Water Parameters Predicting the Seasonal and Spatial Dynamics of the Vibrio Harveyi- and Splendidus-Clade Pathogens
Abstract
1. Introduction
2. Materials and Methods
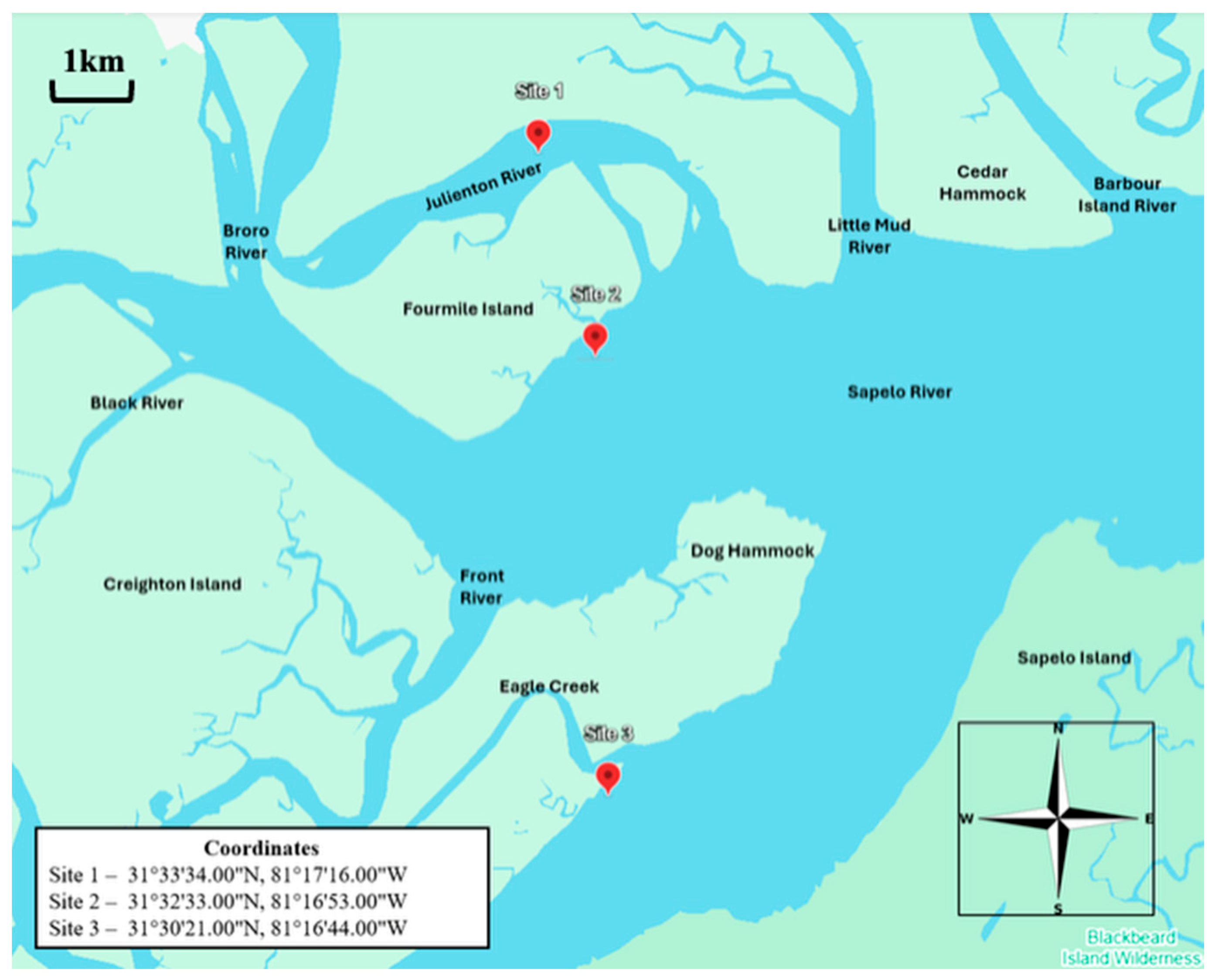
2.1. Field Sites and Sampling Events
2.2. Water Collection, Processing, and Storage of Samples
2.3. Sediment Collection, Processing, and Storage of Samples
2.4. Oyster & Clam Collection, Processing, and Storage of Samples
2.5. Environmental Parameters
2.6. Primers and PCR Protocols
2.7. Statistical Treatment of Data
3. Results
3.1. Frequency of Detection of the Targeted Vibrio Species
3.2. Seasonal and Spatial Dynamics of V. splendidus and the Harveyi-Clade Species
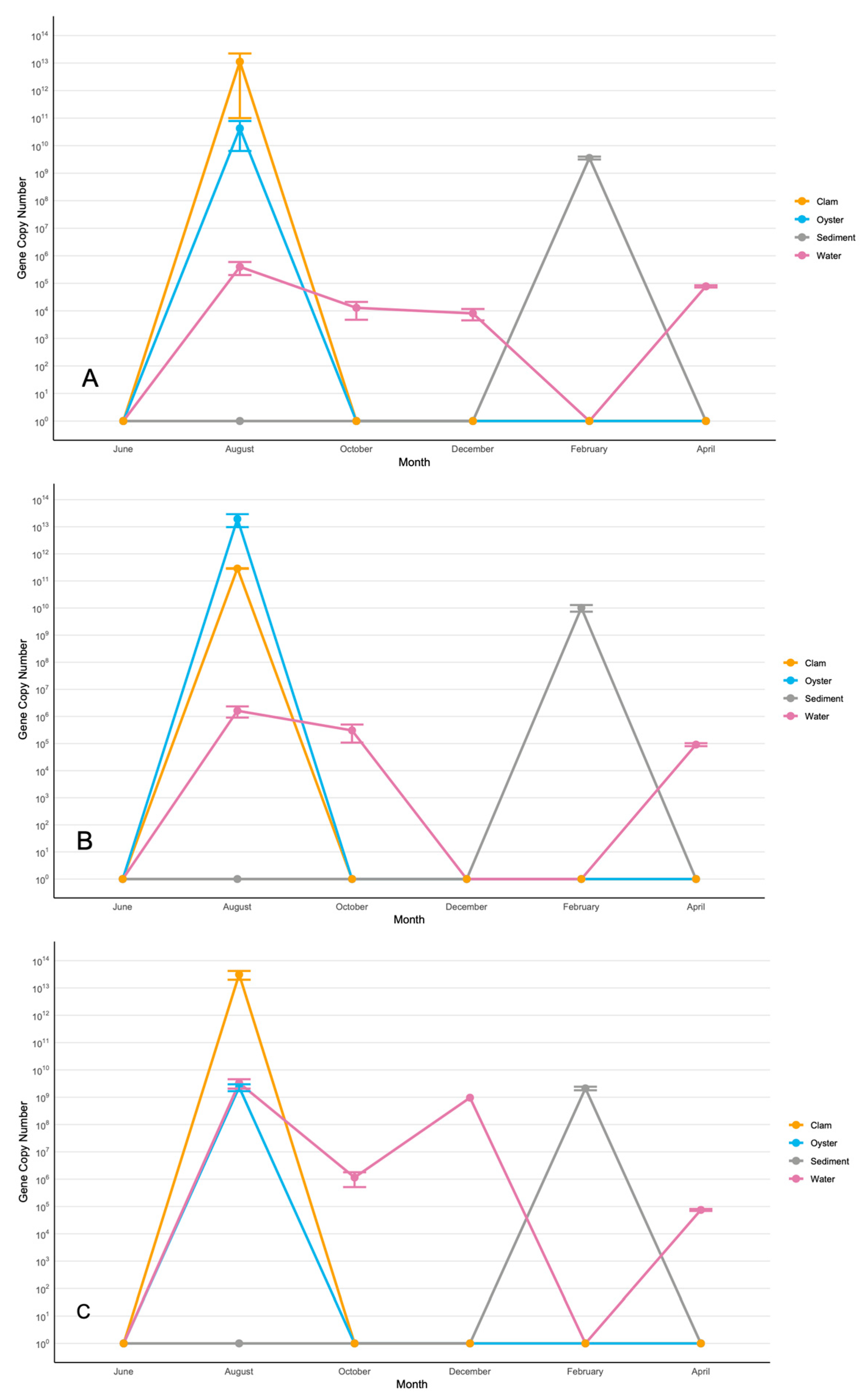
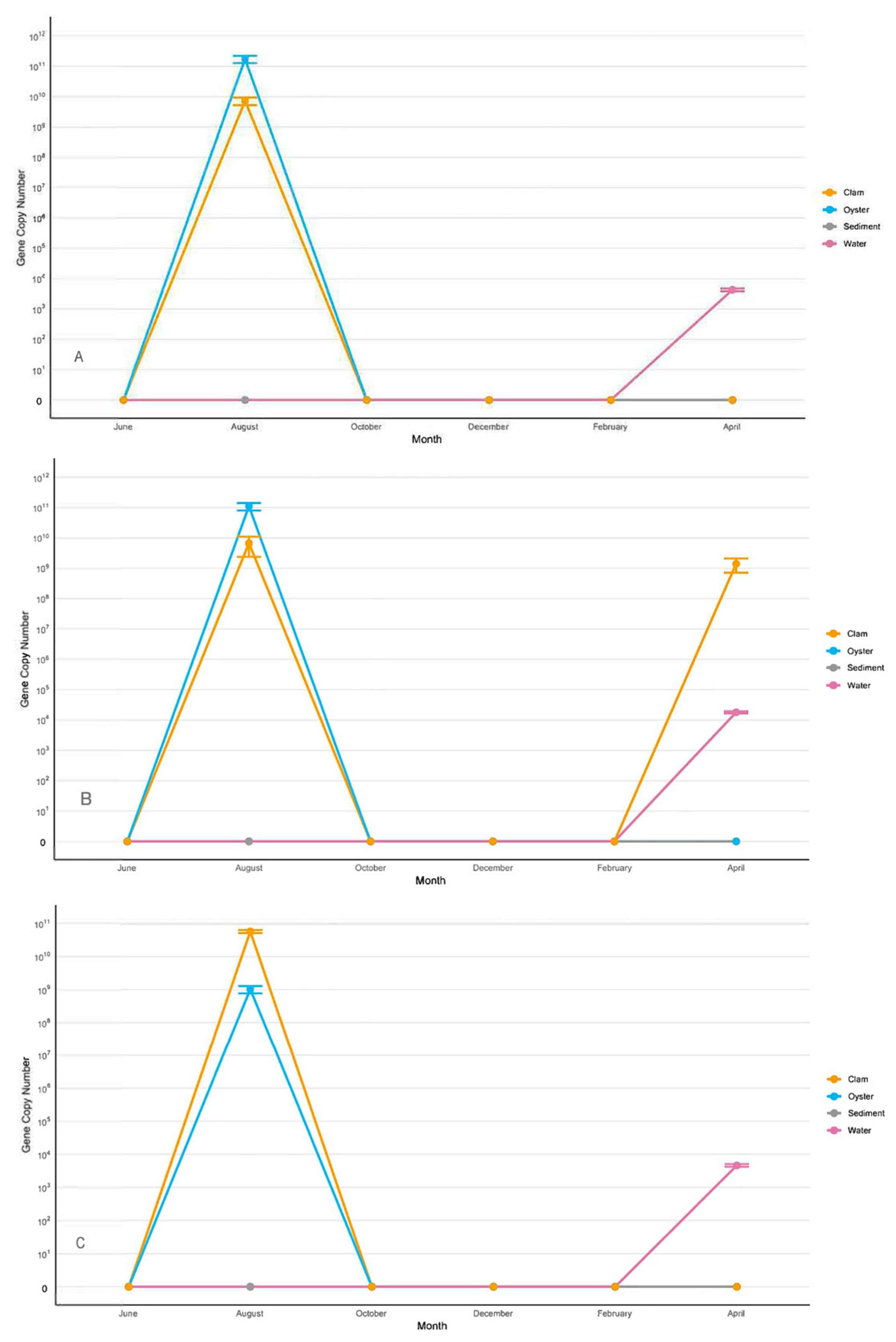
3.2.1. Vibrio splendidus
3.2.2. Vibrio alginolyticus
3.2.3. Vibrio harveyi/Vibrio campbellii
3.2.4. Vibrio parahaemolyticus
3.3. Impact of Water Parameters on the Distribution and Abundance of Targeted Vibrio Species
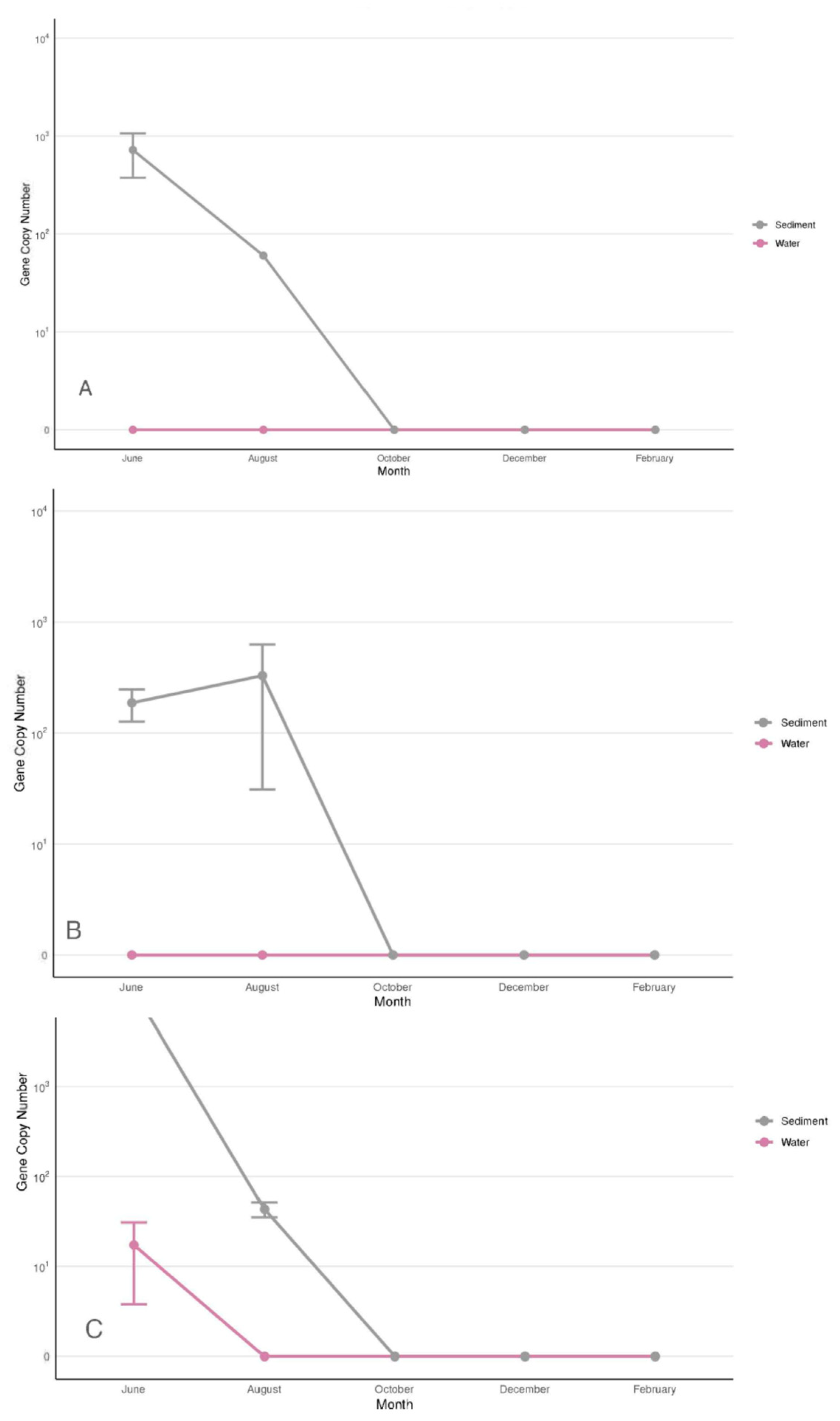
3.4. The Harveyi-Clade Carriers of Virulence Genes
4. Discussion
4.1. Shared Patterns of the Targeted Vibrio Species
4.2. V. splendidus
4.3. V. alginolyticus
4.4. Vibrio harveyi/Vibrio campbellii
4.5. Vibrio parahaemolyticus
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Culot, A.; Grosset, N.; Bruey, Q.; Auzou, M.; Giard, J.C.; Favard, B.; Wakatsuki, A.; Baron, S.; Frouel, S.; Teacher, C.; et al. Isolation of Harveyi clade Vibrio spp. collected in aquaculture farms: How can the identification be addressed? J. Microbiol. Methods 2021, 180, 106106. [Google Scholar] [CrossRef]
- Darshanee Ruwandeepika, H.A.; Sanjeewa Prasad Jayaweera, T.; Paban Bhowmick, P.; Karunasagar, I.; Bossier, P.; Defoirdt, T. Pathogenesis, virulence factors and virulence regulation of vibrios belonging to the Harveyi clade. Rev. Aquac. 2012, 4, 59–74. [Google Scholar] [CrossRef]
- Baker-Austin, C.; Oliver, J.D.; Alam, M.; Ali, A.; Waldor, M.K.; Qadri, F.; Martinez-Urtaza, J. Vibrio spp. infections. Nat. Rev. Dis. Prim. 2018, 4, 1–19. [Google Scholar] [CrossRef]
- Loo, K. The burden of Vibrio sp. Infections—A scoping review. Prog. Microbes Mol. Biol. 2023, 6. [Google Scholar] [CrossRef]
- Barkovskii, A.L.; Brown, C. Environmental Drivers of the Divergence of Harveyi Clade Pathogens with Distinctive Virulence Gene Profiles. Microorganisms 2024, 12, 2234. [Google Scholar] [CrossRef]
- Ruwandeepika, H.A.D.; Defoirdt, T.; Bhowmick, P.P.; Karunasagar, I.; Bossier, P. In vitro and in vivo expression of virulence genes in Vibrio isolates belonging to the Harveyi clade in relation to their virulence towards gnotobiotic brine shrimp (Artemia franciscana). Environ. Microbiol. 2011, 12, 506–517. [Google Scholar] [CrossRef] [PubMed]
- Letchumanan, V.; Chan, K.G.; Lee, L.H. Vibrio parahaemolyticus: A review on the pathogenesis, prevalence, and advance molecular identification techniques. Front. Microbiol. 2014, 5, 705. [Google Scholar] [CrossRef] [PubMed]
