Neutralizing Antibodies vs. Viruses: Interacting Mechanisms and Escape Tactics
Abstract
1. Introduction
2. The Action Mechanisms of VNAs
2.1. Direct Neutralization Mechanisms
2.1.1. Mechanisms Targeting Viral Entry
Steric Blockade of Receptor-Binding Sites
Polyvalency in Enhancing Neutralization
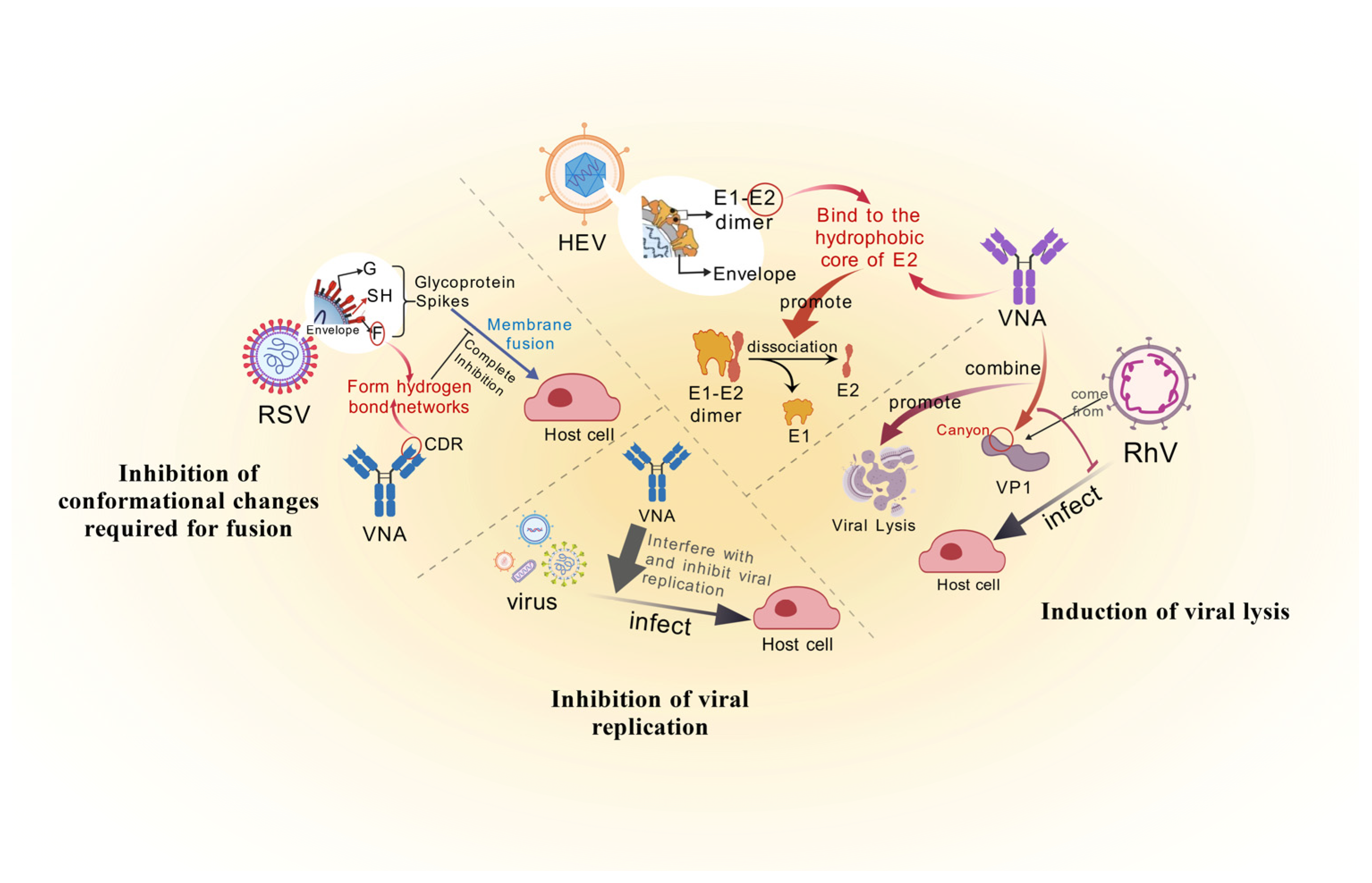
Inhibition of Conformational Changes Required for Fusion
2.1.2. Lysis and Replication Inhibition of Viruses
Induction of Viral Lysis
Inhibition of Viral Replication
2.2. Indirect Effect Functions
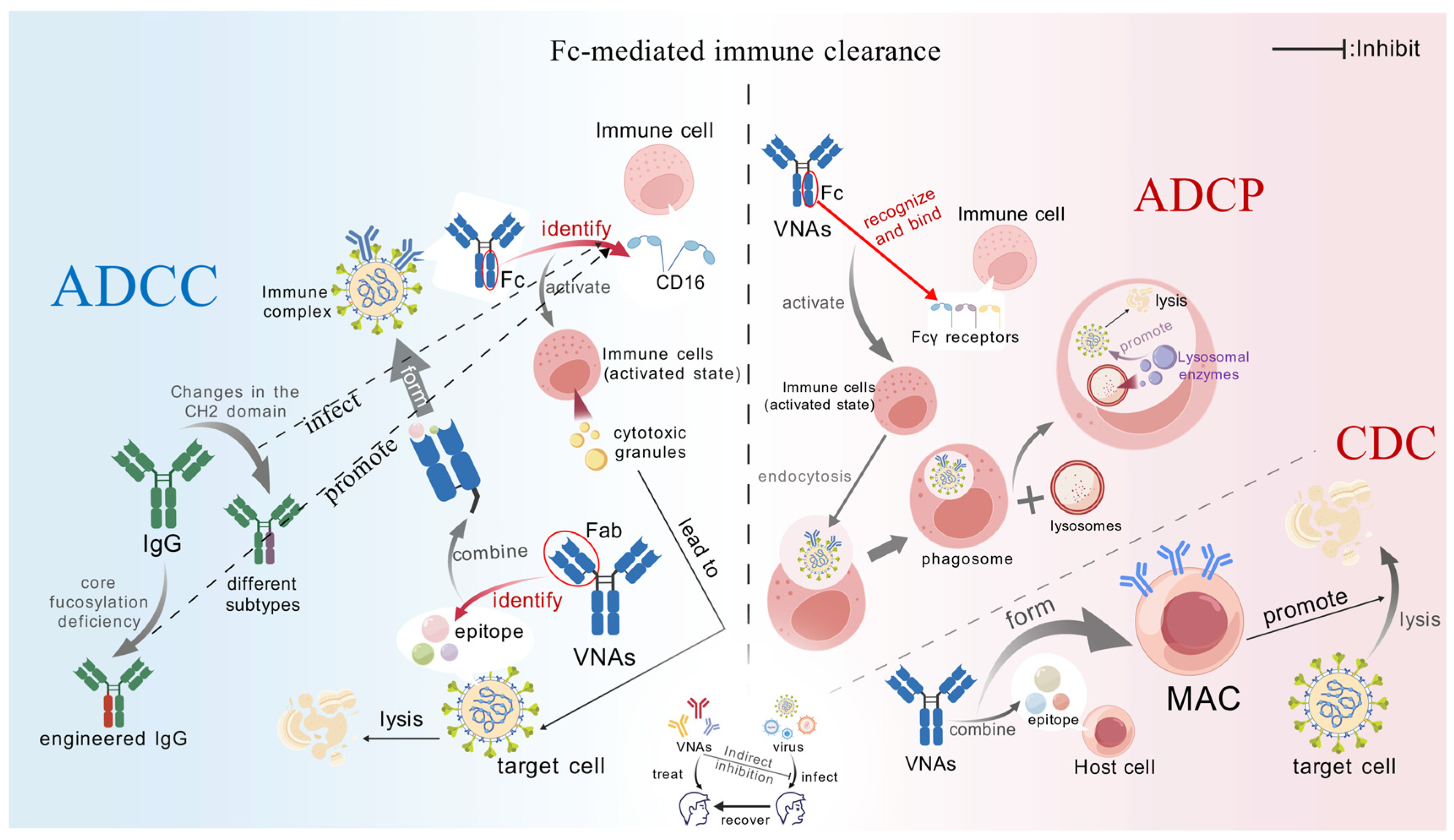
2.2.1. Fc-Mediated Immune Clearance
ADCC
ADCP
CDC
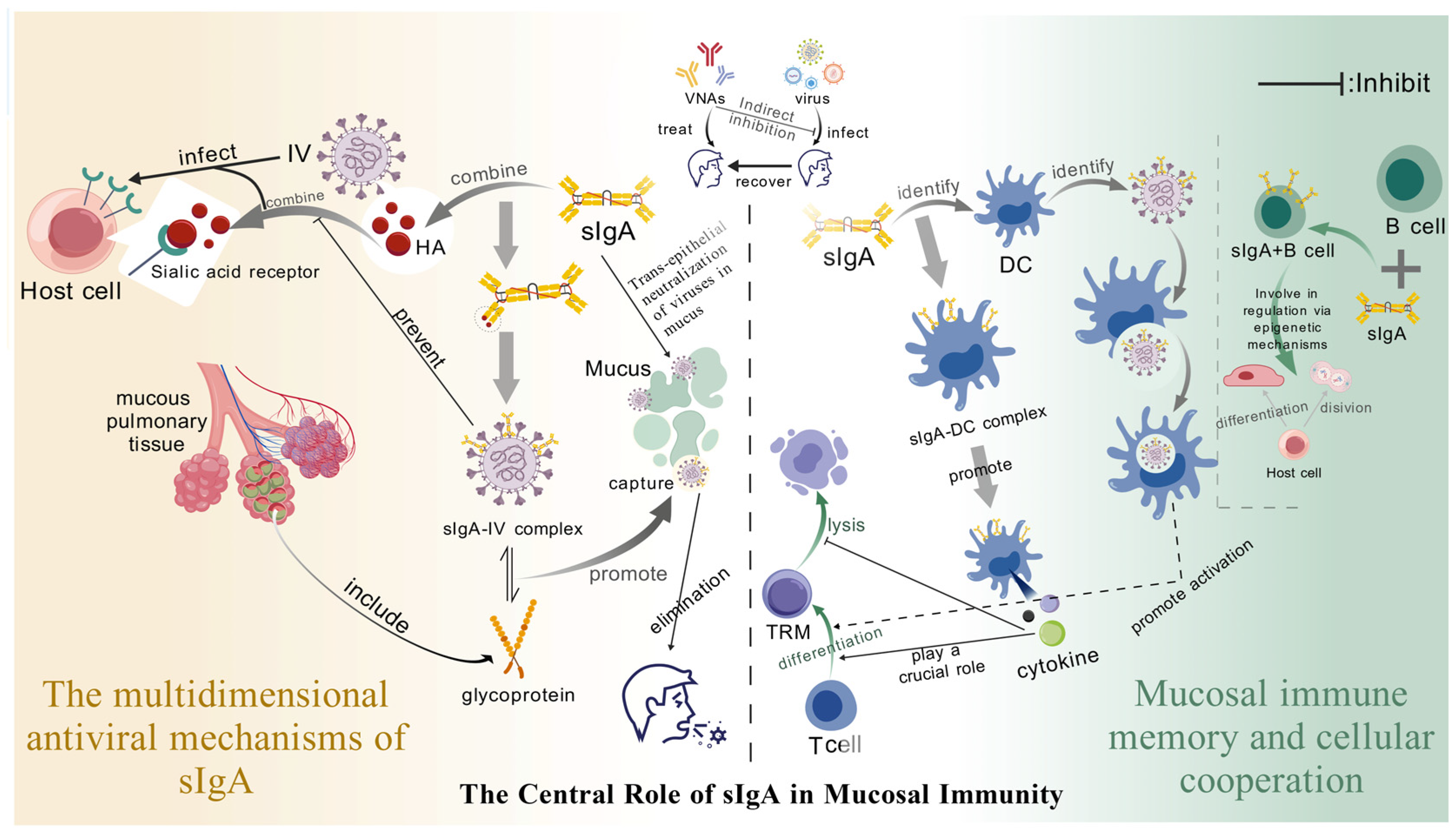
2.2.2. The Central Role of Secretory Immunoglobulin A (sIgA) in Mucosal Immunity
Mucosal Immune Memory Works in Synergy with Cells
3. The Mechanisms by Which Viruses Evade Neutralizing Antibodies
3.1. Antigen Variation
3.1.1. High-Frequency Point Mutations (Point Drift)
3.1.2. Antigenic Shift
3.2. Glycosylation Shielding
3.3. Dynamic Configuration
3.4. Physical Masking and Molecular Camouflage
3.5. ADE
3.6. Immunosuppression and Antibody Interference
3.7. Escape Mutations Under Herd Immunity Pressure
3.8. The Synergy of Escape Strategies
4. Perspectives and Closing Remarks
4.1. Rational Design of Antibodies Driven by Computational Biology
4.2. Immunoengineering Empowers the Innovation of Antibody Function and Delivery
4.3. Individualized Treatment Strategies in the Context of Precision Medicine
4.4. Future Directions and R&D Challenges
- (i)
- Biological challenges: Rapid viral mutations and antigenic diversity demand iterative antibody design. Before the arrival of new variant viruses, can we shift from reactive antibody design (responding to new variants) to proactive/predictive design anticipating viral evolution (AI-guided approaches)? With the crossdisciplinary integration and the development of AI technology, VNAs are gradually moving from “passively following virus evolution” to a new paradigm of “active prediction-active design”. Generative AI platforms (e.g., EVEscape, AlphaFold-Multimer, RFdiffusion) now enable proactive prediction of epitope evolution and antibody sequences months before new variants emerge, facilitating ‘zero-lag’ antibody library generation [148]. Integrated with microfluidics-coupled B-cell single-cell omics and CRISPR editing, these AI designs undergo functional validation within 2–3 weeks, accelerating the design–testing cycle [149].
- (ii)
- Technological and translational challenges: The development of antibody drugs has long faced challenges such as large-scale production, long-term safety, and cost control [150]. During the process of implementation, significant progress has been made in existing technologies. For instance, the half-life engineering (e.g., YTE mutation, as in nirsevimab) has extended the dosing interval from weeks to months, significantly reducing the medical burden in scenarios like virus prevention for premature infants [151], while new delivery systems such as combined dosing regimens, micro-needle arrays, and nasal dry powder inhalation devices are gradually replacing traditional intravenous infusion, enhancing the accessibility of drugs during the pandemic. Moreover, emerging production platforms also demonstrate great potential. For instance, research has shown that the mRNA in vitro transcription-liposome encapsulation method can achieve “digital” antibody production within a short period of time [152]. The economic advantages of the monoclonal antibody production platform are prominent, and in the future, it will provide more affordable localized production solutions for low-income countries.
- (iii)
- Logistical and regulatory challenges: Regulatory frameworks must evolve to address novel modalities. mRNA-encoded antibodies and highly engineered formats (e.g., multi-specifics, Fc-modified antibodies) pose unique evaluation complexities for agencies like the FDA and EMA. Overcoming these demands integrating computational biology, synthetic immunology, and clinical insights.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Abbreviations | Full names |
| VNAs | Antiviral neutralizing antibodies |
| ADE | Antibody-dependent enhancement |
| cryo-EM | Cryo-electron microscopy |
| CDR | Complementarity-determining region |
| RBD | Receptor-binding domain |
| ACE2 | Angiotensin-converting enzyme 2 |
| IV | Influenza virus |
| HA | Hemagglutinin |
| WNV | West Nile virus |
| NTD | N-terminal domain |
| BsAb | Bispecific antibody |
| RSV | Respiratory syncytial virus |
| HEV | Hepatitis E Virus |
| RhV | Rhinovirus |
| SAXS | Small-angle X-ray scattering |
| cryo-ET | Cryo-electron tomography |
| 6-HB | Six-helix bundle |
| ADCC | Antibody-dependent cell-mediated cytotoxicity |
| CD16 | FcγRIII |
| ADCP | Antibody-dependent cellular phagocytosis |
| MAC | Membrane attack complex |
| CDC | Complement dependent cytotoxicity |
| sIgA | Secretory immunoglobulin A |
| DC | Dendritic cell |
| TRM | Tissue-resident memory T |
| non-NAbs | Non neutralizing antibodies |
| weak-NAbs | Weak neutralizing antibodies |
| NK | Natural killer |
| FcμR | Fcμ receptor |
| SC | Secretory component |
| pIgR | Polymeric immunoglobulin receptor |
| TRM | Tissue-resident memory T cells |
| IL-15 | Interleukin-15 |
| RdRps | RNA polymerases |
| HCV | Hepatitis C virus |
| EBV | Epstein–Barr virus |
| MHC-I | Major histocompatibility complex class I |
References
- Vanblargan, L.A.; Goo, L.; Pierson, T.C. Deconstructing the Antiviral Neutralizing-Antibody Response: Implications for Vaccine Development and Immunity. Microbiol. Mol. Biol. Rev. 2016, 80, 989–1010. [Google Scholar] [CrossRef]
- Mian, I.S.; Bradwell, A.R.; Olson, A.J. Structure, function and properties of antibody binding sites. J. Mol. Biol. 1991, 217, 133–151. [Google Scholar] [CrossRef]
- Burton, D.R. Antiviral neutralizing antibodies: From in vitro to in vivo activity. Nat. Rev. Immunol. 2023, 23, 720–734. [Google Scholar] [CrossRef]
