A Comparative Study of Extended Gentamicin and Tobramycin Release and Antibacterial Efficacy from Palacos and Simplex Acrylic Cements
Abstract
1. Introduction
2. Materials and Methods
2.1. Bone Cements and Bacterial Strains
2.2. Short- and Long-Term Release Rate of Gentamicin and Tobramycin
2.3. Comparative Long-Term Efficacy of Gentamicin and Tobramycin
2.4. Statistical Analysis
3. Results
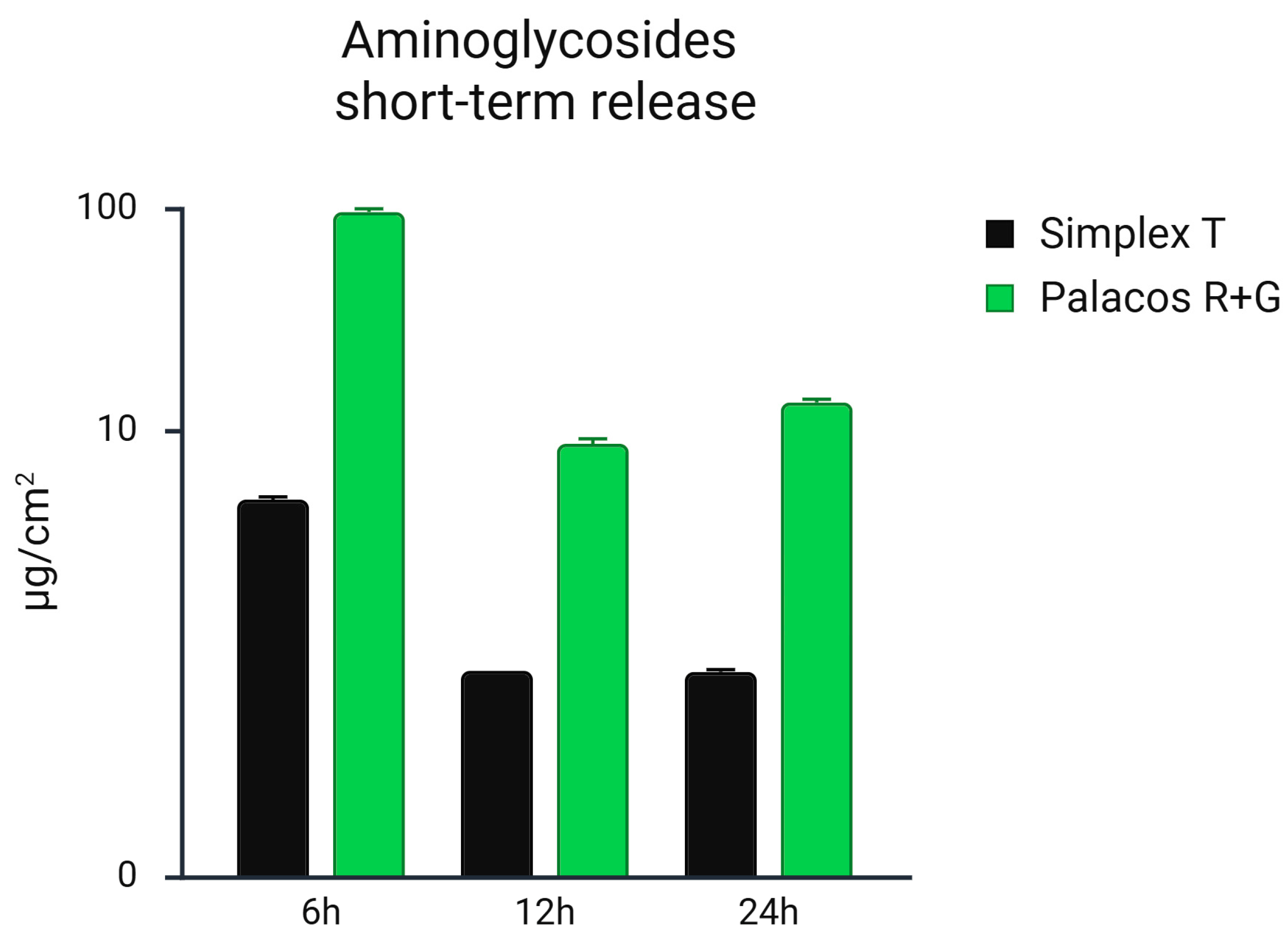
3.1. Comparative Short-Term Release Rate of Gentamicin and Tobramycin
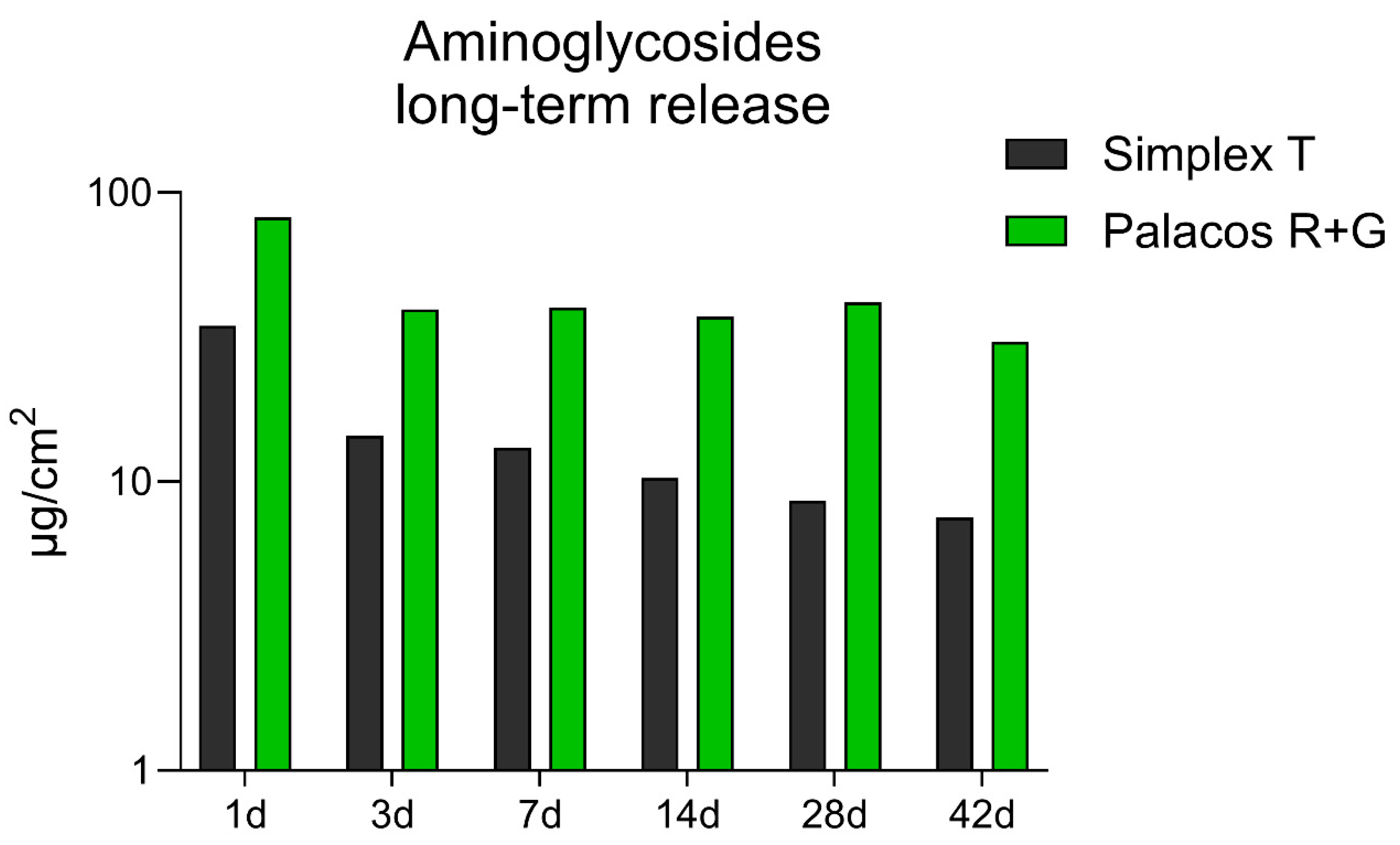
3.2. Comparative Long-Term Release Rate of Gentamicin and Tobramycin
3.3. Antibiotic Susceptibility Tests Carried Out During Comparative Long-Term Tests on Gentamicin and Tobramycin from Palacos® R+G and Simplex® T
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ALBC | Antibiotic-loaded bone cement |
| ELISA | Enzyme-linked immunosorbent assay |
| EUCAST | European Society of Clinical Microbiology and Infectious Diseases |
| HPLC | High-performance liquid chromatography |
| HV | High-viscosity bone cement |
| IZT | Inhibition zone test |
| LV | Low-viscosity bone cement |
| MV | Medium-viscosity bone cement |
| PJI | Periprosthetic joint infection |
| PMMA | Polymethylmethacrylate |
References
- Sebastian, S.; Liu, Y.; Christensen, R.; Raina, D.B.; Tägil, M.; Lidgren, L. Antibiotic containing bone cement in prevention of hip and knee prosthetic joint infections: A systematic review and meta-analysis. J. Orthop. Transl. 2020, 23, 53–60. [Google Scholar] [CrossRef]
- von Hertzberg-Boelch, S.P.; Luedemann, M.; Rudert, M.; Steinert, A.F. PMMA Bone Cement: Antibiotic Elution and Mechanical Properties in the Context of Clinical Use. Biomedicines 2022, 10, 1830. [Google Scholar] [CrossRef] [PubMed]
- Parvizi, J.; Saleh, K.J.; Ragland, P.S.; Pour, A.E.; Mont, M.A. Efficacy of antibiotic-impregnated cement in total hip replacement. Acta Orthop. 2008, 79, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Kendoff, D.O.; Gehrke, T.; Stangenberg, P.; Frommelt, L.; Bösebeck, H. Bioavailability of gentamicin and vancomycin released from an antibiotic containing bone cement in patients undergoing a septic one-stage total hip arthroplasty (THA) revision: A monocentric open clinical trial. HIP Int. 2016, 26, 90–96. [Google Scholar] [CrossRef]
