1. Introduction
SBM, with its high crude protein content (40–48%) and balanced amino acid profile, serves as the primary protein source in poultry feed, contributing approximately 60% of dietary protein [
1]. However, the rapid intensification of livestock farming has driven surging global soybean demand, increasing Chicago Board of Trade (CBOT) soybean futures prices by USD 9.47 per bushel during 2020–2023, thereby significantly reducing profit margins in animal production. This supply-demand imbalance highlights three systemic challenges: (1) Geopolitical Concentration: The United States and Brazil collectively dominate 60% of global soybean production, 90% of which comprises genetically modified varieties [
2]. Meanwhile, China’s import dependency exceeds 80%, exposing its industry to trade volatility, such as tariff disputes that inflate costs; (2) Ecological Pressures: Global soybean cultivation areas expanded by 108% from 2000 to 2019, directly linked to 9% of South American deforestation [
3]. The overall average carbon footprint of Brazilian soybean exports is 0.69 tons of carbon dioxide equivalent per ton of soy equivalent [
4], while excessive SBM use contributes to 41.6% of livestock nitrogen emissions [
5]; (3) Resource Constraints: A 10% reduction in SBM usage could conserve 11.3 million hectares of arable land annually (equivalent to Bulgaria’s land area) and reduce nitrogen emissions by 1.4 million tons [
6]. These challenges underscore the urgent need for sustainable SBM reduction strategies to ensure food security and ecological preservation.
Low-protein diets (LPD) based on the ideal amino acid model and supplemented with synthetic amino acids offer a viable strategy for conserving protein resources. Alterations in dietary composition, particularly protein levels, can influence the gut microbiota structure, thereby modulating nutrient utilization efficiency and environmental outcomes [
7,
8]. While LPD has demonstrated potential to reduce nitrogen emissions in broilers [
9], its application in goose nutrition remains largely underexplored. Notably, global goose production reached 770 million birds in 2023, with China contributing over 97% of this total and an annual growth rate of approximately 1% [
10]. Despite this, research on SBM reduction in geese constitutes less than 3% of total poultry-related studies. As herbivorous poultry, geese exhibit unique digestive adaptations, such as robust cecal fermentation capacity, which may confer greater flexibility in adapting to dietary protein adjustments. Experimental evidence indicates that reducing crude protein levels in gosling diets from 18.5% to 15.5%, while balancing essential amino acids, decreases nitrogen excretion by 23% without compromising growth [
11]. More recent findings suggest that lowering dietary protein and supplementing key amino acids during the gosling phase not only sustains growth performance into the growing period but also consistently reduces nitrogen excretion [
12]. Additionally, prioritizing supplementation of sulfur-containing amino acids, such as methionine, at consistent protein levels has been shown to enhance slaughter rate and muscle quality in geese [
13].
Despite these advances, research on low-protein, amino acid-balanced diets in geese has predominantly focused on goslings and breeding geese [
14], with systematic studies on meat geese (commercial geese) remaining scarce. Meat geese, characterized by a prolonged rearing period and limited suitability for restricted feeding, may exhibit heightened adaptability to LPD [
15], underscoring the practical significance of investigating SBM reduction strategies in this population. However, current studies primarily emphasize single SBM reduction paired with comprehensive amino acid balancing or supplementation with a single amino acid [
16]. While these approaches confirm the feasibility of LPD in goose production, incorporating major grain processing by-products—such as DDGS, broken rice, rice bran, and wheat bran—alongside SBM reduction could diversify dietary composition, substantially lower costs, and enhance resource efficiency. Moreover, precise balancing of limiting amino acids in LPD holds promise for elucidating the amino acid metabolic requirements and nutrient deposition patterns in geese, providing a robust theoretical foundation for future optimization of meat goose diets.
The Sanhua Goose is an excellent meat goose breed widely raised across China, boasting both outstanding growth performance and strong stress resistance. According to the recommendations of the Chinese agricultural industry standards “Nutritional Requirements for Geese” (NY/T 4641-2025 [
17]) and “Feeding Standards for Commercial Meat Geese” (DB37/T 2784-2016 [
18]), the nutritional needs for growing geese aged 4–10 weeks include a dietary metabolizable energy of 11.0–11.5 MJ/kg, a CP level of approximately 14–16%, lysine (0.75–0.9%), methionine + cystine (0.64–0.65%), threonine (0.40–0.60%), and valine (0.61–0.67%) to support normal growth. Building on this, the present study employs a gradient SBM replacement strategy, based on the metabolizable energy levels and apparent digestibility of amino acids in geese for alternative ingredients such as rapeseed meal, DDGS, broken rice, and rice bran. By supplementing crystalline amino acids to achieve balance in key limiting amino acids, a CLPD for meat geese was formulated. Through systematic assessment of its effects on growth performance, nitrogen utilization efficiency, and intestinal development, combined with 16S rRNA gene sequencing to characterize shifts in gut microbiota structure, this research seeks to clarify the nutritional metabolic profiles of geese under reduced SBM conditions and the interplay between amino acid balance and gut microbiota. These insights aim to provide scientific guidance for advancing sustainable waterfowl production.