- Humphries, R.M.; Linscott, A.J. Practical guidance for clinical microbiology laboratories: Diagnosis of bacterial gastroenteritis. Clin. Microbiol. Rev. 2015, 28, 3–31. [Google Scholar] [CrossRef]
- Bryant, T.; Ellenwood, S.; Butters, O.; Saccoccio, F.M. An uncommon cause of soft tissue and knee infection after penetrating injury in a non-immunocompromised adolescent male. SAGE Open Med. Case Rep. 2021, 9, 2050313X2110346. [Google Scholar] [CrossRef]
- Daniels, N.A.; Mackinnon, L.; Bishop, R.D.; Altekruse, S.F.; Ray, B.; Hammond, R.; Thompson, S.; Wilson, S.; Bean, N.H.; Griffin, P.M.; et al. Vibrio parahaemolyticus infections in the United States, 1973–1998. J. Infect. Dis. 2000, 181, 1661–1666. [Google Scholar] [CrossRef]
- Ahmad, A.; Brumble, L.; Maniaci, M. Vibrio parahaemolyticus induced necrotizing fasciitis: An atypical organism causing an unusual presentation. Case Rep. Infect. Dis. 2013, 2013, 216854. [Google Scholar] [CrossRef] [PubMed]
- CDC. Severe Vibrio vulnificus Infections in the United States Associated with Warming Coastal Waters, 1 September 2023. Available online: https://www.cdc.gov/han/2023/han00497.html#:~:text=Vibrio%20are%20bacteria%20that%20cause,About%20150%E2%80%93200%20V.September (accessed on 10 September 2025).
- Gopal, S.; Otta, S.K.; Kumar, S.; Karunasagar, I.; Nishibuchi, M.; Karunasagar, I. The occurrence of Vibrio species in tropical shrimp culture environments; implications for food safety. Int. J. Food Microbiol. 2005, 102, 151–159. [Google Scholar] [CrossRef]
- Gómez-León, J.; Villamil, L.; Lemos, M.L.; Novoa, B.; Figueras, A. Isolation of Vibrio alginolyticus and Vibrio splendidus from aquacultured carpet shell clam (Ruditapes decussatus) larvae associated with mass mortalities. Appl. Environ. Microbiol. 2005, 71, 98–104. [Google Scholar] [CrossRef]
- Yang, B.; Zhai, S.; Li, X.; Tian, J.; Li, Q.; Shan, H.; Liu, S. Identification of Vibrio alginolyticus as a causative pathogen associated with mass summer mortality of the Pacific Oyster (Crassostrea gigas) in China. Aquaculture 2021, 535, 736363. [Google Scholar] [CrossRef]
- Manchanayake, T.; Mohamad, A.; Amir-Danial, Z.; Nor, N.A.; Yong-Kit, C.; Nazarudin, M.F.; Nor, R.M.; Hasnan, Q.; Zamri-Saad, M.; Azmai Amal, M.N.; et al. Oral Adjuvanted Vibrio Vaccine Enhances Antibody Production and Lysozyme Activity in the Serum and Mucus of Marine-cultured Red Hybrid Tilapia (Oreochromis sp.) Against V. harveyi and V. alginolyticus. Fish Shellfish. Immunol. 2025, 110503. [Google Scholar] [CrossRef]
- Slifka, K.J.; Newton, A.E.; Mahon, B.E. Vibrio alginolyticus infections in the USA, 1988–2012. Epidemiol. Infect. 2017, 145, 1491–1499. [Google Scholar] [CrossRef]
- Khan, A.A.; Linkous, B.K.; Lanza, J.T. Vibrio alginolyticus: A rare cause of otitis externa off the coast of Florida. Cureus 2024, 16, e61524. [Google Scholar] [CrossRef] [PubMed]