- Frommelt, L. Principles of systemic antimicrobial therapy in foreign material associated infection in bone tissue, with special focus on periprosthetic infection. Injury 2006, 37 (Suppl. 2), S87–S94. [Google Scholar] [CrossRef] [PubMed]
- Ensing, G.T.; van Horn, J.R.; van der Mei, H.C.; Busscher, H.J.; Neut, D. Copal bone cement is more effective in preventing biofilm formation than Palacos R-G. Clin. Orthop. Relat. Res. 2008, 466, 1492–1498. [Google Scholar] [CrossRef]
- Anagnostakos, K.; Fink, B. Antibiotic-loaded cement spacers—Lessons learned from the past 20 years. Expert Rev. Med. Devices 2018, 15, 231–245. [Google Scholar] [CrossRef]
- Villanueva-Martinez, M.; Rios-Luna, A.; Chana, F.; de Pedro, J.A.; Perez-Caballer, A. Articulating Spacers in Infection of Total Knee Arthroplasty—State of the Art. In Arthroplasty—Update; Kinov, P., Ed.; InTech: London, UK, 2013; ISBN 978-953-51-0995-2. [Google Scholar]
- Dias Carvalho, A.; Ribau, A.; Soares, D.; Santos, A.C.; Abreu, M.; Sousa, R. Combined antibiotic therapy spacers either commercial or handmade are superior to monotherapy—A microbiological analysis at the second stage of revision. J. Bone Jt. Infect. 2021, 6, 305–312. [Google Scholar] [CrossRef]
- Lunz, A.; Schonhoff, M.; Omlor, G.W.; Knappe, K.; Bangert, Y.; Lehner, B.; Renkawitz, T.; Jaeger, S. Enhanced antibiotic release from bone cement spacers utilizing dual antibiotic loading with elevated vancomycin concentrations in two-stage revision for periprosthetic joint infection. Int. Orthop. 2023, 47, 2655–2661. [Google Scholar] [CrossRef] [PubMed]
- Hansen, E.; Kühn, K.-D. (Eds.) Essentials of Cemented Knee Arthroplasty; Springer: Berlin/Heidelberg, Germany, 2022. [Google Scholar] [CrossRef]