- Immink, L.E.; Guthmiller, J.J. Isolation of Rare Antigen-Specific Memory B Cells via Antigen Tetramers. Methods Mol. Biol. 2024, 2826, 95–115. [Google Scholar]
- De Bruin, R.; Spelt, K.; Mol, J.; Koes, R.; Quattrocchio, F. Selection of high-affinity phage antibodies from phage display libraries. Nat. Biotechnol. 1999, 17, 397–399. [Google Scholar] [CrossRef] [PubMed]
- Fishwild, D.M.; O’donnell, S.L.; Bengoechea, T.; Hudson, D.V.; Harding, F.; Bernhard, S.L.; Jones, D.; Kay, R.M.; Higgins, K.M.; Schramm, S.R.; et al. High-avidity human IgG kappa monoclonal antibodies from a novel strain of minilocus transgenic mice. Nat. Biotechnol. 1996, 14, 845–851. [Google Scholar] [CrossRef] [PubMed]
- Jakobovits, A. Production and selection of antigen-specific fully human monoclonal antibodies from mice engineered with human Ig loci. Adv. Drug Deliv. Rev. 1998, 31, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Weinreich, D.M.; Sivapalasingam, S.; Norton, T.; Ali, S.; Gao, H.; Bhore, R.; Musser, B.J.; Soo, Y.; Rofail, D.; Im, J.; et al. REGN-COV2, a Neutralizing Antibody Cocktail, in Outpatients with COVID-19. N. Engl. J. Med. 2021, 384, 238–251. [Google Scholar] [CrossRef]
- Corti, D.; Misasi, J.; Mulangu, S.; Stanley, D.A.; Kanekiyo, M.; Wollen, S.; Ploquin, A.; Doria-Rose, N.A.; Staupe, R.P.; Bailey, M.; et al. Protective monotherapy against lethal Ebola virus infection by a potently neutralizing antibody. Science 2016, 351, 1339–1342. [Google Scholar] [CrossRef]
- Nugent, J.R.; Wood, M.S.; Liu, L.; Bullick, T.; Schapiro, J.M.; Arunleung, P.; Gautham, G.; Getabecha, S.; Morales, C.; Amsden, L.B.; et al. SARS-CoV-2 Omicron subvariant genomic variation associations with immune evasion in Northern California: A retrospective cohort study. medRxiv 2024, 20, e0319218. [Google Scholar] [CrossRef]
- Chen, L.; He, Y.; Liu, H.; Shang, Y.; Guo, G. Potential immune evasion of the severe acute respiratory syndrome coronavirus 2 Omicron variants. Front. Immunol. 2024, 15, 1339660. [Google Scholar] [CrossRef]
- Zheng, M.; Zhou, L.; Huang, Y.; Zhang, X.; Yu, Z.; Yang, C.; Chen, Y.; Ying, D.; Wang, H.; Chen, Z.; et al. Structural basis for the synergetic neutralization of hepatitis E virus by antibody-antibody interaction. Proc. Natl. Acad. Sci. USA 2024, 121, e2408585121. [Google Scholar] [CrossRef]
- Erasmus, J.H.; Archer, J.; Fuerte-Stone, J.; Khandhar, A.P.; Voigt, E.; Granger, B.; Bombardi, R.G.; Govero, J.; Tan, Q.; Durnell, L.A.; et al. Intramuscular Delivery of Replicon RNA Encoding ZIKV-117 Human Monoclonal Antibody Protects against Zika Virus Infection. Mol. Ther. Methods Clin. Dev. 2020, 18, 402–414. [Google Scholar] [CrossRef] [PubMed]
- Barnes, C.O.; Jette, C.A.; Abernathy, M.E.; Dam, K.A.; Esswein, S.R.; Gristick, H.B.; Malyutin, A.G.; Sharaf, N.G.; Huey-Tubman, K.E.; Lee, Y.E.; et al. SARS-CoV-2 neutralizing antibody structures inform therapeutic strategies. Nature 2020, 588, 682–687, Erratum in Nature 2024, 628, E2. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Chen, J.; Zhang, L.; Liu, T.; Ding, M.; Zheng, Y.W.; Zhang, Y. Interfacial subregions of SARS-CoV-2 spike RBD to hACE2 affect intermolecular affinity by their distinct roles played in association and dissociation kinetics. Commun. Biol. 2024, 7, 1621. [Google Scholar] [CrossRef] [PubMed]
- Yuan, M.; Huang, D.; Lee, C.D.; Wu, N.C.; Jackson, A.M.; Zhu, X.; Liu, H.; Peng, L.; van Gils, M.J.; Sanders, R.W.; et al. Structural and functional ramifications of antigenic drift in recent SARS-CoV-2 variants. Science 2021, 373, 818–823. [Google Scholar] [CrossRef]
- Cai, Y.; Zhang, J.; Xiao, T.; Lavine, C.L.; Rawson, S.; Peng, H.; Zhu, H.; Anand, K.; Tong, P.; Gautam, A.; et al. Structural basis for enhanced infectivity and immune evasion of SARS-CoV-2 variants. Science 2021, 373, 642–648. [Google Scholar] [CrossRef]
- Zhou, T.; Georgiev, I.; Wu, X.; Yang, Z.Y.; Dai, K.; Finzi, A.; Kwon, Y.D.; Scheid, J.F.; Shi, W.; Xu, L.; et al. Structural basis for broad and potent neutralization of HIV-1 by antibody VRC01. Science 2010, 329, 811–817. [Google Scholar] [CrossRef]
- Walker, L.M.; Huber, M.; Doores, K.J.; Falkowska, E.; Pejchal, R.; Julien, J.P.; Wang, S.K.; Ramos, A.; Chan-Hui, P.Y.; Moyle, M.; et al. Broad neutralization coverage of HIV by multiple highly potent antibodies. Nature 2011, 477, 466–470. [Google Scholar] [CrossRef]
- Guthmiller, J.J.; Han, J.; Utset, H.A.; Li, L.; Lan, L.Y.; Henry, C.; Stamper, C.T.; McMahon, M.; O’Dell, G.; Fernández-Quintero, M.L.; et al. Broadly neutralizing antibodies target a haemagglutinin anchor epitope. Nature 2022, 602, 314–320. [Google Scholar] [CrossRef]
- Han, T.; Marasco, W.A. Structural basis of influenza virus neutralization. Ann. New York Acad. Sci. 2011, 1217, 178–190. [Google Scholar] [CrossRef]
- Yang, J.R.; Lin, C.H.; Chen, C.J.; Liu, J.L.; Huang, Y.P.; Kuo, C.Y.; Yao, C.Y.; Hsu, L.C.; Lo, J.; Ho, Y.L.; et al. A new antigenic variant of human influenza A (H3N2) virus isolated from airport and community surveillance in Taiwan in early 2009. Virus Res. 2010, 151, 33–38. [Google Scholar] [CrossRef]
- Nogales, A.; Piepenbrink, M.S.; Wang, J.; Ortega, S.; Basu, M.; Fucile, C.F.; Treanor, J.J.; Rosenberg, A.F.; Zand, M.S.; Keefer, M.C.; et al. A Highly Potent and Broadly Neutralizing H1 Influenza-Specific Human Monoclonal Antibody. Sci. Rep. 2018, 8, 4374. [Google Scholar] [CrossRef] [PubMed]
- Jones, K.; Savulescu, A.F.; Brombacher, F.; Hadebe, S. Immunoglobulin M in Health and Diseases: How Far Have We Come and What Next? Front. Immunol. 2020, 11, 595535. [Google Scholar] [CrossRef]
- Adams, C.; Carbaugh, D.L.; Shu, B.; Ng, T.S.; Castillo, I.N.; Bhowmik, R.; Segovia-Chumbez, B.; Puhl, A.C.; Graham, S.; Diehl, S.A.; et al. Structure and neutralization mechanism of a human antibody targeting a complex Epitope on Zika virus. PLoS Pathog. 2023, 19, e1010814. [Google Scholar] [CrossRef]