2. Research Methods and Materials
2.1. Animal Ethics Guidelines
The experimental protocol for this study received approval from the Shanghai Academy of Agricultural Sciences’ Animal Care and Use Committee (SAASPZ0522050, 1 April 2022). It was conducted in compliance with China’s national standards, including the ’Experimental Animal—Guidelines for Welfare’ (GB/T 42011-2022 [
19]). All procedures were designed to minimize distress and discomfort for the geese throughout the study.
2.2. Experimental Animals and Diet Design
A total of 192 healthy 4-week-old Sanhua geese were sourced from the Daluyuan Breeding Cooperative in Lu’an City, Anhui Province, China. After a 3-day acclimatization period, the geese were weighed to determine their initial body weights. They were then randomly assigned to one of four dietary treatment groups based on these initial weights: a control group fed a Corn-Soybean meal Diet (CSD) with 16.5% crude protein (CP) and three Composite Low-Protein Diet (CLPD) groups with 14.0% CP, 11.5% CP, and 9.8% CP, respectively. Each group comprised six replicates, with eight geese per replicate. The CLPDs were formulated to match the amino acid profile of the CSD, with synthetic amino acids added to achieve this balance. To maintain energy adequacy while reducing crude protein levels, the protein-to-energy ratios were adjusted as follows: 14.86 g CP/MJ ME for the 16.5% CP (CSD) group (control), 12.62 g CP/MJ ME for the 14.0% CP (CLPD) group, 10.37 g CP/MJ ME for the 11.5% CP (CLPD) group, and 8.84 g CP/MJ ME for the 9.8% CP (CLPD) group. All dietary formulations adhered to the Nutritional Requirements for Geese in China (NY/T 4641-2025) and the Nutritional Standards for Commercial Meat Geese (DB37/T 2784-2016).
2.3. Feeding Management and Facility Conditions
The experiment was conducted over 6 weeks at the Zhuanghang Comprehensive Experimental Station, Shanghai Academy of Agricultural Sciences, Shanghai, China. Geese were housed in an environmentally controlled facility, with temperatures maintained between 22 and 28 °C and relative humidity ranging from 55 to 65%. Twenty-four raised mesh pens (dimensions: 3.0 m × 2.0 m × 0.8 m) were used, each fitted with plastic mesh flooring featuring 1 cm apertures. Each pen was equipped with trough feeders for ad libitum feed access and nipple drinkers for water supply. Standardized biosecurity measures were enforced, including vaccinations against Newcastle disease and avian influenza, weekly removal of pen waste, and daily monitoring of goose health status.
2.4. Nutrient Composition Determination
The CP content of feed and fecal samples was determined according to the method described in AOAC 984.13 (AOAC, 2005 [
20]). The amino acid profiles were analyzed by Shanghai Kailite Agricultural Product Testing Technology Service Co., Ltd. (Shanghai, China) following the method specified in AOAC Method 994.12 (AOAC, 2019 [
21]). The detailed breakdown of nutrient components in the feed of each treatment group is shown in
Table 1.
2.5. Growth Performance Evaluation
On the day the experiment commenced (4 weeks of age), geese were subjected to a 12-h fasting period prior to weighing, and their initial body weight (IBW) was recorded. On the final day of the experiment (10 weeks of age), the same fasting procedure was applied before weighing to determine their final body weight (FBW), from which the average daily gain (ADG) was calculated. Throughout the experimental period, feed consumption was monitored for each replicate pen, and the feed-to-gain ratio (F/G) was determined for each replicate. The average daily feed intake (ADFI) for each replicate was subsequently calculated based on the F/G and ADG values.