- Ajit Singh, V.; Chun Haw, B.; Haseeb, A.; Shuan Ju Teh, C. Hand-mixed vancomycin versus commercial tobramycin cement revisited: A study on mechanical and antibacterial properties. J. Orthop. Surg. 2019, 27, 2309499019839616. [Google Scholar] [CrossRef] [PubMed]
- Kwong, J.; Abramowicz, M.; Kühn, K.-D.; Foelsch, C.; Hansen, E. High and Low Dosage of Vancomycin in Polymethylmethacrylate Cements: Efficacy and Mechanical Properties. Antibiotics 2024, 318, 818. [Google Scholar] [CrossRef]
- Alt, V.; Bechert, T.; Steinrücke, P.; Wagener, M.; Seidel, P.; Dingeldein, E.; Domann, E.; Schnettler, R. An in vitro assessment of the antibacterial properties and cytotoxicity of nanoparticulate silver bone cement. Biomaterials 2004, 25, 4383–4391. [Google Scholar] [CrossRef] [PubMed]
- Kittinger, C.; Stadler, J.; Kühn, K.D. Evaluation of Gentamicin Release of PMMA Cements Using Different Methods: HPLC, Elution and Inhibition Zone Testing. Antibiotics 2024, 13, 754. [Google Scholar] [CrossRef]
- Atıcı, T.; Şahin, N.; Çavun, S.; Özakin, C.; Kaleli, T. Antibiotic release and antibacterial efficacy in cement spacers and cement beads impregnated with different techniques: In vitro study. Eklem Hast. Cerrahisi 2018, 29, 71–78. [Google Scholar] [CrossRef]
- Janssen, D.M.C.; Willems, P.; Geurts, J.; Arts, C.J.J. Antibiotic release from PMMA spacers and PMMA beads measured with ELISA: Assessment of in vitro samples and drain fluid samples of patients. J. Orthop. Res. 2023, 41, 1831–1839. [Google Scholar] [CrossRef] [PubMed]
- Weisman, D.L.; Olmstead, M.L.; Kowalski, J.J. In vitro evaluation of antibiotic elution from polymethylmethacrylate (PMMA) and mechanical assessment of antibiotic-PMMA composites. Vet. Surg. 2000, 29, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in vitro evaluating antimicrobial activity: A review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef]
- Odekerken, J.C.E.; Logister, D.M.W.; Assabre, L.; Arts, J.J.C.; Walenkamp, G.H.I.M.; Welting, T.J.M. ELISA-based detection of gentamicin and vancomycin in protein-containing samples. SpringerPlus 2015, 4, 614. [Google Scholar] [CrossRef]
