Rev. Immunol. 2013, 31, 163–194. [Google Scholar] [CrossRef] [PubMed]
- Bruchard, M.; Ghiringhelli, F. Deciphering the Roles of Innate Lymphoid Cells in Cancer. Front. Immunol. 2019, 10, 656. [Google Scholar] [CrossRef] [PubMed]
- Voskoboinik, I.; Whisstock, J.C.; Trapani, J.A. Perforin and granzymes: Function, dysfunction and human pathology. Nat. Rev. Immunol. 2015, 15, 388–400. [Google Scholar] [CrossRef] [PubMed]
- Cooper, M.; Fehniger, T.A.; Fuchs, A.; Colonna, M.; Caligiuri, M.A. NK cell and DC interactions. Trends Immunol. 2004, 25, 47–52. [Google Scholar] [CrossRef]
- Nandakumar, S.; Woolard, S.N.; Yuan, D.; Rouse, B.T.; Kumaraguru, U. Natural killer cells as novel helpers in anti-herpes simplex virus immune response. J. Virol. 2008, 82, 10820–10831. [Google Scholar] [CrossRef]
- Schroder, K.; Hertzog, P.J.; Ravasi, T.; Hume, D.A. Interferon-gamma: An overview of signals, mechanisms and functions. J. Leukoc. Biol. 2004, 75, 163–189. [Google Scholar] [CrossRef]
- Quere, P.; Dambrine, G. Development of anti-tumoral cell-mediated cytotoxicity during the course of Marek’s disease in chickens. Ann. Rech. Vet. 1988, 19, 193–201. [Google Scholar]
- Garcia-Camacho, L.; Schat, K.A.; Brooks, R.; Bounous, D.I. Early cell-mediated immune responses to Marek’s disease virus in two chicken lines with defined major histocompatibility complex antigens. Vet. Immunol. Immunop. 2003, 95, 145–153. [Google Scholar] [CrossRef]
- Heller, E.D.; Schat, K.A. Enhancement of natural killer cell activity by Marek’s disease vaccines. Avian Pathol. 1987, 16, 51–60. [Google Scholar] [CrossRef]
- Jansen, C.A.; van de Haar, P.M.; van Haarlem, D.; van Kooten, P.; de Wit, S.; van Eden, W.; Viertlbock, B.C.; Gobel, T.W.; Vervelde, L. Identification of new populations of chicken natural killer (NK) cells. Dev. Comp. Immunol. 2010, 34, 759–767. [Google Scholar] [CrossRef]
- Lupiani, B.; Lee, L.F.; Cui, X.; Gimeno, I.; Anderson, A.; Morgan, R.W.; Silva, R.F.; Witter, R.L.; Kung, H.J.; Reddy, S.M. Marek’s disease virus-encoded Meq gene is involved in transformation of lymphocytes but is dispensable for replication. Proc. Natl. Acad. Sci. USA 2004, 101, 11815–11820. [Google Scholar] [CrossRef] [PubMed]
- Schat, K.; Purchase, H. Cell-culture Methods. In A Laboratory Manual for the Isolation and Identification of Avian Pathogens, 4th ed.; American Association of Avian Pathologists: Kennett Square, PA, USA, 1998. [Google Scholar]
- Gobel, T.W.; Chen, C.L.; Shrimpf, J.; Grossi, C.E.; Bernot, A.; Bucy, R.P.; Auffray, C.; Cooper, M.D. Characterization of avian natural killer cells and their intracellular CD3 protein complex. Eur. J. Immunol. 1994, 24, 1685–1691. [Google Scholar] [CrossRef] [PubMed]
- Bertzbach, L.D.; Pfaff, F.; Pauker, V.I.; Kheimar, A.M.; Höper, D.; Härtle, S.; Karger, A.; Kaufer, B.B. The Transcriptional Landscape of Marek’s Disease Virus in Primary Chicken B Cells Reveals Novel Splice Variants and Genes. Viruses 2019, 11, 264. [Google Scholar] [CrossRef] [PubMed]
- Conradie, A.M.; Bertzbach, L.D.; Bhandari, N.; Parcells, M.; Kaufer, B.B. A Common Live-Attenuated Avian Herpesvirus Vaccine Expresses a Very Potent Oncogene. mSphere 2019, 4, e00658-19. [Google Scholar] [CrossRef]
- Engel, A.T.; Selvaraj, R.K.; Kamil, J.P.; Osterrieder, N.; Kaufer, B.B. Marek’s disease viral interleukin-8 promotes lymphoma formation through targeted recruitment of B cells and CD4+ CD25+ T cells. J. Virol. 2012, 86, 8536–8545. [Google Scholar] [CrossRef]