The following formulas were used to assess growth performance parameters:
2.6. Relative Organ Weight Determination
On the final day of the experiment, eight geese with similar body weights were selected from each treatment group, totaling 32 geese. Specifically, 1 representative goose (matching the average body weight of the pen) was selected from each of the 6 pens (replicates) in the treatment group, and an additional 2 geese were supplementarily selected from the same treatment group to ensure consistency with the overall average body weight of the group. After an 8-h fasting period, the geese were anesthetized through a wing vein injection of 30 mg/kg sodium pentobarbital (Sinopharm Group, Shanghai, China). Once fully anesthetized, they were euthanized by exsanguination via the jugular vein, following ethical guidelines for animal welfare. The abdominal cavity was then opened, and the heart, liver, spleen, gizzard, and abdominal fat pad were carefully dissected and weighed. All organ weights were recorded by a single technician to minimize measurement variation. Relative organ weight was calculated using the formula:
2.7. Determination of Apparent Digestibility Nutrient
Representative feed samples were collected from each treatment group throughout the trial to accurately reflect the nutritional composition of the diets consumed by the geese. For excreta collection, one goose per replicate pen, selected based on body weight closest to the group mean (total of 6 geese per treatment group), was used. These geese were acclimated to individual metabolic cages for a 3-day adaptation period, during which they received the experimental diet ad libitum to facilitate environmental adjustment and minimize stress-induced variability in excretion patterns. Following adaptation, excreta were collected continuously and completely over a 48-h period. Any feathers or spilled feed mixed in the excreta were carefully removed, and the samples were treated with 10% hydrochloric acid for nitrogen fixation to prevent ammonia volatilization. The treated excreta were immediately stored at −20 °C to inhibit microbial degradation and nutrient loss. For analysis, both feed and excreta samples were thawed, homogenized, and dried at 65 °C in a forced-air oven to constant weight (72 h). The dried samples were then ground through a 1-mm sieve using a laboratory mill and stored in airtight containers at room temperature until further analysis. Crude protein content was determined using the Kjeldahl method according to AOAC 984.13 (AOAC, 2005 [
20]), and amino acid profiles were analyzed via ion-exchange chromatography following AOAC 994.12 (AOAC, 2019 [
21]). The acid-insoluble ash (AIA) content was determined following the GB/T 23742-2009 standard [
22] and the equivalent AOAC 975.12 method. First, the samples were ashed at 600 °C, followed by acid treatment to separate and quantify the insoluble fraction.
Apparent digestibility (AD) was calculated as:
where AIA_feed and AIA_feces are the AIA percentages in feed and feces, respectively, and nutrient_feed and nutrient_feces are the concentrations of crude protein or specific amino acids in feed and feces, respectively.
2.8. Blood Biochemistry and Amino Acid Profile Analysis
On the final day of the experiment, following an 8-h fasting period, 5 mL blood samples were collected from the brachial vein of geese. The samples were allowed to clot at 4 °C for 2 h and then centrifuged at 3000 rpm for 10 min to separate the serum. The resulting serum samples were stored at −80 °C and subsequently transported to Shanghai Renjie Biotechnology Co., Ltd. (Shanghai, China) for biochemical analysis. Serum biochemical parameters, including total protein (TP), albumin (Alb), globulin (Glob), total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), uric acid (UA), serum creatinine (SCR), glucose (Glu), total bilirubin (TBIL), indirect bilirubin (IBIL), alanine aminotransferase (ALT), aspartate aminotransferase (AST), and alkaline phosphatase (ALP), were measured using an automatic biochemical analyzer (Model 7180, Hitachi, Tokyo, Japan), with all procedures conducted in strict accordance with the manufacturer’s instructions.
2.9. Determination of Amino Acid Profiles in Serum and Breast-Leg Muscle Tissues
Serum samples were collected as described in
Section 2.7 and stored at −80 °C until analysis. Following euthanasia, approximately 5 g of tissue was excised from the same anatomical locations in the breast and leg muscles of each goose. These samples were immediately placed in cryogenic tubes, snap-frozen in liquid nitrogen, and maintained at −80 °C until further processing. The amino acid composition of serum and muscle tissues was determined using a Hitachi automatic amino acid analyzer (Model: LA8080, Hitachi High-Tech Corporation, Tokyo, Japan), in accordance with the standards outlined in GB/T 18246-2019 [
23] and AOAC Method 994.12 (AOAC, 2019). Briefly, sample pretreatment involved hydrochloric acid extraction for serum to isolate free amino acids, followed by precipitation removal and pH adjustment for purification; for muscle, proteins were first hydrolyzed with acid to release amino acids, followed by similar purification steps to eliminate impurities. Subsequently, target amino acids were separated via cation-exchange chromatography based on differences in their charge properties. Post-column derivatization with ninhydrin was performed online, and the derivatives were detected photometrically at dual wavelengths (570 nm for primary amino acids and 440 nm for secondary amino acids such as proline). Finally, amino acids were identified qualitatively by retention time and quantified using the external standard method with calibration curves to determine concentrations in serum and muscle.
2.10. Morphological Observation of the Small Intestine
Following euthanasia, the abdominal cavity was opened, and the duodenum, jejunum, and ileum were carefully isolated, rinsed with phosphate-buffered saline (PBS), and fixed in 4% paraformaldehyde solution. After 12 h, the fixative was replaced, and the samples were sent to Beijing Lanyi Technology Co., Ltd., Beijing, China, for histological processing. The fixed samples were subjected to gradient ethanol dehydration, paraffin embedding, sectioning at 5 μm, H&E staining, and mounting. Sections were examined using an Olympus BX-41TF microscope (Olympus Corporation, Tokyo, Japan). For each intestinal segment, six sections were analyzed, and measurements of villus height (VH), crypt depth (CD), and muscularis mucosae thickness were obtained from morphologically intact areas. The VH/CD ratio was calculated, and each parameter was measured five times across different areas of the section, with the average value being used for analysis.