- Czuban, M.; Wulsten, D.; Wang, L.; Di Luca, M.; Trampuz, A. Release of different amphotericin B formulations from PMMA bone cements and their activity against Candida biofilm. Colloids Surf. B Biointerfaces 2019, 183, 110406. [Google Scholar] [CrossRef]
- Huys, G.; D’Haene, K.; Swings, J. Influence of the culture medium on antibiotic susceptibility testing of food-associated lactic acid bacteria with the agar overlay disc diffusion method. Lett. Appl. Microbiol. 2002, 34, 402–406. [Google Scholar] [CrossRef]
- Kok, M.; Hankemeier, T.; van Hasselt, J.G.C. Nutrient conditions affect antimicrobial pharmacodynamics in Pseudomonas aeruginosa. Microbiol. Spectr. 2025, 13, e0140924. [Google Scholar] [CrossRef]
- Karaglani, M.; Molla Moustafa, R.; Panagopoulou, M.; Chatzaki, E.; Drosos, G. Antibiotic Release and Mechanical Performance of PMMA Bone Cement: Findings from In Vitro Studies. Preprints 2024. [Google Scholar] [CrossRef]
- McLaren, A.C.; Nugent, M.; Economopoulos, K.; Kaul, H.; Vernon, B.L.; McLemore, R. Hand-mixed and premixed antibiotic-loaded bone cement have similar homogeneity. Clin. Orthop. Relat. Res. 2009, 467, 1693–1698. [Google Scholar] [CrossRef]
- Levack, A.E.; Turajane, K.; Yang, X.; Miller, A.O.; Carli, A.V.; Bostrom, M.P.; Wellman, D.S. Thermal Stability and in Vitro Elution Kinetics of Alternative Antibiotics in Polymethylmethacrylate (PMMA) Bone Cement. J. Bone Jt. Surg. 2021, 103, 1694–1704. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-H.; Tai, C.-L.; Chen, S.-Y.; Chang, C.-H.; Chang, Y.-H.; Hsieh, P.-H. Elution and Mechanical Strength of Vancomycin-Loaded Bone Cement: In Vitro Study of the Influence of Brand Combination. PLoS ONE 2016, 11, e0166545. [Google Scholar] [CrossRef]
- van de Belt, H.; Neut, D.; Schenk, W.; van Horn, J.R.; van der Mei, H.C.; Busscher, H.J. Staphylococcus aureus biofilm formation on different gentamicin-loaded polymethylmethacrylate bone cements. Biomaterials 2001, 22, 1607–1611. [Google Scholar] [CrossRef] [PubMed]
