Aquatic Chlamydiae: A Review of Their Roles in Fish Health
Abstract
1. Introduction
1.1. Importance of Fish Farms and Financial Risks Associated with Fish Diseases
1.2. Gill Functions and Gill Diseases
1.3. Etiologies of Gill Diseases
1.4. Epitheliocystis Disease
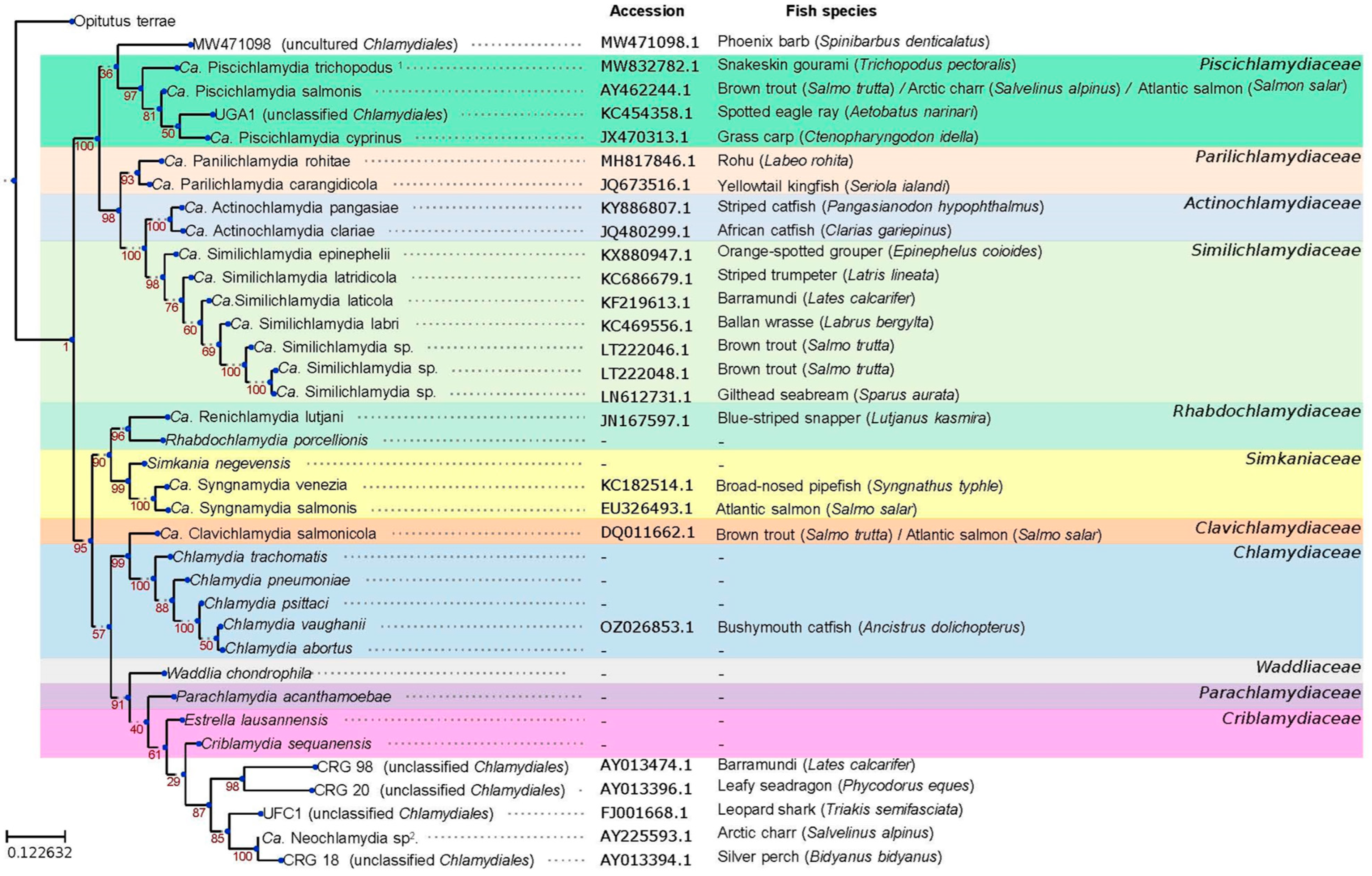
1.5. The Chlamydiota Order
2. Chlamydia-Related Bacteria as Causative Agents of Epitheliocystis
2.1. Chlamydia-Related Bacteria Associated with Epitheliocystis
2.2. Recently Discovered Chlamydia-Related Bacteria Associated with Fish
- Ca. Panilichlamydia rohitae
- Ca. Piscichlamydia trichopodus
- Uncultured member of the Chlamydiota order in cyprinids
- Chlamydia vaughanii
| Fish Species | Family-Level Lineage | Chlamydiota Species | Accession | References |
|---|---|---|---|---|
| African catfish (Clarias gariepinus) * | Actinochlamydiaceae | Ca. Actinochlamydia clariae | JQ480299 | [39] |
| Arctic charr (Salvelinus alpinus) * | Piscichlamydiaceae | Ca. Piscichlamydia salmonis | AY462244 | [40] |
| Parachlamydiaceae | Ca. Neochlamydia sp. | 100% identical to AY225593.1. | [10] | |
| Atlantic Salmon (Salmo salar) ** | Clavichlamydiaceae | Ca. Clavichlamydia salmonicola | DQ011662 | [41,42,43] |
| Simkaniaceae | Ca. Syngnamydia salmonis | EU326493 | [44] | |
| Piscichlamydiaceae | Ca. Piscichlamydia salmonis | AY462244 | [15,43,45,46] | |
| Ballan wrasse (Labrus bergylta) ** | Parilichlamydiaceae | Ca. Similichlamydia labri | KC469556 | [47] |
| Barramundi (Lates calcarifer) ** | Parilichlamydiaceae | Ca. Similichlamydia laticola | KF219613 | [48] |
| not determined | CRG 98 (unclassified Chlamydiales) | AY013474 | [37] | |
| Blue-striped snapper (Lutjanus kasmira) ** | Rhabdochlamydiaceae | Ca. Renichlamydia lutjani | JN167597 | [49] |
| Broad-nosed pipefish (Syngnathus typhle) ** | Simkaniaceae | Ca. Syngnamydia venezia | KC182514 | [50] |
| Brown trout (Salmo trutta) * | Piscichlamydiaceae | Ca. Piscichlamydia salmonis | AY462244 | [43,51] |
| Clavichlamydiaceae | Ca. Clavichlamydia salmonicola | DQ011662 | [41,42,43,46,51,52] | |
| Parilichlamydiaceae | Ca. Similichlamydia sp. | LT222046 LT222048 | [51] | |