2.11. Cecal Microbiota Analysis
During the slaughter process, cecal contents were collected from each goose. The samples were immediately transferred into cryogenic tubes and snap-frozen in liquid nitrogen, then sent to Beijing Biomarker Technologies Co., Ltd. (Beijing, China) for 16S rRNA gene sequencing. Microbial DNA was extracted from the cecal contents using the Tiangen DP328 Stool DNA Kit (Tiangen Biotech, Beijing, China). Following an assessment of DNA quality and concentration, the V3-V4 region of the bacterial 16S rRNA gene was amplified by PCR with the specific primers F (5′-ACTCCTACGGGAGGCAGCA-3′) and R (5′-GGACTACHVGGGTWTCTAAT-3′). The resulting amplicons were purified via gel electrophoresis and used to construct sequencing libraries. After quality control, paired-end sequencing was performed on the Illumina MiSeq 6000 platform. Raw sequencing data were processed using DADA2 for quality filtering, denoising, merging, and chimera removal to generate high-quality operational taxonomic units (OTUs). These OTUs were taxonomically annotated against the SILVA 138 database and analyzed to assess the diversity, composition, and relative abundance of the cecal microbiota in goslings.
5. Discussion
To mitigate the challenges posed by the meat goose industry’s heavy reliance on SBM—including raw material price volatility, geopolitical risks, and environmental pressures—this study developed and evaluated a sustainable low-protein compound diet system. This approach entailed a progressive reduction in dietary SBM content, supplemented by alternative feedstuffs such as rapeseed meal, corn distillers DDGS, broken rice, and rice bran, with dietary formulations optimized through restrictive amino acid balancing. Results revealed that this low-protein feeding strategy significantly enhanced the growth performance of meat geese. Specifically, geese in the CLPD groups (B, C, and D) exhibited a markedly higher ADG and improved feed conversion ratio compared to the control group. These findings suggest that, by refining dietary composition and ensuring amino acid equilibrium, CLPD can sustain or even augment growth outcomes in meat geese while substantially improving nitrogen utilization efficiency. This strategy offers a practical avenue for reducing SBM dependency, providing valuable implications for the sustainable advancement of poultry production.
The observed improvements in growth performance are intricately linked to a suite of physiological and metabolic adaptations. As dietary protein levels declined, the apparent digestibility of crude protein and multiple amino acids in geese increased progressively. This enhancement is likely attributable to the elevated bioavailability of synthetic amino acids, which, existing in free form, are readily absorbed by the intestine without requiring extensive enzymatic degradation [
24]. Such rapid uptake accelerates protein turnover, ensuring an adequate amino acid supply for muscle development. Additionally, the diminished inclusion of SBM likely alleviated the adverse effects of trypsin inhibitors inherent in SBM [
25], thereby enhancing endogenous protease activity and boosting overall feed digestibility. A notable shift in the dietary energy-to-protein ratio prompted a reallocation of metabolic resources. Protein synthesis, encompassing transcription, translation, and folding, demands considerable ATP, whereas lipid synthesis is comparatively energy-efficient. Consequently, CLPD redirected energy toward rapid fat deposition rather than muscle protein accretion [
26], accounting for the accelerated body weight gain and elevated abdominal fat proportion in geese fed these diets. Increased serum low-density lipoprotein cholesterol levels, which may indicate heightened cholesterol transport demands, could further support this potential metabolic shift [
27]. Although we balanced the limiting amino acids (lysine, methionine, and threonine), previous studies suggest that a further reduction in dietary protein levels (below 10%) might lead to relative deficiencies in certain essential amino acids, such as leucine and arginine. These potential deficiencies could plausibly impair the efficiency of ribosomal protein synthesis downstream of the mTORC1 signaling pathway, potentially reducing the overall efficiency of protein synthesis in the organism, as reported in prior research [
28]. As a result, unutilized amino acids are metabolized and released into the bloodstream, leading to elevated blood amino acid concentrations and a concurrent decrease in amino acid deposition in muscle tissue. This observation is consistent with the primary findings of our experimental study.
At the muscular level, responses to CLPD displayed distinct site-specific patterns. In breast muscle, concentrations of glycine, alanine, and aromatic amino acids were significantly diminished. Concurrently, rapid fat synthesis and deposition, coupled with sustained insulin elevation, may induce “anabolic resistance” in fast-twitch fibers, potentially diminishing mTORC1 pathway responsiveness to amino acids and channeling carbon skeletons toward adipose tissue, as hypothesized in previous literature [
29]. This mechanism elucidates the heightened sensitivity of breast muscle to LPD. Conversely, leg muscle, dominated by slow-twitch fibers, exhibited greater metabolic stability, with elevated tyrosine and phenylalanine levels detected only in the group with the most substantial SBM reduction (group D). This anomaly likely stems from insufficient phenylalanine intake due to lower SBM levels, constraining tetrahydrobiopterin synthesis—a critical cofactor for phenylalanine hydroxylase—and impeding phenylalanine conversion to tyrosine [
30]. These differential responses highlight the potential of targeted supplementation with branched-chain and methyl-donor amino acids, alongside comprehensive optimization of the dietary amino acid profile, to enhance breast muscle protein synthesis efficiency and maintain leg muscle aromatic amino acid homeostasis while reducing SBM reliance, ultimately preserving meat quality.