- Haldar, S.; Chatterjee, S.; Sugimoto, N.; Das, S.; Chowdhury, N.; Hinenoya, A.; Asakura, M.; Yamasaki, S. Identification of Vibrio campbellii isolated from diseased farm-shrimps from south India and establishment of its pathogenic potential in an artemia model. Microbiology 2011, 157, 179–188. [Google Scholar] [CrossRef]
- Kumar, S.; Kumar, C.B.; Rajendran, V.; Abishaw, N.; Anand, P.S.S.; Kannapan, S.; Nagaleekar, V.K.; Vijayan, K.K.; Alavandi, S.V. Delineating virulence of Vibrio campbellii: A predominant luminescent bacterial pathogen in Indian shrimp hatcheries. Sci. Rep. 2021, 11, 15831. [Google Scholar] [CrossRef]
- Zhang, X.H.; He, X.; Austin, B. Vibrio harveyi: A serious pathogen of fish and invertebrates in mariculture. Mar. Life Sci. Technol. 2020, 2, 231–245. [Google Scholar] [CrossRef] [PubMed]
- Soto-Rodríguez, S.A.; Gómez-Gil, B.; Lozano, R. ‘Bright-red’ syndrome in pacific white shrimp Litopenaeus vannamei is caused by Vibrio harveyi. Dis. Aquat. Org. 2010, 92, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Brehm, T.T.; Berneking, L.; Rohde, H.; Chistner, M.; Schlickewei, C.; Sena Martins, M.; Schmiedel, S. Wound infection with Vibrio harveyi following a traumatic leg amputation after a motorboat propeller injury in Mallorca, Spain: A case report and review of literature. BMC Infect. Dis. 2020, 20, 104. [Google Scholar] [CrossRef] [PubMed]
- Cano-Gomez, A.; Høj, L.; Owens, L.; Andreakis, N. Multilocus sequence analysis provides basis for fast and reliable identification of Vibrio harveyi-related species and reveals previous misidentification of important marine pathogens. Syst. Appl. Microbiol. 2011, 34, 561–565. [Google Scholar] [CrossRef]
- Srisangthong, I.; Sangseedum, C.; Chaichanit, N.; Surachat, K.; Suanyuk, N.; Mittraparp-arthorn, P. Characterization and genome analysis of Vibrio campbellii lytic bacteriophage OPA17. Microbiol. Spectr. 2023, 11, 01623. [Google Scholar] [CrossRef]
- Renault, T.; Pons, A.; Roux, F.L. Two Vibrio splendidus related strains collaborate to kill Crassostrea gigas: Taxonomy and host alterations. Dis. Aquat. Org. 2004, 62, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Lacoste, A.; Jalabert, F.; Malham, S.K.; Cueff, A.; Gélébart, F.; Cordevant, C.; Lange, M.; Poulet, S.A. A Vibrio splendidus strain is associated with summer mortality of juvenile oysters Crassostrea gigas in the Bay of Morlaix (North Brittany, France). Dis. Aquat. Org. 2001, 46, 139–145. [Google Scholar] [CrossRef]
- Thompson, J.R.; Randa, M.A.; Marcelino, L.A.; Tomita-Mitchell, A.; Lim, E.; Polz, M.F. Diversity and Dynamics of a North Atlantic Coastal Vibrio Community. Appl. Environ. Microbiol. 2004, 70, 4103–4110. [Google Scholar] [CrossRef]
- Prescott, J.; Barkovskii, A.L. In situ dynamics of Vibrio parahaemolyticus and Vibrio vulnificus in water, sediment and triploid Crassostrea virginica oysters cultivated in floating gear. J. Appl. Microbiol. 2022, 132, 3343–3354. [Google Scholar] [CrossRef]
- MacKnight, S.D. Selection of bottom sediment sampling stations. In Handbook of Techniques for Aquatic Sediments Sampling; CRC Press Inc.: Boca Raton, FL, USA, 1994; pp. 17–28. [Google Scholar]