- Egger, V.; Dammerer, D.; Degenhart, G.; Pallua, J.D.; Schmölz, W.; Thaler, M.; Kühn, K.-D.; Nogler, M.; Putzer, D. Does the Addition of Low-Dose Antibiotics Compromise the Mechanical Properties of Polymethylmethacrylate (PMMA)? Polymers 2024, 16, 2378. [Google Scholar] [CrossRef]
- Kühn, K.-D. PMMA Cements; Springer: Berlin/Heidelberg, Germany, 2014; pp. 131–133, 143–144, 146–148, 150–159. [Google Scholar] [CrossRef]
- Meeker, D.G.; Cooper, K.B.; Renard, R.L.; Mears, S.C.; Smeltzer, M.S.; Barnes, C.L. Comparative Study of Antibiotic Elution Profiles From Alternative Formulations of Polymethylmethacrylate Bone Cement. J. Arthroplast. 2019, 34, 1458–1461. [Google Scholar] [CrossRef]
- Fink, B.; Vogt, S.; Reinsch, M.; Büchner, H. Sufficient release of antibiotic by a spacer 6 weeks after implantation in two-stage revision of infected hip prostheses. Clin. Orthop. Relat. Res. 2011, 469, 3141–3147. [Google Scholar] [CrossRef]
- Stryker Howmedica Osteonics. Antibiotic Simplex with Tobramycin: Instructions for Use 2025. Available online: https://www.stryker.com/content/dam/stryker/ifus/canada/169981.pdf (accessed on 4 August 2025).
- Heraeus Medical GmbH. PALACOS R+G: Instruction for Use 2025. Available online: https://ifu.heraeus-medical.com (accessed on 4 August 2025).
- Steixner, S.J.M.; Spiegel, C.; Dammerer, D.; Wurm, A.; Nogler, M.; Coraça-Huber, D.C. Influence of Nutrient Media Compared to Human Synovial Fluid on the Antibiotic Susceptibility and Biofilm Gene Expression of Coagulase-Negative Staphylococci In Vitro. Antibiotics 2021, 10, 790. [Google Scholar] [CrossRef] [PubMed]
- European Society of Clinical Microbiology and Infectious Diseases—EUCAST. Antimicrobial Susceptibility Testing. EUCAST Disk Diffusion Method. 2025. Available online: https://www.eucast.org/ (accessed on 25 March 2025).
- European Society of Clinical Microbiology and Infectious Diseases—EUCAST. Clinical Breakpoints—Breakpoints and Guidance 2025. Available online: https://www.eucast.org/clinical_breakpoints?utm_source=chatgpt.com (accessed on 25 March 2025).