- Da Vela, S.; Svergun, D.I. Methods, development and applications of small-angle X-ray scattering to characterize biological macromolecules in solution. Curr. Res. Struct. Biol. 2020, 2, 164–170. [Google Scholar] [CrossRef]
- Cao, Y.; Jian, F.; Zhang, Z.; Yisimayi, A.; Hao, X.; Bao, L.; Yuan, F.; Yu, Y.; Du, S.; Wang, J.; et al. Rational identification of potent and broad sarbecovirus-neutralizing antibody cocktails from SARS convalescents. Cell Rep. 2022, 41, 111845. [Google Scholar] [CrossRef]
- Wilkins, D.; Yuan, Y.; Chang, Y.; Aksyuk, A.A.; Núñez, B.S.; Wählby-Hamrén, U.; Zhang, T.; Abram, M.E.; Leach, A.; Villafana, T.; et al. Durability of neutralizing RSV antibodies following nirsevimab administration and elicitation of the natural immune response to RSV infection in infants. Nat. Med. 2023, 29, 1172–1179, Erratum in Nat. Med. 2024, 30, 1785.. [Google Scholar] [CrossRef] [PubMed]
- Zou, G.; Cao, S.; Gao, Z.; Yie, J.; Wu, J.Z. Current state and challenges in respiratory syncytial virus drug discovery and development. Antivir. Res. 2024, 221, 105791. [Google Scholar] [CrossRef] [PubMed]
- Dubois, R.M. Tenacious Researchers Identify a Weakness in All Ebolaviruses. mBio 2018, 9, e02249-18. [Google Scholar] [CrossRef]
- Monteiro, A.; Yu, K.O.A.; Hicar, M.D. Peptide-based Fusion Inhibitors for Preventing the Six-helix Bundle Formation of Class I Fusion Proteins: HIV and Beyond. Curr. HIV Res. 2021, 19, 465–475. [Google Scholar] [CrossRef]
- Houde, D.; Peng, Y.; Berkowitz, S.A.; Engen, J.R. Post-translational modifications differentially affect IgG1 conformation and receptor binding. Mol. Cell. Proteom. 2010, 9, 1716–1728. [Google Scholar] [CrossRef] [PubMed]
- Ubah, O.C.; Lake, E.W.; Gunaratne, G.S.; Gallant, J.P.; Fernie, M.; Robertson, A.J.; Marchant, J.S.; Bold, T.D.; Langlois, R.A.; Matchett, W.E.; et al. Mechanisms of SARS-CoV-2 neutralization by shark variable new antigen receptors elucidated through X-ray crystallography. Nat. Commun. 2021, 12, 7325. [Google Scholar] [CrossRef]
- Schoof, M.; Faust, B.; Saunders, R.A.; Sangwan, S.; Rezelj, V.; Hoppe, N.; Boone, M.; Billesbølle, C.B.; Puchades, C.; Azumaya, C.M.; et al. An ultrapotent synthetic nanobody neutralizes SARS-CoV-2 by stabilizing inactive Spike. Science 2020, 370, 1473–1479. [Google Scholar] [CrossRef] [PubMed]
- Nan, X.; Li, Y.; Zhang, R.; Wang, R.; Lv, N.; Li, J.; Chen, Y.; Zhou, B.; Wang, Y.; Wang, Z.; et al. Exploring distinct modes of inter-spike cross-linking for enhanced neutralization by SARS-CoV-2 antibodies. Nat. Commun. 2024, 15, 10578. [Google Scholar] [CrossRef] [PubMed]
- Al Qaraghuli, M.M.; Kubiak-Ossowska, K.; Ferro, V.A.; Mulheran, P.A. Antibody-protein binding and conformational changes: Identifying allosteric signalling pathways to engineer a better effector response. Sci. Rep. 2020, 10, 13696. [Google Scholar] [CrossRef]
- Smith, T.J.; Olson, N.H.; Cheng, R.H.; Liu, H.; Baker, T.S. Structure of human rhinovirus complexed with Fab fragments from a neutralizing antibody. J. Virol. 1993, 67, 1148–1158. [Google Scholar] [CrossRef]
- Dong, Y.; Liu, Y.; Jiang, W.; Smith, T.J.; Xu, Z.; Rossmann, M.G. Antibody-induced uncoating of human rhinovirus B14. Proc. Natl. Acad. Sci. USA 2017, 114, 8017–8022. [Google Scholar] [CrossRef]
- Ruihan, L.; Runan, Z.; Yuan, Q. Infections. Research progress on NS1 protein of influenza A viruses. J. Microbes Infect. 2019, 14, 39–45. [Google Scholar]
- Sootichote, R.; Puangmanee, W.; Benjathummarak, S.; Kowaboot, S.; Yamanaka, A.; Boonnak, K.; Ampawong, S.; Chatchen, S.; Ramasoota, P.; Pitaksajjakul, P. Potential Protective Effect of Dengue NS1 Human Monoclonal Antibodies against Dengue and Zika Virus Infections. Biomedicines 2023, 11, 227. [Google Scholar] [CrossRef]
- Gay, C.L.; James, K.S.; Tuyishime, M.; Falcinelli, S.D.; Joseph, S.B.; Moeser, M.J.; Allard, B.; Kirchherr, J.L.; Clohosey, M.; Raines, S.L.M.; et al. Stable Latent HIV Infection and Low-level Viremia Despite Treatment with the Broadly Neutralizing Antibody VRC07-523LS and the Latency Reversal Agent Vorinostat. J. Infect. Dis. 2022, 225, 856–861. [Google Scholar] [CrossRef]
- Chan, K.R.; Ong, E.Z.; Mok, D.Z.; Ooi, E.E. Fc receptors and their influence on efficacy of therapeutic antibodies for treatment of viral diseases. Expert Rev. Anti-Infect. Ther. 2015, 13, 1351–1360. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Gunasekaran, K.; Wang, W.; Razinkov, V.; Sekirov, L.; Leng, E.; Sweet, H.; Foltz, I.; Howard, M.; Rousseau, A.M.; et al. Asymmetrical Fc engineering greatly enhances antibody-dependent cellular cytotoxicity (ADCC) effector function and stability of the modified antibodies. J. Biol. Chem. 2014, 289, 3571–3590. [Google Scholar] [CrossRef] [PubMed]
- de Taeye, S.W.; Bentlage, A.E.H.; Mebius, M.M.; Meesters, J.I.; Lissenberg-Thunnissen, S.; Falck, D.; Sénard, T.; Salehi, N.; Wuhrer, M.; Schuurman, J.; et al. FcγR Binding and ADCC Activity of Human IgG Allotypes. Front. Immunol. 2020, 11, 740. [Google Scholar] [CrossRef]
- Nagashima, H.; Ootsubo, M.; Fukazawa, M.; Motoi, S.; Konakahara, S.; Masuho, Y. Enhanced antibody-dependent cellular phagocytosis by chimeric monoclonal antibodies with tandemly repeated Fc domains. J. Biosci. Bioeng. 2011, 111, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Simmons, M.; Putnak, R.; Sun, P.; Burgess, T.; Marasco, W.A. Antibody Prophylaxis Against Dengue Virus 2 Infection in Non-Human Primates. Am. J. Trop. Med. Hyg. 2016, 95, 1148–1156. [Google Scholar] [CrossRef]
- Sarker, A.; Dhama, N.; Gupta, R.D. Dengue virus neutralizing antibody: A review of targets, cross-reactivity, and antibody-dependent enhancement. Front. Immunol. 2023, 14, 1200195. [Google Scholar] [CrossRef]
- Taylor, R.P. T cells reinforce NK cell-mediated ADCC. Blood 2024, 143, 1786–1787. [Google Scholar] [CrossRef]
- Ravetch, J.V.; Bolland, S. IgG Fc receptors. Annu. Rev. Immunol. 2001, 19, 275–290. [Google Scholar] [CrossRef]