- Torresi, M.; Acciari, V.A.; Piano, A.; Serratore, P.; Prencipe, V.; Migliorati, G. Detection of Vibrio splendidus and related species in Chamelea gallina sampled in the Adriatic along the Abruzzi coastline. Vet. Ital. 2011, 47, 371–378. [Google Scholar]
- Nordstrom, J.L.; Vickery, M.C.L.; Blackstone, G.M.; Murray, S.L.; DePaola, A. Development of a multiplex real-time PCR assay with an internal amplification control for the detection of total and pathogenic Vibrio parahaemolyticus bacteria in oysters. Appl. Environ. Microbiol. 2007, 73, 5840–5847. [Google Scholar] [CrossRef]
- Kim, H.J.; Ryu, J.O.; Lee, S.Y.; Kim, E.S.; Kim, H.Y. Multiplex PCR for detection of the Vibrio genus and five pathogenic Vibrio species with primer sets designed using comparative genomics. BMC Microbiol. 2015, 15, 239. [Google Scholar] [CrossRef] [PubMed]
- Kelly, M.T. Effect of temperature and salinity on Vibrio (beneckea) vulnificus occurrence in a Gulf coast environment. Appl. Environ. Microbiol. 1982, 44, 820–824. [Google Scholar] [CrossRef] [PubMed]
- Pfeffer, C.S.; Hite, M.; Oliver, J.D. Ecology of Vibrio vulnificus in estuarine waters of eastern North Carolina. Appl. Environ. Microbiol. 2003, 69, 3526–3531. [Google Scholar] [CrossRef] [PubMed]
- Gradoville, M.; Crump, B.; Häse, C.; White, A. Environmental controls of oyster-pathogenic Vibrio spp. in Oregon estuaries and a shellfish hatchery. Appl. Environ. Microbiol. 2018, 84, e02156-17. [Google Scholar] [CrossRef]
- Cantet, F.; Hervio-Heath, D.; Caro, A.; Mennec, C.L.; Monteil, C.; Quéméré, C.; Jolivet-Gougeon, A.; Colwell, R.R.; Monfort, P. Quantification of Vibrio parahaemolyticus, Vibrio vulnificus, and Vibrio cholerae in French Mediterranean coastal lagoons. Res. Microbiol. 2013, 164, 867–874. [Google Scholar] [CrossRef]
- Barkovskii, A.L.; Thomas, M.; Hurley, D.; Teems, C. Environmental factors responsible for the incidence of antibiotic resistance genes in pristine Crassostrea virginica reefs. Mar. Pollut. Bull. 2012, 64, 2692–2698. [Google Scholar] [CrossRef]
- Velez, K.C.; Leighton, R.E.; Decho, A.W.; Pinckney, J.L.; Norman, R.S. Modeling pH and temperature effects as climatic hazards in Vibrio vulnificus and Vibrio parahaemolyticus planktonic growth and biofilm formation. GeoHealth 2023, 7, e2022GH000769. [Google Scholar] [CrossRef]
- Stoodley, P.; Cargo, R.; Rupp, C.J.; Wilson, S.; Klapper, I. Biofilm material properties as related to shear-induced deformation and detachment phenomena. J. Ind. Microbiol. Biotechnol. 2002, 29, 361–367. [Google Scholar] [CrossRef]
- Yamahara, K.M.; Sassoubre, L.M.; Goodwin, K.D.; Boehm, A.B. Occurrence and persistence of bacterial pathogens and indicator organisms in beach sand along the California coast. Appl. Environ. Microbiol. 2012, 78, 1733–1745. [Google Scholar] [CrossRef]