- Sohani, Z.N.; Lieu, A.; Semret, M.; Cheng, M.P.; Simic, N.; Bamba, R.; Patel, M.; Lawandi, A.; Lee, T.C. Comparison of ciprofloxacin and aminoglycoside susceptibility testing for ceftriaxone non-susceptible Enterobacterales by disk diffusion and VITEK 2 vs. broth microdilution using updated Clinical and Laboratory Standards Institute breakpoints. BMC Microbiol. 2025, 25, 175. [Google Scholar] [CrossRef]
- Heller, D.N.; Peggins, J.O.; Nochetto, C.B.; Smith, M.L.; Chiesa, O.A.; Moulton, K. LC/MS/MS measurement of gentamicin in bovine plasma, urine, milk, and biopsy samples taken from kidneys of standing animals. J. Chromatogr. B 2005, 821, 22–30. [Google Scholar] [CrossRef]
- Lode, H. Tobramycin: A review of therapeutic uses and dosing schedules. Curr. Ther. Res. 1998, 59, 420–453. [Google Scholar] [CrossRef]
- Alt, V.; Bechert, T.; Steinrücke, P.; Wagener, M.; Seidel, P.; Dingeldein, E.; Domann, E.; Schnettler, R. In vitro testing of antimicrobial activity of bone cement. Antimicrob. Agents Chemother. 2004, 48, 4084–4088. [Google Scholar] [CrossRef]
- Kühn, K.-D.; Lieb, E.; Berberich, C. PMMA Bone Cement: What is the role of local antibiotics? Maitrise Orthop. 2016, 243, 1–15, Commission Paritaire 1218/86410.. [Google Scholar]
- Dietz, M.J.; McGowan, B.M.; Thomas, D.D.; Hunt, E.R.; Stewart, E.; Squire, M.W. Does Cement Viscosity Impact Antibiotic Elution and In Vitro Efficacy Against Common Prosthetic Joint Infection Pathogens? Clin. Orthop. Relat. Res. 2024, 483, 488–497. [Google Scholar] [CrossRef] [PubMed]
- Federspil, P.; Schätzle, W.; Tiesler, E. Pharmacokinetics and ototoxicity of gentamicin, tobramycin, and amikacin. J. Infect. Dis. 1976, 134 (Suppl. S1), S200–S205. [Google Scholar] [CrossRef] [PubMed]
- Brogden, R.N.; Pinder, R.M.; Sawyer, P.R.; Speight, T.M.; Avery, G.S. Tobramycin: A review of its antibacterial and pharmacokinetic properties and therapeutic use. Drugs 1976, 12, 166–200. [Google Scholar] [CrossRef] [PubMed]
- Fleischmann, W.A.; Greenwood-Quaintance, K.E.; Patel, R. In Vitro Activity of Plazomicin Compared to Amikacin, Gentamicin, and Tobramycin against Multidrug-Resistant Aerobic Gram-Negative Bacilli. Antimicrob. Agents Chemother. 2020, 64, e01711-19. [Google Scholar] [CrossRef]
- Fiel, S.B.; Roesch, E.A. The use of tobramycin for Pseudomonas aeruginosa: A review. Expert Rev. Respir. Med. 2022, 16, 503–509. [Google Scholar] [CrossRef]
- Prié, H.; Meyssonnier, V.; Kerroumi, Y.; Heym, B.; Lidove, O.; Marmor, S.; Zeller, V. Pseudomonas aeruginosa prosthetic joint-infection outcomes: Prospective, observational study on 43 patients. Front. Med. 2022, 9, 1039596. [Google Scholar] [CrossRef]
- Hsu, Y.-H.; Hu, C.; Hsieh, P.-H.; Shih, H.-N.; Ueng, S.W.N.; Chang, Y. Vancomycin and Ceftazidime in Bone Cement as a Potentially Effective Treatment for Knee Periprosthetic Joint Infection. J. Bone Jt. Surg. 2017, 99, 223–231. [Google Scholar] [CrossRef]
- Carli, A.V.; Bhimani, S.; Yang, X.; de Mesy Bentley, K.L.; Ross, F.P.; Bostrom, M.P.G. Vancomycin-Loaded Polymethylmethacrylate Spacers Fail to Eradicate Periprosthetic Joint Infection in a Clinically Representative Mouse Model. J. Bone Jt. Surg. 2018, 100, e76. [Google Scholar] [CrossRef] [PubMed]
- Tseng, T.-H.; Chang, C.-H.; Chen, C.-L.; Chiang, H.; Hsieh, H.-Y.; Wang, J.-H.; Young, T.-H. A simple method to improve the antibiotic elution profiles from polymethylmethacrylate bone cement spacers by using rapid absorbable sutures. BMC Musculoskelet. Disord. 2022, 23, 916. [Google Scholar] [CrossRef] [PubMed]
- Stryker Howmedica Osteonics. Bone Cement Matters: Simplex P Bone Cements. Available online: https://necod.com.ar/catalogoPdf/7/l.pdf (accessed on 4 August 2025).