- Bournazos, S.; Wang, T.T.; Dahan, R.; Maamary, J.; Ravetch, J.V. Signaling by Antibodies: Recent Progress. Annu. Rev. Immunol. 2017, 35, 285–311. [Google Scholar] [CrossRef] [PubMed]
- Kallolimath, S.; Sun, L.; Palt, R.; Föderl-Höbenreich, E.; Hermle, A.; Voss, L.; Kleim, M.; Nimmerjahn, F.; Gach, J.S.; Hitchcock, L.; et al. IgG1 versus IgG3: Influence of antibody-specificity and allotypic variance on virus neutralization efficacy. Front. Immunol. 2024, 15, 1490515. [Google Scholar] [CrossRef] [PubMed]
- Shields, R.L.; Lai, J.; Keck, R.; O’connell, L.Y.; Hong, K.; Meng, Y.G.; Weikert, S.H.; Presta, L.G. Lack of fucose on human IgG1 N-linked oligosaccharide improves binding to human Fcgamma RIII and antibody-dependent cellular toxicity. J. Biol. Chem. 2002, 277, 26733–26740. [Google Scholar] [CrossRef] [PubMed]
- Mulangu, S.; Dodd, L.E.; Davey, R.T., Jr.; Tshiani Mbaya, O.; Proschan, M.; Mukadi, D.; Lusakibanza Manzo, M.; Nzolo, D.; Tshomba Oloma, A.; Ibanda, A.; et al. A Randomized, Controlled Trial of Ebola Virus Disease Therapeutics. N. Engl. J. Med. 2019, 381, 2293–2303. [Google Scholar] [CrossRef]
- Giron, L.B.; Liu, Q.; Adeniji, O.S.; Yin, X.; Kannan, T.; Ding, J.; Lu, D.Y.; Langan, S.; Zhang, J.; Azevedo, J.; et al. Immunoglobulin G N-glycan markers of accelerated biological aging during chronic HIV infection. Nat. Commun. 2024, 15, 3035. [Google Scholar] [CrossRef]
- Su, S.; Zhao, J.; Xing, Y.; Zhang, X.; Liu, J.; Ouyang, Q.; Chen, J.; Su, F.; Liu, Q.; Song, E. Immune Checkpoint Inhibition Overcomes ADCP-Induced Immunosuppression by Macrophages. Cell 2018, 175, 442–457.E23. [Google Scholar] [CrossRef] [PubMed]
- Abdeldaim, D.T.; Schindowski, K. Fc-Engineered Therapeutic Antibodies: Recent Advances and Future Directions. Pharmaceutics 2023, 15, 2402. [Google Scholar] [CrossRef]
- Shi, Y.; Sun, Y.; Seki, A.; Rutz, S.; Koerber, J.T.; Wang, J. A real-time antibody-dependent cellular phagocytosis assay by live cell imaging. J. Immunol. Methods 2024, 531, 113715. [Google Scholar] [CrossRef]
- Wilkinson, I.; Anderson, S.; Fry, J.; Julien, L.A.; Neville, D.; Qureshi, O.; Watts, G.; Hale, G. Fc-engineered antibodies with immune effector functions completely abolished. PLoS ONE 2021, 16, e0260954. [Google Scholar] [CrossRef]
- Oganesyan, V.; Damschroder, M.M.; Woods, R.M.; Cook, K.E.; Wu, H.; Dall’acqua, W.F. Structural characterization of a human Fc fragment engineered for extended serum half-life. Mol. Immunol. 2009, 46, 1750–1755. [Google Scholar] [CrossRef]
- Matsumoto, M.L. Molecular Mechanisms of Multimeric Assembly of IgM and IgA. Annu. Rev. Immunol. 2022, 40, 221–247. [Google Scholar] [CrossRef]
- Sharp, T.H.; Boyle, A.L.; Diebolder, C.A.; Kros, A.; Koster, A.J.; Gros, P. Insights into IgM-mediated complement activation based on in situ structures of IgM-C1-C4b. Proc. Natl. Acad. Sci. USA 2019, 116, 11900–11905. [Google Scholar] [CrossRef]
- Svilenov, H.L.; Bester, R.; Sacherl, J.; Absmeier, R.; Peters, C.; Protzer, U.; Brockmeyer, C.; Buchner, J. Multimeric ACE2-IgM fusions as broadly active antivirals that potently neutralize SARS-CoV-2 variants. Commun. Biol. 2022, 5, 1237. [Google Scholar] [CrossRef] [PubMed]
- Sopp, J.M.; Peters, S.J.; Rowley, T.F.; Oldham, R.J.; James, S.; Mockridge, I.; French, R.R.; Turner, A.; Beers, S.A.; Humphreys, D.P.; et al. On-target IgG hexamerisation driven by a C-terminal IgM tail-piece fusion variant confers augmented complement activation. Commun. Biol. 2021, 4, 1031. [Google Scholar] [CrossRef]
- He, J.; Chen, Y.; Li, Y.; Feng, Y. Molecular mechanisms and therapeutic interventions in acute kidney injury: A literature review. BMC Nephrol. 2025, 26, 144. [Google Scholar] [CrossRef]
- Doorduijn, D.J.; Lukassen, M.V.; Van‘t Wout, M.F.L.; Franc, V.; Ruyken, M.; Bardoel, B.W.; Heck, A.J.R.; Rooijakkers, S.H.M. Soluble MAC is primarily released from MAC-resistant bacteria that potently convert complement component C5. eLife 2022, 11, e77503. [Google Scholar] [CrossRef]
- Saito, S.; Namisaki, H.; Hiraishi, K.; Takahashi, N.; Iida, S. A stable engineered human IgG3 antibody with decreased aggregation during antibody expression and low pH stress. Protein Sci. 2019, 28, 900–909. [Google Scholar] [CrossRef]
- Blank, A.; Hohmann, N.; Dettmer, M.; Manka-Stuhlik, A.; Mikus, G.; Stoll, F.; Stützle-Schnetz, M.; Thomas, D.; Exner, E.; Schmitt-Bormann, B.; et al. First-in-human, randomized, double-blind, placebo-controlled, dose escalation trial of the anti-herpes simplex virus monoclonal antibody HDIT101 in healthy volunteers. Clin. Transl. Sci. 2022, 15, 2366–2377. [Google Scholar] [CrossRef] [PubMed]
- Brezski, R.J.; Vafa, O.; Petrone, D.; Tam, S.H.; Powers, G.; Ryan, M.H.; Luongo, J.L.; Oberholtzer, A.; Knight, D.M.; Jordan, R.E. Tumor-associated and microbial proteases compromise host IgG effector functions by a single cleavage proximal to the hinge. Proc. Natl. Acad. Sci. USA 2009, 106, 17864–17869. [Google Scholar] [CrossRef] [PubMed]
- Kraivong, R.; Punyadee, N.; Liszewski, M.K.; Atkinson, J.P.; Avirutnan, P. Dengue and the Lectin Pathway of the Complement System. Viruses 2021, 13, 1219. [Google Scholar] [CrossRef]
- Shresta, S. Role of complement in dengue virus infection: Protection or pathogenesis? mBio 2012, 3, e00003-12. [Google Scholar] [CrossRef]
- Saito, S.; Sano, K.; Suzuki, T.; Ainai, A.; Taga, Y.; Ueno, T.; Tabata, K.; Saito, K.; Wada, Y.; Ohara, Y.; et al. IgA tetramerization improves target breadth but not peak potency of functionality of anti-influenza virus broadly neutralizing antibody. PLoS Pathog. 2019, 15, e1007427. [Google Scholar] [CrossRef]
- Corthésy, B. Multi-faceted functions of secretory IgA at mucosal surfaces. Front. Immunol. 2013, 4, 185. [Google Scholar] [CrossRef]
- Göritzer, K.; Strasser, R.; Ma, J.K. Stability Engineering of Recombinant Secretory IgA. Int. J. Mol. Sci. 2024, 25, 6856. [Google Scholar] [CrossRef] [PubMed]