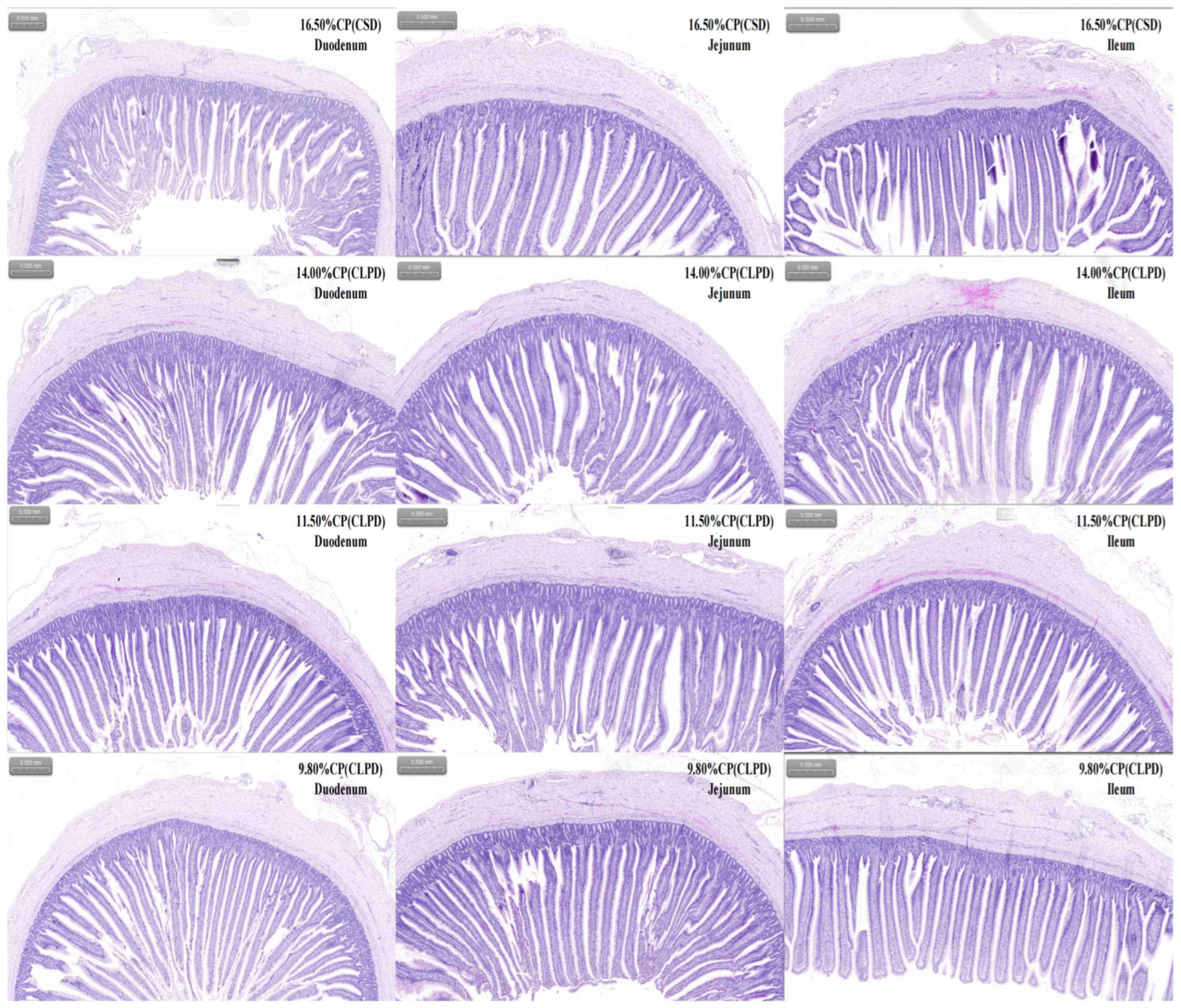
The influence of CLPD on intestinal morphology also exhibited pronounced segment-specificity, predominantly affecting the duodenum and jejunum, with minimal impact on the ileum. In the duodenum, groups C and D displayed significantly greater villus height and reduced crypt depth relative to groups A and B, culminating in an elevated V/C in group D—a trait closely associated with improved nutrient absorption [
31]. Additionally, the thickened mucosal muscularis in group D likely bolstered intestinal mechanical strength and peristalsis [
32]. In the jejunum, CLPD similarly fostered villus elongation, yet mucosal muscularis thickness declined with decreasing protein levels, possibly reflecting adaptive responses to altered mechanical demands, the mechanisms of which merit further exploration. By contrast, ileal morphology remained largely unaltered, with only minor shifts in muscularis thickness in select groups, consistent with its primary role in water and electrolyte absorption [
33]. Remarkably, despite progressive SBM reduction, intestinal morphology showed no detrimental effects; instead, enhancements in duodenal and jejunal parameters emerged. This improvement likely arises from the combined reduction in SBM-derived antinutritional factors and optimized amino acid provision via synthetic supplements, synergistically enhancing nutrient absorption and feed efficiency in the proximal intestine.