| Bushymouth catfish (Ancistrus dolichopterus) * | Chlamydiaceae | Chlamydia vaughanii | OZ026853.1 | [35] |
| Common carp (Cyprinus carpio) * | Parachlamydiaceae | Ca. Neochlamydia sp. | not available | [53] |
| Parachlamydiaceae | Ca. Protochlamydia sp. | not available | [53] | |
| Piscichlamydiaceae | Ca. Piscichlamydia sp. | not available | [53] | |
| Gibel carp (Carassius auratus) * | Parachlamydiaceae | Ca. Neochlamydia sp. | not available | [53] |
| Parachlamydiaceae | Ca. Protochlamydia sp. | not available | [53] | |
| Piscichlamydiaceae | Ca. Piscichlamydia sp. | not available | [53] | |
| Gilthead seabream (Sparus aurata) ** | Parilichlamydiaceae | Ca. Similichlamydia sp. | LN612731 | [54] |
| Grass carp (Ctenopharyngodon idella) * | Piscichlamydiaceae | Ca. Piscichlamydia cyprinus | JX470313 | [55] |
| Leafy seadragon (Phycodorus eques) ** | not determined | CRG 20 (unclassified Chlamydiales) | AY013396 | [37] |
| Leopard shark (Triakis semifasciata) ** | not determined | UFC1 (unclassified Chlamydiales) | FJ001668 | [56] |
| Orange-spotted grouper (Epinephelus coioides) ** | Parilichlamydiaceae | Ca. Similichlamydia epinephelii | KX880947 | [57] |
| Phoenix barb (Spinibarbus denticalatus) * | not determined | MW471098 (uncultured Chlamydiales) | MW471098 | [34] |
| Rohu (Labeo rohita) * | Parilichlamydiaceae | Ca. Panilichlamydia rohitae | MH817846 | [31] |
| Silver perch (Bidyanus bidyanus) * | not determined | CRG 18 (unclassified Chlamydiales) | AY013394 | [37] |
| Snakeskin gourami (Trichopodus pectoralis) * | new family-level lineage | Ca. Piscichlamydia trichopodus 1 | MW832782 | [32] |
| Spotted eagle ray (Aetobatus narinari) ** | not determined | UGA1 (unclassified Chlamydiales) | KC454358 | [58] |
| Striped catfish (Pangasianodon hypophthalmus) * | Actinochlamydiaceae | Ca. Actinochlamydia pangasiae | KY886807.1 | [59] |
| Striped trumpeter (Latris lineata) ** | Parilichlamydiaceae | Ca. Similichlamydia latridicola | KC686679 | [60] |
| Yellowtail kingfish (Seriola ialandi) ** | Parilichlamydiaceae | Ca. Parilichlamydia carangidicola | JQ673516 | [61] |