- Marrero, K.; Sánchez, A.; Rodríguez-Ulloa, A.; González, L.J.; Castellanos-Serra, L.; Paz-Lago, D.; Campos, J.; Rodríguez, B.L.; Suzarte, E.; Ledón, T.; et al. Anaerobic growth promotes synthesis of colonization factors encoded at the Vibrio pathogenicity island in Vibrio cholerae El Tor. Res. Microbiol. 2009, 160, 48–56. [Google Scholar] [CrossRef]
- Phippen, B.L.; Oliver, J.D. Role of anaerobiosis in capsule production and biofilm formation in Vibrio vulnificus. Infect. Immun. 2015, 83, 551–559. [Google Scholar] [CrossRef]
- Gamble, M.D.; Lovell, C.R. Infaunal burrows are enrichment zones for Vibrio parahaemolyticus. Appl. Environ. Microbiol. 2011, 77, 3703–3714. [Google Scholar] [CrossRef]
- Klein, S.L.; Lovell, C.R. The hot oyster: Levels of virulent Vibrio parahaemolyticus strains in individual oysters. FEMS Microbiol. Ecol. 2017, 93, fiw232. [Google Scholar] [CrossRef]
- Pérez-Cataluña, A.; Lucena, T.; Tarazona, E.; Arahal, D.R.; Macián, M.C.; Pujalte, M.J. An MLSA approach for the taxonomic update of the Splendidus clade, a lineage containing several fish and shellfish pathogenic Vibrio spp. Syst. Appl. Microbiol. 2016, 39, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Norfolk, W.A.; Shue, C.; Henderson, W.M.; Glinski, D.A.; Lipp, E.K. Vibrio alginolyticus growth kinetics and the metabolic effects of iron. Microbiol. Spectr. 2023, 11, e02680-23. [Google Scholar] [CrossRef]
- Oberbeckmann, S.; Wichels, A.; Wiltshire, K.H.; Gerdts, G. Occurrence of Vibrio parahaemolyticus and Vibrio alginolyticus in the German Bight over a seasonal cycle. Antonie Van Leeuwenhoek 2011, 100, 291–307. [Google Scholar] [CrossRef] [PubMed]
- Balebona, M.; Andreu, M.; Bordas, M.; Zorrilla, I.; Moriñigo, M.; Borrego, J. Pathogenicity of Vibrio alginolyticus for cultured gilt-head sea bream (Sparus aurata L.). Appl. Environ. Microbiol. 1998, 64, 4269–4275. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Zhao, Y.; Zhang, Y.; Liu, Y.; Wang, G.; He, Z.; Cao, W.; Han, T.; Zhang, X.; Zhang, Z.; et al. Population response of intestinal microbiota to acute Vibrio alginolyticus infection in half-smooth tongue sole (Cynoglossus semilaevis). Front. Microbiol. 2023, 14, 1178575. [Google Scholar] [CrossRef]
- Kataržytė, M.; Gyraitė, G.; Kalvaitienė, G.; Vaičiūtė, D.; Budrytė, O.; Bučas, M. Potentially Pathogenic Vibrio spp. in Algal Wrack Accumulations on Baltic Sea Sandy Beaches. Microorganisms 2024, 12, 2101. [Google Scholar] [CrossRef]
- King, W.L.; Siboni, N.; Kahlke, T.; Green, T.; Labbate, M.; Seymour, J.R. A new high throughput sequencing assay for characterizing the diversity of natural Vibrio communities and its application to a pacific oyster mortality event. Front. Microbiol. 2019, 10, 2907. [Google Scholar] [CrossRef]
- Xiao, J.; Liu, L.; Ke, Y.; Li, X.; Liu, Y.; Pan, Y.; Yan, S.; Wang, Y. Shrimp AHPND-causing plasmids encoding the Pirab toxins as mediated by pirab-tn903 are prevalent in various Vibrio species. Sci. Rep. 2017, 7, 42177. [Google Scholar] [CrossRef] [PubMed]
- Mohamad, N.; Amal, M.N.A.; Saad, M.Z.; Yasin, I.S.M.; Zulkiply, N.A.; Mustafa, M.; Nasruddin, N.S. Virulence-associated genes and antibiotic resistance patterns of Vibrio spp. isolated from cultured marine fishes in Malaysia. BMC Vet. Res. 2019, 15, 176. [Google Scholar] [CrossRef]