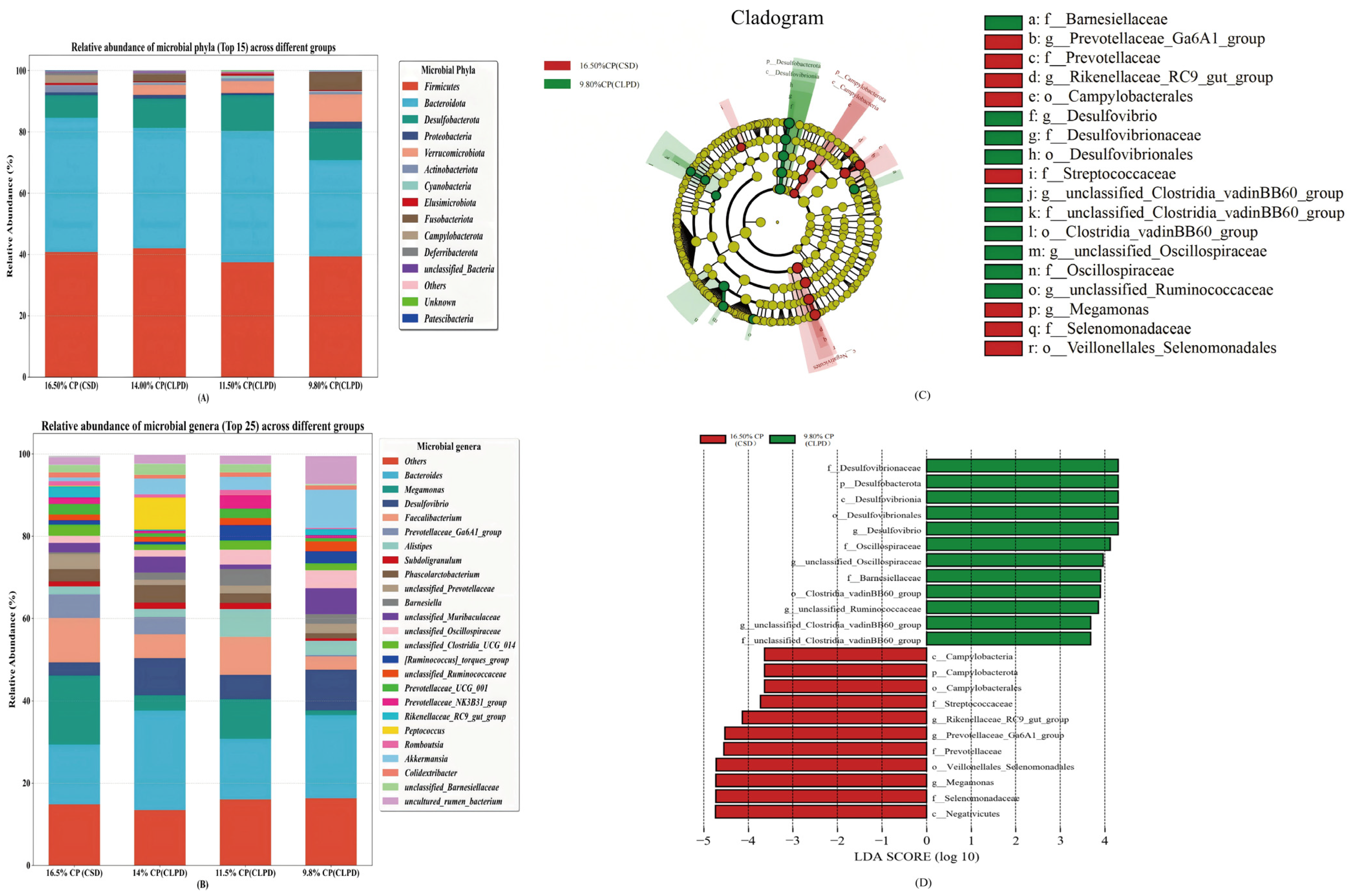
Using 16S rRNA sequencing, this study assessed the effects of CLPD on cecal microbiota, elucidating how SBM reduction modulates gut microbial communities to bolster growth and metabolic efficiency. Dietary treatments exerted no significant influence on cecal microbial alpha diversity, corroborating prior evidence that moderate protein reductions preserve microbial richness and stability [
34]. However, beta diversity analyses revealed distinct microbial community structures across groups, with the greatest divergence between the 16.5%CP (CSD) and the 9.8%CP (CLPD) groups, indicating that substantial protein level alterations drive microbial restructuring.