- Kumar Bharathkar, S.; Stadtmueller, B.M. Structural and Biochemical Requirements for Secretory Component Interactions with Dimeric IgA. J. Immunol. 2024, 213, 226–234. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, G.; Li, Y.; Zhu, Q.; Shen, H.; Gao, N.; Xiao, J. Structural insights into secretory immunoglobulin A and its interaction with a pneumococcal adhesin. Cell Res. 2020, 30, 602–609. [Google Scholar] [CrossRef] [PubMed]
- Raskova Kafkova, L.; Brokesova, D.; Krupka, M.; Stehlikova, Z.; Dvorak, J.; Coufal, S.; Fajstova, A.; Srutkova, D.; Stepanova, K.; Hermanova, P.; et al. Secretory IgA N-glycans contribute to the protection against E. coli O55 infection of germ-free piglets. Mucosal Immunol. 2021, 14, 511–522. [Google Scholar] [CrossRef] [PubMed]
- Mazanec, M.B.; Kaetzel, C.S.; Lamm, M.E.; Fletcher, D.; Nedrud, J.G. Intracellular neutralization of virus by immunoglobulin A antibodies. Proc. Natl. Acad. Sci. USA 1992, 89, 6901–6905. [Google Scholar] [CrossRef]
- Mazanec, M.B.; Coudret, C.L.; Fletcher, D.R. Intracellular neutralization of influenza virus by immunoglobulin A anti-hemagglutinin monoclonal antibodies. J. Virol. 1995, 69, 1339–1343. [Google Scholar] [CrossRef]
- Rocha-Zavaleta, L.; Barrios, T.; García-Carrancá, A.; Valdespino, V.; Cruz-Talonia, F. Cervical secretory immunoglobulin A to human papillomavirus type 16 (HPV16) from HPV16-infected women inhibit HPV16 virus-like particles-induced hemagglutination of mouse red blood cells. FEMS Immunol. Med. Microbiol. 2001, 31, 47–51. [Google Scholar] [CrossRef]
- Qin, D.; Li, Y.; Chen, X.; Li, L.; Wang, G.; Hou, X.; Yu, L. Secretory IgA-ETEC F5 Immune Complexes Promote Dendritic Cell Differentiation and Prime T Cell Proliferation in the Mouse Intestine. Life 2023, 13, 1936. [Google Scholar] [CrossRef]
- Crowl, J.T.; Heeg, M.; Ferry, A.; Milner, J.J.; Omilusik, K.D.; Toma, C.; He, Z.; Chang, J.T.; Goldrath, A.W. Tissue-resident memory CD8+ T cells possess unique transcriptional, epigenetic and functional adaptations to different tissue environments. Nat. Immunol. 2022, 23, 1121–1131. [Google Scholar] [CrossRef]
- Richards, A.; Baranova, D.; Mantis, N.J. The prospect of orally administered monoclonal secretory IgA (SIgA) antibodies to prevent enteric bacterial infections. Hum. Vaccines Immunother. 2022, 18, 1964317. [Google Scholar] [CrossRef] [PubMed]
- Lycke, N. Recent progress in mucosal vaccine development: Potential and limitations. Nat. Rev. Immunol. 2012, 12, 592–605. [Google Scholar] [CrossRef] [PubMed]
- Langel, S.N.; Otero, C.E.; Steppe, J.T.; Williams, C.A.; Travieso, T.; Chang, J.; Webster, H.; Williamson, L.E.; Crowe, J.E., Jr.; Greenberg, H.B.; et al. Breast milk delivery of an engineered dimeric IgA protects neonates against rotavirus. Mucosal Immunol. 2025, 18, 441–452. [Google Scholar] [CrossRef]
- Omatola, C.A.; Olaniran, A.O. Rotaviruses: From Pathogenesis to Disease Control-A Critical Review. Viruses 2022, 14, 875. [Google Scholar] [CrossRef] [PubMed]
- Shao, W.; Wang, Y.; Fang, Q.; Shi, W.; Qi, H. Epigenetic recording of stimulation history reveals BLIMP1-BACH2 balance in determining memory B cell fate upon recall challenge. Nat. Immunol. 2024, 25, 1432–1444. [Google Scholar] [CrossRef]
- Luo, X.; Hou, X.; Wang, Y.; Li, Y.; Yu, S.; Qi, H. An interleukin-9-ZBTB18 axis promotes germinal center development of memory B cells. Immunity 2025, 175, 442–457.e23. [Google Scholar] [CrossRef]
- Dominguez, P.M.; Teater, M.; Chambwe, N.; Kormaksson, M.; Redmond, D.; Ishii, J.; Vuong, B.; Chaudhuri, J.; Melnick, A.; Vasanthakumar, A.; et al. DNA Methylation Dynamics of Germinal Center B Cells Are Mediated by AID. Cell Rep. 2015, 12, 2086–2098. [Google Scholar] [CrossRef]
- Xiao, F.; Rui, K.; Shi, X.; Wu, H.; Cai, X.; Lui, K.O.; Lu, Q.; Ballestar, E.; Tian, J.; Zou, H.; et al. Epigenetic regulation of B cells and its role in autoimmune pathogenesis. Cell. Mol. Immunol. 2022, 19, 1215–1234. [Google Scholar] [CrossRef]
- Yu, B.; Qi, Y.; Li, R.; Shi, Q.; Satpathy, A.T.; Chang, H.Y. B cell-specific XIST complex enforces X-inactivation and restrains atypical B cells. Cell 2021, 184, 1790–1803.e17. [Google Scholar] [CrossRef]
- Wang, Y.T.; Branche, E.; Xie, J.; Mcmillan, R.E.; Ana-Sosa-Batiz, F.; Lu, H.H.; Li, Q.H.; Clark, A.E.; Valls Cuevas, J.M.; Viramontes, K.M.; et al. Zika but not Dengue virus infection limits NF-κB activity in human monocyte-derived dendritic cells and suppresses their ability to activate T cells. Nat. Commun. 2025, 16, 2695. [Google Scholar] [CrossRef]
- He, P.; Liu, B.; Gao, X.; Yan, Q.; Pei, R.; Sun, J.; Chen, Q.; Hou, R.; Li, Z.; Zhang, Y.; et al. SARS-CoV-2 Delta and Omicron variants evade population antibody response by mutations in a single spike epitope. Nat. Microbiol. 2022, 7, 1635–1649. [Google Scholar] [CrossRef]
- Kaku, Y.; Okumura, K.; Padilla-Blanco, M.; Kosugi, Y.; Uriu, K.; Hinay, A.A., Jr.; Chen, L.; Plianchaisuk, A.; Kobiyama, K.; Ishii, K.J.; et al. Virological characteristics of the SARS-CoV-2 JN.1 variant. Lancet Infect. Dis. 2024, 24, e82. [Google Scholar] [CrossRef]
- Das, S.R.; Hensley, S.E.; Ince, W.L.; Brooke, C.B.; Subba, A.; Delboy, M.G.; Russ, G.; Gibbs, J.S.; Bennink, J.R.; Yewdell, J.W. Defining influenza A virus hemagglutinin antigenic drift by sequential monoclonal antibody selection. Cell Host Microbe 2013, 13, 314–323. [Google Scholar] [CrossRef]
- Maurer, D.P.; Vu, M.; Schmidt, A.G. Antigenic drift expands influenza viral escape pathways from recalled humoral immunity. Immunity 2025, 58, 716–727.e6. [Google Scholar] [CrossRef]
- Kaverin, N.V.; Rudneva, I.A.; Govorkova, E.A.; Timofeeva, T.A.; Shilov, A.A.; Kochergin-Nikitsky, K.S.; Krylov, P.S.; Webster, R.G. Epitope mapping of the hemagglutinin molecule of a highly pathogenic H5N1 influenza virus by using monoclonal antibodies. J. Virol. 2007, 81, 12911–12917. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Hong, W.; Shi, H.; Wang, Z.; He, C.; Lei, H.; Yan, H.; Alu, A.; Ao, D.; Chen, Z.; et al. A recombinant protein vaccine induces protective immunity against SARS-CoV-2 JN.1 and XBB-lineage subvariants. Signal Transduct. Target. Ther. 2025, 10, 58. [Google Scholar] [CrossRef] [PubMed]