- Ball, A.S.; Chaparian, R.R.; van Kessel, J.C. Quorum sensing gene regulation by LuxR/HapR master regulators in Vibrios. J. Bacteriol. 2017, 199, e00105-17. [Google Scholar] [CrossRef]
- Zhong, Y.; Zhang, X.H.; Chen, J.; Chi, Z.; Sun, B.; Li, Y.; Austin, B. Overexpression, purification, characterization, and pathogenicity of Vibrio harveyi hemolysin VHH. Infect. Immun. 2006, 74, 6001–6005. [Google Scholar] [CrossRef]
- Raghunath, P. Roles of thermostable direct hemolysin (TDH) and TDH-related hemolysin (TRH) in Vibrio parahaemolyticus. Front. Microbiol. 2015, 5, 805. [Google Scholar] [CrossRef]
- Sheikh, H.I.; Najiah, M.; Fadhlina, A.; Laith, A.A.; Nor, M.M.; Jalal, K.C.A.; Kasan, N.A. Temperature upshift mostly but not always enhances the growth of Vibrio species: A systematic review. Front. Mar. Sci. 2022, 9, 959830. [Google Scholar] [CrossRef]
- Zhang, X.; Austin, B. Haemolysins in Vibrio species. J. Appl. Microbiol. 2005, 98, 1011–1019. [Google Scholar] [CrossRef] [PubMed]

| Site/Targeted Species | Va | Vs | Vp | Vc & Vh |
|---|---|---|---|---|
| Site 1 | ||||
| June Sediment | − | − | − | + |
| August Sediment | − | − | + | + |
| October Sediment | − | − | − | − |
| December Sediment | − | − | − | − |
| February Sediment | − | + | + | − |
| April Sediment | − | − | − | N/A |
| June Water | − | − | − | − |
| August Water | − | + | + | − |
| October Water | − | + | + | − |
| December Water | − | + | + | − |
| February Water | − | − | + | − |
| April Water | + | + | − | N/A |
| Site 2 | ||||
| June Sediment | − | − | − | + |
| August Sediment | − | − | + | + |
| October Sediment | − | − | + | − |
| December Sediment | − | − | − | − |
| February Sediment | − | + | + | − |
| April Sediment | − | − | − | N/A |
| June Water | − | − | − | − |
| August Water | − | + | + | − |
| October Water | − | + | + | − |
| December Water | − | + | + | − |
| February Water | − | − | + | − |
| April Water | + | + | − | N/A |
| Site 3 | ||||
| June Sediment | − | − | − | + |
| August Sediment | − | − | + | + |
| October Sediment | − | − | + | − |
| December Sediment | − | − | − | − |
| February Sediment | − | + | + | − |
| April Sediment | − | − | − | N/A |
| June Water | − | − | − | + |
| August Water | − | + | + | − |
| October Water | − | + | − | − |
| December Water | − | + | + | − |
| February Water | − | − | + | − |
| April Water | + | + | − | N/A |
| Detection Frequency | 3/36 (8.33%) | 15/36 (41.67%) | 19/36 (52.78%) | 7/36 (19.44%) |
| Sediment Total | 0/18 (0%) | 3/18 (16.67%) | 8/18 (44.44%) | 6/18 (33.33%) |
| Water Total | 3/18 (16.67%) | 12/18 (66.67%) | 11/18 (61.11%) | 1/18 (5.55%) |
| Site/Targeted Species | Va | Vs | Vp |
|---|---|---|---|
| Site 1 | |||
| June Clam | − | − | + |
| August Clam | + | + | + |
| October Clam | − | − | + |
| December Clam | − | − | + |
| February Clam | − | − | + |
| April Clam | − | − | + |