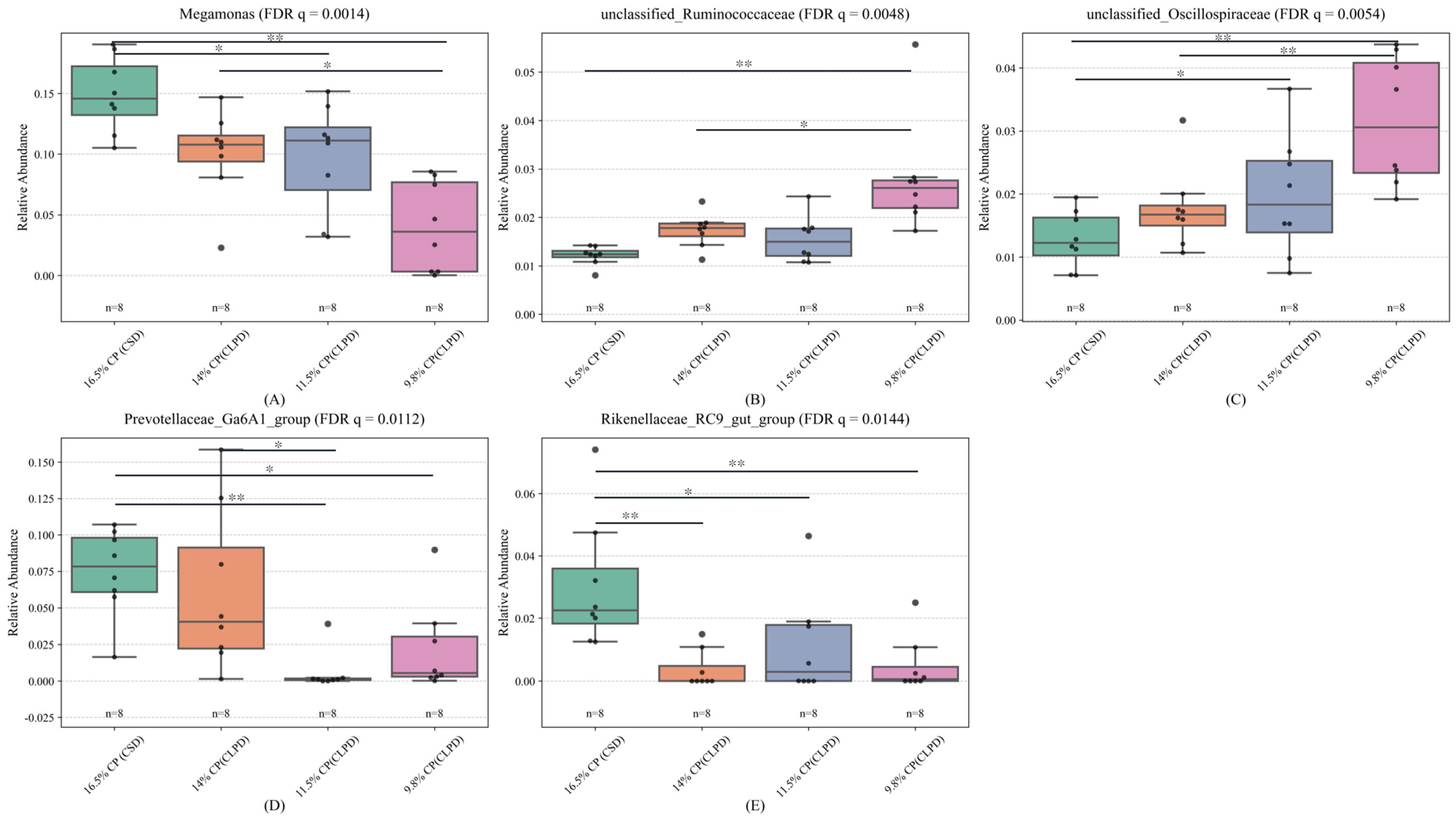
Megamonas, potentially linked to fat deposition and energy metabolism, declined in abundance under the high energy-to-protein ratio of CLPD, consistent with observations in laying hens on high-energy diets [
35]. Previous studies have shown that the improvement of cecal microflora in geese is accompanied by a significant increase in the relative abundance of
Oscillospiraceae and
Ruminococcaceae. As important producers of short-chain fatty acids (SCFAs) in the intestine [
36,
37], the butyric acid produced by
Oscillospiraceae and
Ruminococcaceae helps to directly support the function of intestinal epithelial cells and maintain the intestinal barrier function [
38]. This study found that a low-protein, diversified diet is conducive to the colonization of these bacteria in the cecum of geese, promoting their decomposition of cellulose in the diet and improving the feed conversion efficiency.