- Dai, W.; Li, X.; Liu, Z.; Zhang, C. Identification of four neutralizing antigenic sites on the enterovirus D68 capsid. J. Virol. 2023, 97, e0160023. [Google Scholar] [CrossRef]
- Burton, D.R.; Hangartner, L. Broadly Neutralizing Antibodies to HIV and Their Role in Vaccine Design. Annu. Rev. Immunol. 2016, 34, 635–659. [Google Scholar] [CrossRef]
- Zhang, F.; Schmidt, F.; Muecksch, F.; Wang, Z.; Gazumyan, A.; Nussenzweig, M.C.; Gaebler, C.; Caskey, M.; Hatziioannou, T.; Bieniasz, P.D. SARS-CoV-2 spike glycosylation affects function and neutralization sensitivity. mBio 2024, 15, e0167223. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Liang, Q.; He, X.; Zhao, C.; Ren, W.; Yang, Z.; Wang, Z.; Ding, Q.; Deng, H.; Wang, T.; et al. Loss of Spike N370 glycosylation as an important evolutionary event for the enhanced infectivity of SARS-CoV-2. Cell Res. 2022, 32, 315–318. [Google Scholar] [CrossRef]
- Idris, F.; Muharram, S.H.; Diah, S. Glycosylation of dengue virus glycoproteins and their interactions with carbohydrate receptors: Possible targets for antiviral therapy. Arch. Virol. 2016, 161, 1751–1760. [Google Scholar] [CrossRef]
- Mondotte, J.A.; Lozach, P.Y.; Amara, A.; Gamarnik, A.V. Essential role of dengue virus envelope protein N glycosylation at asparagine-67 during viral propagation. J. Virol. 2007, 81, 7136–7148. [Google Scholar] [CrossRef]
- Deng, L.; Cao, H.; Li, G.; Zhou, K.; Fu, Z.; Zhong, J.; Wang, Z.; Yang, X. Progress on Respiratory Syncytial Virus Vaccine Development and Evaluation Methods. Vaccines 2025, 13, 304. [Google Scholar] [CrossRef]
- Radzimanowski, J.; Effantin, G.; Weissenhorn, W. Conformational plasticity of the Ebola virus matrix protein. Protein Sci. 2014, 23, 1519–1527. [Google Scholar] [CrossRef]
- Li, K.; Huang, B.; Wu, M.; Zhong, A.; Li, L.; Cai, Y.; Wang, Z.; Wu, L.; Zhu, M.; Li, J.; et al. Dynamic changes in anti-SARS-CoV-2 antibodies during SARS-CoV-2 infection and recovery from COVID-19. Nat. Commun. 2020, 11, 6044. [Google Scholar] [CrossRef]
- Bhattacharya, M.; Chatterjee, S.; Lee, S.S.; Dhama, K.; Chakraborty, C. Antibody evasion associated with the RBD significant mutations in several emerging SARS-CoV-2 variants and its subvariants. Drug Resist. Updates 2023, 71, 101008. [Google Scholar] [CrossRef]
- Tzarum, N.; Wilson, I.A.; Law, M. The Neutralizing Face of Hepatitis C Virus E2 Envelope Glycoprotein. Front. Immunol. 2018, 9, 1315. [Google Scholar] [CrossRef]
- Tanner, J.E.; Coinçon, M.; Leblond, V.; Hu, J.; Fang, J.M.; Sygusch, J.; Alfieri, C. Peptides designed to spatially depict the Epstein-Barr virus major virion glycoprotein gp350 neutralization epitope elicit antibodies that block virus-neutralizing antibody 72A1 interaction with the native gp350 molecule. J. Virol. 2015, 89, 4932–4941. [Google Scholar] [CrossRef]
- Wessel, A.W.; Kose, N.; Bombardi, R.G.; Roy, V.; Chantima, W.; Mongkolsapaya, J.; Edeling, M.A.; Nelson, C.A.; Bosch, I.; Alter, G.; et al. Antibodies targeting epitopes on the cell-surface form of NS1 protect against Zika virus infection during pregnancy. Nat. Commun. 2020, 11, 5278. [Google Scholar] [CrossRef]
- Troyer, Z.; Alhusaini, N.; Tabler, C.O.; Sweet, T.; De Carvalho, K.I.L.; Schlatzer, D.M.; Carias, L.; King, C.L.; Matreyek, K.; Tilton, J.C. Extracellular vesicles carry SARS-CoV-2 spike protein and serve as decoys for neutralizing antibodies. J. Extracell. Vesicles 2021, 10, e12112. [Google Scholar] [CrossRef]
- Rydell, G.E.; Prakash, K.; Norder, H.; Lindh, M. Hepatitis B surface antigen on subviral particles reduces the neutralizing effect of anti-HBs antibodies on hepatitis B viral particles in vitro. Virology 2017, 509, 67–70. [Google Scholar] [CrossRef]
- Bdeir, N.; Lüddecke, T.; Maaß, H.; Schmelz, S.; Rand, U.; Jacobsen, H.; Metzdorf, K.; Kulkarni, U.; Cossmann, A.; Stankov, M.V.; et al. Reverse mutational scanning of SARS-CoV-2 spike BA.2.86 identifies epitopes contributing to immune escape from polyclonal sera. Nat. Commun. 2025, 16, 809. [Google Scholar] [CrossRef]
- Wang, X.; Lu, L.; Jiang, S. SARS-CoV-2 evolution from the BA.2.86 to JN.1 variants: Unexpected consequences. Trends Immunol. 2024, 45, 81–84. [Google Scholar] [CrossRef]
- Guha, D.; Ayyavoo, V. Innate immune evasion strategies by human immunodeficiency virus type 1. Isrn Aids 2013, 2013, 954806. [Google Scholar] [CrossRef]
- Lilley, B.N.; Ploegh, H.L.; Tirabassi, R.S. Human cytomegalovirus open reading frame TRL11/IRL11 encodes an immunoglobulin G Fc-binding protein. J. Virol. 2001, 75, 11218–11221. [Google Scholar] [CrossRef]
- Bai, L.; Xu, J.; Zeng, L.; Zhang, L.; Zhou, F. A review of HSV pathogenesis, vaccine development, and advanced applications. Mol. Biomed. 2024, 5, 35. [Google Scholar] [CrossRef]
- Dai, X.; Zhou, Z.H. Structure of the herpes simplex virus 1 capsid with associated tegument protein complexes. Science 2018, 360, eaao7298. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Y.; Li, Y.; Huang, F.; Luo, B.; Yuan, Y.; Xia, B.; Ma, X.; Yang, T.; Yu, F.; et al. The ORF8 protein of SARS-CoV-2 mediates immune evasion through down-regulating MHC-I. Proc. Natl. Acad. Sci. USA 2021, 118, e2024202118. [Google Scholar] [CrossRef]
- Lei, R.; Liang, W.; Ouyang, W.O.; Hernandez Garcia, A.; Kikuchi, C.; Wang, S.; Mcbride, R.; Tan, T.J.C.; Sun, Y.; Chen, C.; et al. Epistasis mediates the evolution of the receptor binding mode in recent human H3N2 hemagglutinin. Nat. Commun. 2024, 15, 5175. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, J.; Johnson, B.A.; Xia, H.; Ku, Z.; Schindewolf, C.; Widen, S.G.; An, Z.; Weaver, S.C.; Menachery, V.D.; et al. Delta spike P681R mutation enhances SARS-CoV-2 fitness over Alpha variant. Cell Rep. 2022, 39, 110829. [Google Scholar] [CrossRef]