- Schumacher, D.; Tischer, B.K.; Fuchs, W.; Osterrieder, N. Reconstitution of Marek’s disease virus serotype 1 (MDV-1) from DNA cloned as a bacterial artificial chromosome and characterization of a glycoprotein B-negative MDV-1 mutant. J. Virol. 2000, 74, 11088–11098. [Google Scholar] [CrossRef]
- Ariaans, M.P.; van de Haar, P.M.; Lowenthal, J.W.; van Eden, W.; Hensen, E.J.; Vervelde, L. ELISPOT and intracellular cytokine staining: Novel assays for quantifying T cell responses in the chicken. Dev. Comp. Immunol. 2008, 32, 1398–1404. [Google Scholar] [CrossRef]
- Tai, S.S.; Hearn, C.; Umthong, S.; Agafitei, O.; Cheng, H.H.; Dunn, J.R.; Niikura, M. Expression of Marek’s Disease Virus Oncoprotein Meq During Infection in the Natural Host. Virology 2017, 503, 103–113. [Google Scholar] [CrossRef]
- Petherbridge, L.; Howes, K.; Baigent, S.J.; Sacco, M.A.; Evans, S.; Osterrieder, N.; Nair, V. Replication-competent bacterial artificial chromosomes of Marek’s disease virus: Novel tools for generation of molecularly defined herpesvirus vaccines. J. Virol. 2003, 77, 8712–8718. [Google Scholar] [CrossRef]
- Schermuly, J.; Greco, A.; Hartle, S.; Osterrieder, N.; Kaufer, B.B.; Kaspers, B. In vitro model for lytic replication, latency, and transformation of an oncogenic alphaherpesvirus. Proc. Natl. Acad. Sci. USA 2015, 112, 7279–7284. [Google Scholar] [CrossRef]
- Schat, K.A.; Markowski-Grimsrud, C.J. Immune responses to Marek’s disease virus infection. Curr. Top. Microbiol. Immunol. 2001, 255, 91–120. [Google Scholar] [PubMed]
- Bertzbach, L.D.; Kheimar, A.; Ali, F.A.Z.; Kaufer, B.B. Viral Factors Involved in Marek’s Disease Virus (MDV) Pathogenesis. Curr. Clin. Microbiol. Rep. 2018, 5, 238–244. [Google Scholar] [CrossRef]
- Osterrieder, N.; Kamil, J.P.; Schumacher, D.; Tischer, B.K.; Trapp, S. Marek’s disease virus: From miasma to model. Nat. Rev. Microbiol. 2006, 4, 283–294. [Google Scholar] [CrossRef] [PubMed]
- Hayakawa, Y.; Smyth, M.J. CD27 dissects mature NK cells into two subsets with distinct responsiveness and migratory capacity. J. Immunol. 2006, 176, 1517–1524. [Google Scholar] [CrossRef] [PubMed]
- Jansen, C.A.; de Geus, E.D.; van Haarlem, D.A.; van de Haar, P.M.; Londt, B.Z.; Graham, S.P.; Gobel, T.W.; van Eden, W.; Brookes, S.M.; Vervelde, L. Differential lung NK cell responses in avian influenza virus infected chickens correlate with pathogenicity. Sci. Rep. 2013, 3, 2478. [Google Scholar] [CrossRef] [PubMed]
- Sid, H.; Schusser, B. Applications of Gene Editing in Chickens: A New Era Is on the Horizon. Front. Genet. 2018, 9, 456. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bertzbach, L.D.; van Haarlem, D.A.; Härtle, S.; Kaufer, B.B.; Jansen, C.A. Marek’s Disease Virus Infection of Natural Killer Cells. Microorganisms 2019, 7, 588. https://doi.org/10.3390/microorganisms7120588
Bertzbach LD, van Haarlem DA, Härtle S, Kaufer BB, Jansen CA. Marek’s Disease Virus Infection of Natural Killer Cells. Microorganisms. 2019; 7(12):588. https://doi.org/10.3390/microorganisms7120588
Chicago/Turabian StyleBertzbach, Luca D., Daphne A. van Haarlem, Sonja Härtle, Benedikt B. Kaufer, and Christine A. Jansen. 2019. "Marek’s Disease Virus Infection of Natural Killer Cells" Microorganisms 7, no. 12: 588. https://doi.org/10.3390/microorganisms7120588
APA StyleBertzbach, L. D., van Haarlem, D. A., Härtle, S., Kaufer, B. B., & Jansen, C. A. (2019). Marek’s Disease Virus Infection of Natural Killer Cells. Microorganisms, 7(12), 588. https://doi.org/10.3390/microorganisms7120588