Prevotellaceae_Ga6A1_group, implicated in cellulose degradation for high-fiber digestion [
39], decreased possibly due to DDGS inclusion, mirroring findings from brewer’s grain supplementation in Landes geese [
37].
Rikenellaceae_RC9_gut_group, engaged in bile acid, protein, lipid, and carbohydrate metabolism [
40], diminished with reduced crude fiber and increased energy feed proportions in LPD [
41]. In contrast,
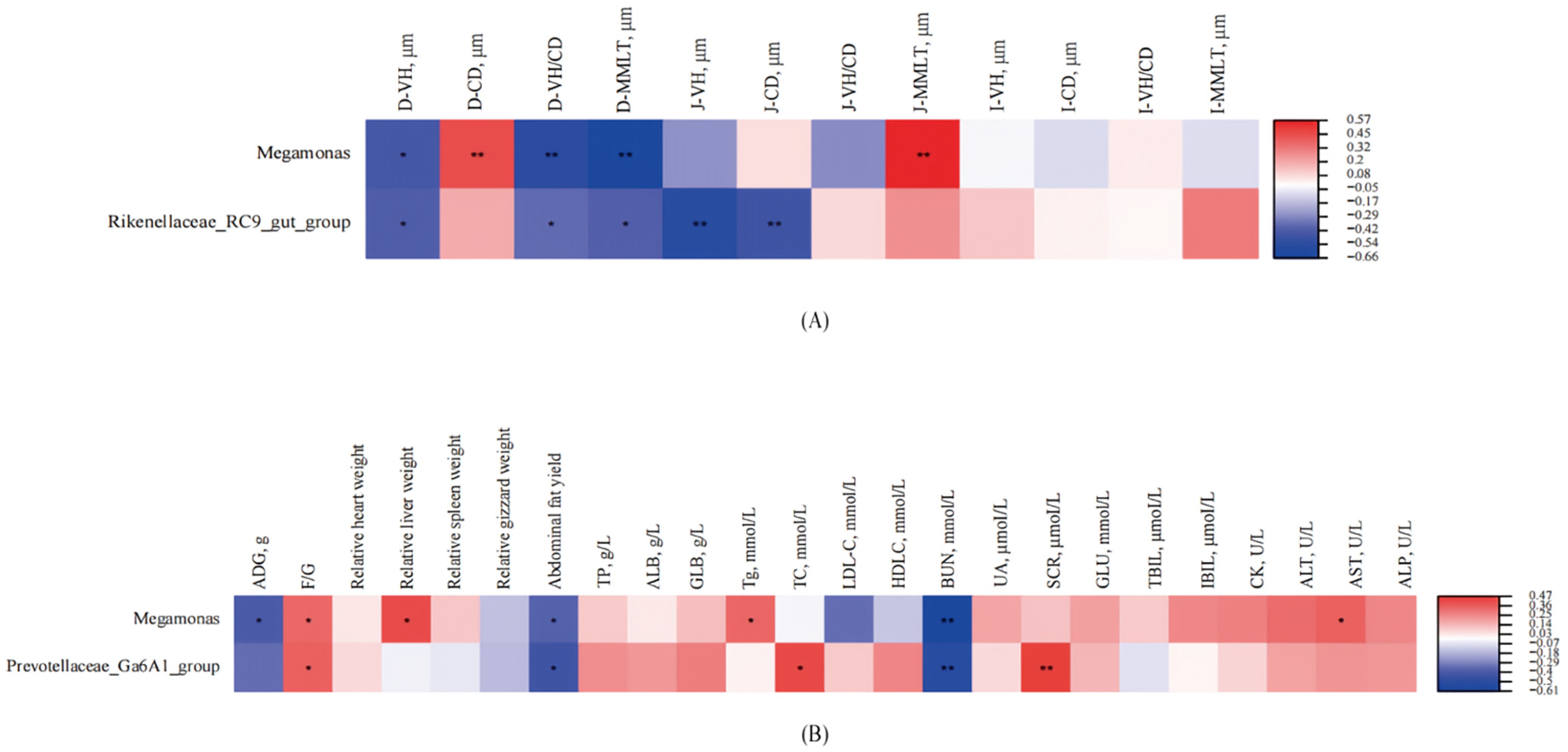
Megamonas positively correlated with F/G and BUN and negatively with ADG, abdominal fat, and intestinal morphology, implying nitrogen wastage and intestinal suppression in high-protein contexts [
42].
Prevotellaceae_Ga6A1_group positively correlated with serum cholesterol and creatinine, hinting at lipid and renal regulatory roles, while
Rikenellaceae_RC9_gut_group negatively correlated with intestinal morphology, reinforcing high-protein diets’ inhibitory effects. Collectively, CLPD with amino acid balancing selectively modulates cecal microbiota, enhancing growth, intestinal health, and nitrogen efficiency, thus supporting sustainable poultry production.