| June Oyster | − | − | + |
| August Oyster | + | + | + |
| October Oyster | − | − | + |
| December Oyster | − | − | + |
| February Oyster | − | − | + |
| April Oyster | − | − | + |
| Site 2 | |||
| June Clam | − | − | + |
| August Clam | + | + | + |
| October Clam | − | − | + |
| December Clam | − | − | + |
| February Clam | − | − | + |
| April Clam | + | − | + |
| June Oyster | − | − | + |
| August Oyster | + | + | + |
| October Oyster | − | − | + |
| December Oyster | − | − | + |
| February Oyster | − | − | + |
| April Oyster | − | − | + |
| Site 3 | |||
| June Clam | − | − | − |
| August Clam | + | + | + |
| October Clam | − | − | + |
| December Clam | − | − | + |
| February Clam | − | − | + |
| April Clam | − | − | − |
| June Oyster | − | − | + |
| August Oyster | + | + | + |
| October Oyster | − | − | + |
| December Oyster | − | − | + |
| February Oyster | − | − | + |
| April Oyster | − | − | + |
| Detection Frequency | 7/36 (19.44%) | 6/36 (16.67%) | 34/36 (94.44%) |
| Clam Total | 4/18 (22.22%) | 3/18 (16.67%) | 16/18 (88.89%) |
| Oyster Total | 3/18 (16.67%) | 3/18 (16.67%) | 18/18 (100%) |
| Pearson Coeff. | pH | Temp. (°C) | Salinity (ppt) | Turbidity (NTU) | dOxygen (mg/L) | Conduct (mS/cm) | TDS (g/L) | Water Density (g/cm3) |
|---|---|---|---|---|---|---|---|---|
| Vp | −0.425 | 0.597 * | 0.331 | −0.210 | −0.375 | 0.256 | 0.259 | −0.127 |
| Va | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| Vs | −0.351 | 0.408 | 0.455 | −0.070 | −0.182 | 0.434 | 0.386 | 0.070 |
| Vc & Vh | −0.745 ** | 0.462 | −0.325 | 0.456 | −0.485 | −0.372 | −0.430 | −0.598 * |
| Pearson Coefficient | pH | Temp (°C) | Salinity (ppt) | Turbidity (NTU) | Dissolved Oxygen (mg/L) | Conduct (mS/cm) | TDS (g/L) | Water Density (g/cm3) |
|---|---|---|---|---|---|---|---|---|
| Vp | −0.455 | 0.636 * | 0.315 | −0.215 | −0.409 | 0.227 | 0.240 | −0.166 |
| Va | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| Vs | 0.602 * | −0.134 | −0.816 *** | −0.867 *** | −0.305 | −0.736 ** | −0.738 ** | −0.365 |
| Vc & Vh | −0.724 ** | 0.437 | −0.334 | 0.460 | −0.467 | −0.378 | −0.435 | −0.588 * |
| Pearson Coefficient | pH | Temp (°C) | Salinity (ppt) | Turbidity (NTU) | dOxygen (mg/L) | Conduct (mS/cm) | TDS (g/L) | Water Density (g/cm3) |
|---|---|---|---|---|---|---|---|---|
| Vp | −0.196 | 0.579 * | −0.064 | −0.601 * | −0.559 * | −0.108 | −0.107 | −0.339 |
| Va | −0.463 | 0.632 * | 0.303 | −0.205 | −0.415 | 0.224 | 0.225 | −0.171 |
| Vs | −0.464 | 0.632 * | 0.303 | −0.205 | −0.416 | 0.224 | 0.225 | −0.172 |
| Pearson Coefficient | pH | Temp (°C) | Salinity (ppt) | Turbidity (NTU) | dOxygen (mg/L) | Conduct (mS/cm) | TDS (g/L) | Water Density (g/cm3) |
|---|---|---|---|---|---|---|---|---|
| Vp | −0.464 | 0.632 * | 0.303 | −0.205 | −0.415 | 0.224 | 0.225 | −0.171 |
| Va | −0.464 | 0.632 * | 0.303 | −0.205 | −0.415 | 0.224 | 0.225 | −0.171 |
| Vs | −0.464 | 0.632 * | 0.303 | −0.205 | −0.415 | 0.224 | 0.225 | −0.171 |