- Fölsch, C.; Schirmer, J.; Glameanu, C.; Ishaque, B.; Fonseca Ulloa, C.A.; Harz, T.; Rickert, M.; Martin, J.R.; Scherberich, J.; Steinbart, J.; et al. Cement Viscosity and Application Time Lead to Significant Changes in Cement Penetration and Contact Surface Area. Arthroplast. Today 2024, 30, 101476. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Moreno, J.; Merino, V.; Nácher, A.; Rodrigo, J.L.; Climente, M.; Merino-Sanjuán, M. Antibiotic-loaded Bone Cement as Prophylaxis in Total Joint Replacement. Orthop. Surg. 2017, 9, 331–341. [Google Scholar] [CrossRef]

| Bone Cement | Simplex® T | Palacos® R+G |
|---|---|---|
| Cement Viscosity | Low Viscosity | High viscosity |
| Polymer powder [g]/Liquid monomer [mL] | 40:20 | 40:20 |
| Monomer Components | MMA, DmpT, HQ | MMA, DmpT, HQ, E141 |
| Polymer Combination | PMMA, MA-S, BPO | PMMA, MA-MMA, E141 |
| Radiopacifier Contained | Barium sulfate | Zirconium dioxide |
| Antibiotic Contained | Tobramycin | Gentamicin |
| Antibiotic Amount [g] per 40 g | 1.0 | 0.5 |
| Antibiotic Concentration [%] | 2.5 | 1.25 |
| Antibiotics | Structure | Staphylococcus aureus (DSM 799) | Staphylococcus epidermidis (DSM 1798) | Escherichia coli (DSM 1576) |
|---|---|---|---|---|
| Gentamicin | 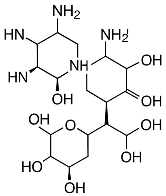 | + | + | + |
| Tobramycin | 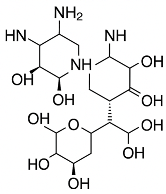 | + | + | + |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coraça-Huber, D.; Humez, M.; Kühn, K.-D. A Comparative Study of Extended Gentamicin and Tobramycin Release and Antibacterial Efficacy from Palacos and Simplex Acrylic Cements. Microorganisms 2025, 13, 2174. https://doi.org/10.3390/microorganisms13092174
Coraça-Huber D, Humez M, Kühn K-D. A Comparative Study of Extended Gentamicin and Tobramycin Release and Antibacterial Efficacy from Palacos and Simplex Acrylic Cements. Microorganisms. 2025; 13(9):2174. https://doi.org/10.3390/microorganisms13092174
Chicago/Turabian StyleCoraça-Huber, Débora, Martina Humez, and Klaus-Dieter Kühn. 2025. "A Comparative Study of Extended Gentamicin and Tobramycin Release and Antibacterial Efficacy from Palacos and Simplex Acrylic Cements" Microorganisms 13, no. 9: 2174. https://doi.org/10.3390/microorganisms13092174
APA StyleCoraça-Huber, D., Humez, M., & Kühn, K.-D. (2025). A Comparative Study of Extended Gentamicin and Tobramycin Release and Antibacterial Efficacy from Palacos and Simplex Acrylic Cements. Microorganisms, 13(9), 2174. https://doi.org/10.3390/microorganisms13092174