- Chakraborty, C.; Sharma, A.R.; Bhattacharya, M.; Lee, S.S. A Detailed Overview of Immune Escape, Antibody Escape, Partial Vaccine Escape of SARS-CoV-2 and Their Emerging Variants with Escape Mutations. Front. Immunol. 2022, 13, 801522. [Google Scholar] [CrossRef]
- Jaki, L.; Weigang, S.; Kern, L.; Kramme, S.; Wrobel, A.G.; Grawitz, A.B.; Nawrath, P.; Martin, S.R.; Dähne, T.; Beer, J.; et al. Total escape of SARS-CoV-2 from dual monoclonal antibody therapy in an immunocompromised patient. Nat. Commun. 2023, 14, 1999. [Google Scholar] [CrossRef]
- Gupta, A.; Konnova, A.; Smet, M.; Berkell, M.; Savoldi, A.; Morra, M.; Van Averbeke, V.; De Winter, F.H.; Peserico, D.; Danese, E.; et al. Host immunological responses facilitate development of SARS-CoV-2 mutations in patients receiving monoclonal antibody treatments. J. Clin. Investig. 2023, 133, e166032. [Google Scholar] [CrossRef]
- Arora, P.; Kempf, A.; Nehlmeier, I.; Schulz, S.R.; Jäck, H.M.; Pöhlmann, S.; Hoffmann, M. Omicron sublineage BQ.1.1 resistance to monoclonal antibodies. Lancet Infect. Dis. 2023, 23, 22–23, Erratum in Lancet Infect. Dis. 2023, 23, e47.. [Google Scholar] [CrossRef]
- Kumar, S.K.; Sathrasala, S.; Krishna, J.S.; Sreekanth, P.; Singh, A.D.; Ratnamani, M.S.; Kalal, I.G.; Tallapaka, K.B.; Kumar, G.P.; Sasidhar, M.V.; et al. Vaccine-elicited immune pressure and SARS-CoV-2 mutational dynamics in breakthrough infections. Gene Rep. 2024, 35, 101899. [Google Scholar] [CrossRef]
- Bayarri-Olmos, R.; Sutta, A.; Rosbjerg, A.; Mortensen, M.M.; Helgstrand, C.; Nielsen, P.F.; Pérez-Alós, L.; González-García, B.; Johnsen, L.B.; Matthiesen, F.; et al. Unraveling the impact of SARS-CoV-2 mutations on immunity: Insights from innate immune recognition to antibody and T cell responses. Front. Immunol. 2024, 15, 1412873. [Google Scholar] [CrossRef]
- Parums, D.V. Editorial: The XBB.1.5 (‘Kraken’) Subvariant of Omicron SARS-CoV-2 and its Rapid Global Spread. Med. Sci. Monit. 2023, 29, e939580. [Google Scholar] [CrossRef]
- Brandolini, M.; Gatti, G.; Grumiro, L.; Zannoli, S.; Arfilli, V.; Cricca, M.; Dirani, G.; Denicolò, A.; Marino, M.M.; Manera, M.; et al. Omicron Sub-Lineage BA.5 and Recombinant XBB Evasion from Antibody Neutralisation in BNT162b2 Vaccine Recipients. Microorganisms 2023, 11, 191. [Google Scholar] [CrossRef]
- Pasala, C.; Sharma, S.; Roychowdhury, T.; Moroni, E.; Colombo, G.; Chiosis, G. N-Glycosylation as a Modulator of Protein Conformation and Assembly in Disease. Biomolecules 2024, 14, 282. [Google Scholar] [CrossRef]
- Hu, C.; Zhou, Y.; Meng, X.; Li, J.; Chen, J.; Ying, Z.; Xie, X.S.; Hu, Y.; Cao, Y.; Jin, R. Safety and Intranasal Retention of a Broad-Spectrum Anti-SARS-CoV-2 Monoclonal Antibody SA55 Nasal Spray in Healthy Volunteers: A Phase I Clinical Trial. Pharmaceutics 2024, 17, 43. [Google Scholar] [CrossRef]
- Liu, Q.; Acharya, P.; Dolan, M.A.; Zhang, P.; Guzzo, C.; Lu, J.; Kwon, A.; Gururani, D.; Miao, H.; Bylund, T.; et al. Quaternary contact in the initial interaction of CD4 with the HIV-1 envelope trimer. Nat. Struct. Mol. Biol. 2017, 24, 370–378. [Google Scholar] [CrossRef]
- Yin, R.; Pierce, B.G. Evaluation of AlphaFold Antibody-Antigen Modeling with Implications for Improving Predictive Accuracy. Protein Sci. 2024, 33, e4865. [Google Scholar] [CrossRef]
- Murin, C.D.; Bruhn, J.F.; Bornholdt, Z.A.; Copps, J.; Stanfield, R.; Ward, A.B. Structural Basis of Pan-Ebolavirus Neutralization by an Antibody Targeting the Glycoprotein Fusion Loop. Cell Rep. 2018, 24, 2723–2732.e4. [Google Scholar] [CrossRef]
- Bahai, A.; Asgari, E.; Mofrad, M.R.K.; Kloetgen, A.; McHardy, A.C. EpitopeVec: Linear epitope prediction using deep protein sequence embeddings. Bioinformatics 2021, 37, 4517–4525. [Google Scholar] [CrossRef]
- Wu, W.L.; Chiang, C.Y.; Lai, S.C.; Yu, C.Y.; Huang, Y.L.; Liao, H.C.; Liao, C.L.; Chen, H.W.; Liu, S.J. Monoclonal antibody targeting the conserved region of the SARS-CoV-2 spike protein to overcome viral variants. JCI Insight 2022, 7, e157597. [Google Scholar] [CrossRef]
- Dai, J.-M.; Zhang, X.-Q.; Dai, J.-Y.; Yang, X.-M.; Chen, Z.-N. Modified Therapeutic Antibodies: Improving Efficacy. Engineering 2021, 7, 1529–1540. [Google Scholar] [CrossRef]
- Falconer, D.J.; Subedi, G.P.; Marcella, A.M.; Barb, A.W. Antibody Fucosylation Lowers the FcγRIIIa/CD16a Affinity by Limiting the Conformations Sampled by the N162-Glycan. ACS Chem. Biol. 2018, 13, 2179–2189. [Google Scholar] [CrossRef]
- Cheng, H.; Shen, L.; Liu, X.; Huo, J.; Wang, S.; Niu, Q.; Yang, H.; Wu, Y.; Wang, X.; Zhang, G. NK cell membrane/MnO2 hybrid nanoparticle-adjuvanted intranasal vaccines synergistically boost protective immunity against H1N1 influenza infection. Chem. Eng. J. 2024, 500, 157381. [Google Scholar] [CrossRef]
- Sun, J.; Zheng, T.; Jia, M.; Wang, Y.; Yang, J.; Liu, Y.; Yang, P.; Xie, Y.; Sun, H.; Tong, Q.; et al. Dual receptor-binding, infectivity, and transmissibility of an emerging H2N2 low pathogenicity avian influenza virus. Nat. Commun. 2024, 15, 10012. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Wang, W.; Tian, J.; Qi, C.; Cai, Z.; Yan, W.; Xuan, S.; Shang, A. Engineered mRNA-expressed bispecific antibody prevent intestinal cancer via lipid nanoparticle delivery. Bioengineered 2021, 12, 12383–12393. [Google Scholar] [CrossRef]
- Gilchuk, P.; Bombardi, R.G.; Erasmus, J.H.; Tan, Q.; Nargi, R.; Soto, C.; Abbink, P.; Suscovich, T.J.; Durnell, L.A.; Khandhar, A.; et al. Integrated pipeline for the accelerated discovery of antiviral antibody therapeutics. Nat. Biomed. Eng. 2020, 4, 1030–1043. [Google Scholar] [CrossRef]
- Yang, X.; Chi, H.; Wu, M.; Wang, Z.; Lang, Q.; Han, Q.; Wang, X.; Liu, X.; Li, Y.; Wang, X.; et al. Discovery and characterization of SARS-CoV-2 reactive and neutralizing antibodies from humanized CAMouse(HG) mice through rapid hybridoma screening and high-throughput single-cell V(D)J sequencing. Front. Immunol. 2022, 13, 992787. [Google Scholar]