| Pearson Coefficient Water | Site 1—Water | Site 2—Water | Site 3—Water | Site 1—Sediment | Site 2—Sediment | Site 3—Sediment |
|---|---|---|---|---|---|---|
| Va ToxR | NA | NA | NA | NA | NA | NA |
| Va LuxR | NA | NA | NA | NA | NA | NA |
| Va Srp | NA | NA | NA | NA | NA | NA |
| Va vhhA | NA | NA | NA | NA | NA | NA |
| Va Vhp | NA | NA | NA | NA | NA | NA |
| Va vhh | NA | NA | NA | NA | NA | NA |
| Vs toxR | −0.266628 | −0.178348 | 0.614342 * | −0.369999 | −0.308843 | −0.237613 |
| Vs luxR | 0.576408 * | −0.309255 | 0.956181 *** | −0.300033 | −0.243797 | −0.379674 |
| Vs srp | −0.267089 | −0.276635 | 0.911818 *** | −0.314119 | −0.029936 | −0.251624 |
| Vs vhhA | 0.636287 * | 0.336443 | 0.956532 *** | −0.253705 | −0.418526 | −0.255721 |
| Vs vhp | 0.914971 *** | −0.288870 | 0.956312 *** | −0.305423 | −0.359860 | −0.244952 |
| Vs vhh | −0.182311 | 0.029843 | 0.940769 *** | −0.237935 | −0.099112 | −0.315662 |
| Vp toxR | −0.326406 | −0.198623 | 0.675464 ** | −0.376410 | 0.993103 *** | −0.237613 |
| Vp luxR | 0.517126 * | −0.329730 | 0.994786 *** | 0.985776 *** | 0.997616 *** | −0.379674 |
| Vp srp | −0.326671 | −0.298497 | 0.961058 *** | −0.031231 | 0.962990 *** | −0.251624 |
| Vp vhhA | 0.577854 * | 0.319396 | 0.994962 *** | −0.245787 | 0.821006 *** | −0.255721 |
| Vp vhp | 0.908499 *** | −0.309028 | 0.994787 *** | −0.232470 | 0.042736 | −0.244952 |
| Vp vhh | −0.238271 | 0.003321 | 0.985684 *** | −0.215360 | 0.524304 * | −0.315662 |
| Vh/Vc toxR | −0.250000 | 0.991771 *** | 0.564862 * | 0.864884 *** | 0.798366 *** | −0.252737 |
| Vh/Vc luxR | 0.590599 * | 0.999925 *** | −0.171827 | −0.004137 | 0.839814 *** | 0.863118 *** |
| Vh/Vc srp | −0.250494 | 0.998538 *** | −0.198096 | 0.989971 *** | 0.820817 *** | −0.241597 |
| Vh/Vc vhhA | 0.649705 ** | 0.790431 *** | −0.171463 | 0.997152 *** | 0.998322 *** | −0.234020 |
| Vh/Vc vhp | 0.915609 *** | 0.999913 *** | −0.172967 | 0.983395 *** | −0.072447 | −0.259005 |
| Vh/Vc vhh | −0.165745 | 0.565125 * | −0.097561 | 0.998677 *** | −0.031420 | −0.336497 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Royer, K.; Barkovskii, A.L. Water Parameters Predicting the Seasonal and Spatial Dynamics of the Vibrio Harveyi- and Splendidus-Clade Pathogens. Microorganisms 2025, 13, 2167. https://doi.org/10.3390/microorganisms13092167
Royer K, Barkovskii AL. Water Parameters Predicting the Seasonal and Spatial Dynamics of the Vibrio Harveyi- and Splendidus-Clade Pathogens. Microorganisms. 2025; 13(9):2167. https://doi.org/10.3390/microorganisms13092167
Chicago/Turabian StyleRoyer, Karagan, and Andrei L. Barkovskii. 2025. "Water Parameters Predicting the Seasonal and Spatial Dynamics of the Vibrio Harveyi- and Splendidus-Clade Pathogens" Microorganisms 13, no. 9: 2167. https://doi.org/10.3390/microorganisms13092167
APA StyleRoyer, K., & Barkovskii, A. L. (2025). Water Parameters Predicting the Seasonal and Spatial Dynamics of the Vibrio Harveyi- and Splendidus-Clade Pathogens. Microorganisms, 13(9), 2167. https://doi.org/10.3390/microorganisms13092167