- Gaudinski, M.R.; Coates, E.E.; Novik, L.; Widge, A.; Houser, K.V.; Burch, E.; Holman, L.A.; Gordon, I.J.; Chen, G.L.; Carter, C.; et al. Safety, tolerability, pharmacokinetics, and immunogenicity of the therapeutic monoclonal antibody mAb114 targeting Ebola virus glycoprotein (VRC 608): An open-label phase 1 study. Lancet 2019, 393, 889–898, Erratum in Lancet 2020, 395, 1694.. [Google Scholar] [CrossRef]
- Costa, G.L.; Sautto, G.A. Exploring T-Cell Immunity to Hepatitis C Virus: Insights from Different Vaccine and Antigen Presentation Strategies. Vaccines 2024, 12, 890. [Google Scholar] [CrossRef] [PubMed]
- Walker, R.S.K.; Pretorius, I.S. Applications of Yeast Synthetic Biology Geared towards the Production of Biopharmaceuticals. Genes 2018, 9, 340. [Google Scholar] [CrossRef]
- Zhao, N.; Song, Y.; Xie, X.; Zhu, Z.; Duan, C.; Nong, C.; Wang, H.; Bao, R. Synthetic biology-inspired cell engineering in diagnosis, treatment, and drug development. Signal Transduct. Target. Ther. 2023, 8, 112. [Google Scholar] [CrossRef]
- Gaudreault, F.; Sulea, T.; Corbeil, C.R. AI-augmented physics-based docking for antibody-antigen complex prediction. Bioinformatics 2025, 41, btaf129. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Ma, H.; Wang, Z.; Wang, S.; He, Y.; Chang, Y.; Zong, H.; Tang, H.; Wang, L.; Ke, Y.; et al. Rapid restoration of potent neutralization activity against the latest Omicron variant JN.1 via AI rational design and antibody engineering. Proc. Natl. Acad. Sci. USA 2025, 122, e2406659122. [Google Scholar] [CrossRef] [PubMed]
- Kozlowski, S.; Swann, P. Current and future issues in the manufacturing and development of monoclonal antibodies. Adv. Drug Deliv. Rev. 2006, 58, 707–722. [Google Scholar] [CrossRef]
- Schneider, E.L.; Hearn, B.R.; Pfaff, S.J.; Fontaine, S.D.; Reid, R.; Ashley, G.W.; Grabulovski, S.; Strassberger, V.; Vogt, L.; Jung, T.; et al. Approach for Half-Life Extension of Small Antibody Fragments That Does Not Affect Tissue Uptake. Bioconjugate Chem. 2016, 27, 2534–2539. [Google Scholar] [CrossRef] [PubMed]
- Rybakova, Y.; Kowalski, P.S.; Huang, Y.; Gonzalez, J.T.; Heartlein, M.W.; Derosa, F.; Delcassian, D.; Anderson, D.G. mRNA Delivery for Therapeutic Anti-HER2 Antibody Expression In Vivo. Mol. Ther. 2019, 27, 1415–1423. [Google Scholar] [CrossRef] [PubMed]

| Escape Mechanisms | Specific Modalities | Action Principle | Influence |
|---|---|---|---|
| Antigen variation | High-frequency point mutation (antigenic drift) | The virus accumulates point mutations through low-fidelity replication, changing the amino acid sequence of key neutralizing epitopes and weakening antibody binding ability. | Weakening the effectiveness of neutralizing antibodies increases the difficulty of developing vaccines and antibody therapies. |
| Antigenic shift | Viruses acquire completely new epitopes through genome recombination or genotype transformation, thereby evading existing antibody responses. | It deactivates existing antibodies and increases the risk of transmission and infection. | |
| Glycosylation shielding | Glycosylation | Viruses add glycan chains around key epitopes to form steric or charge shielding that prevents antibody binding. | Prevent antibody binding and enhance the ability of the virus to escape. |
| Dynamic configuration | Tablet hiding and dynamic exposure | The viral surface protein hides neutralizing epitopes through conformational rearrangement or dynamic fluctuations and is only exposed at specific stages of infection. | It is difficult to be continuously recognized by antibodies, increasing the chance of immune escape. |
| Physical masking and molecular camouflage | Physical shielding epitopes and the evasion of antibody recognition | Viruses use external molecules to physically mask the epitopes, or they use molecular signals to deceive, thereby resisting antibody recognition. | Block antibody recognition and reduce neutralization efficiency. |
| ADE effect | Antibody-dependent enhancement | When inefficient antibodies are present, the virus particles can passively enter the cells through the FcγR pathway to enhance infection. | Enhance the ability to infect and make antibody therapy ineffective. |
| Immunosuppression and antibody interference | Competition of immunoreactive agents or antibodies | Viruses reduce antibody effectiveness by secreting immunosuppressants or inducing antibody competition. | Interferes with immune response and weakens antibody efficacy. |
| Escape mutations under herd immunity pressure | Mutations under population immunization selection pressure | Viruses produce adaptive mutations under the pressure of herd immunity to escape herd immunity response. | Increases the risk of transmission and infection, challenging herd immunity strategies. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, H.; Liu, Y.; Song, Y.; Chen, L.; Zhang, L.; Li, R.; Nie, X.; Zhu, G.; Ding, X.; Wang, L. Neutralizing Antibodies vs. Viruses: Interacting Mechanisms and Escape Tactics. Microorganisms 2025, 13, 2199. https://doi.org/10.3390/microorganisms13092199
Lu H, Liu Y, Song Y, Chen L, Zhang L, Li R, Nie X, Zhu G, Ding X, Wang L. Neutralizing Antibodies vs. Viruses: Interacting Mechanisms and Escape Tactics. Microorganisms. 2025; 13(9):2199. https://doi.org/10.3390/microorganisms13092199
Chicago/Turabian StyleLu, Hao, Yichen Liu, Yue Song, Longxin Chen, Limeng Zhang, Runting Li, Xiaoning Nie, Guoqiang Zhu, Xueyan Ding, and Linqing Wang. 2025. "Neutralizing Antibodies vs. Viruses: Interacting Mechanisms and Escape Tactics" Microorganisms 13, no. 9: 2199. https://doi.org/10.3390/microorganisms13092199
APA StyleLu, H., Liu, Y., Song, Y., Chen, L., Zhang, L., Li, R., Nie, X., Zhu, G., Ding, X., & Wang, L. (2025). Neutralizing Antibodies vs. Viruses: Interacting Mechanisms and Escape Tactics. Microorganisms, 13(9), 2199. https://doi.org/10.3390/microorganisms13092199