3. Chlamydia-Related Bacteria May Play a Role in Multifactorial Complex Gill Disease
3.1. Proliferative Gill Disease
3.2. Proliferative Gill Inflammation
3.3. Proliferative Gill Inflammation and Epitheliocystis
4. Chlamydia-Related Bacteria in Fish Gills
4.1. Gills Microbiome
4.2. Chlamydia-Related Bacteria as Symbionts of Fish
5. Permissiveness of Cell Lines Derived from Fish to Chlamydia-Related Bacteria and Chlamydia vaughanii
5.1. Waddlia chondrophila, Estrella lausannensis and Parachlamydia acanthamoebae
5.2. Chlamydia vaughanii
6. A Zebrafish Model of Infection by Chlamydia-Related Bacteria
6.1. An Attractive Model of Infection
6.2. Waddlia chondrophila in Zebrafish
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. The State of World Fisheries and Aquaculture. Towards Blue Transformation; FAO: Rome, Italy, 2022. [Google Scholar]
- Tavares-Dias, M.; Martins, M.L. An overall estimation of losses caused by diseases in the Brazilian fish farms. J. Parasit. Dis. 2017, 41, 913–918. [Google Scholar] [CrossRef]
- Shinn, A.; Pratoomyot, J.; Bron, J.; Paladini, G.; Brooker, E.; Brooker, A. Economic impacts of aquatic parasites on global finfish production. Glob. Aquac. Advocate 2015, 2015, 58–61. [Google Scholar]
- Evans, D.H.; Piermarini, P.M.; Choe, K.P. The Multifunctional Fish Gill: Dominant Site of Gas Exchange, Osmoregulation, Acid-Base Regulation, and Excretion of Nitrogenous Waste. Physiol. Rev. 2005, 85, 97–177. [Google Scholar] [CrossRef]
- Herrero, A.; Thompson, K.D.; Ashby, A.; Rodger, H.D.; Dagleish, M.P. Complex Gill Disease: An Emerging Syndrome in Farmed Atlantic Salmon (Salmo salar L.). J. Comp. Pathol. 2018, 163, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Boerlage, A.S.; Ashby, A.; Herrero, A.; Reeves, A.; Gunn, G.J.; Rodger, H.D. Epidemiology of marine gill diseases in Atlantic salmon (Salmo salar) aquaculture: A review. Rev. Aquac. 2020, 12, 2140–2159. [Google Scholar] [CrossRef]
- Plehn, M. Praktikum der fischkrankheiten; Schweizerbart (E. Nägele) gmbh: Stuttgart, Germany, 1924; Volume 1. [Google Scholar]
- Hoffman, G.L.; Dunbar, C.E.; Wolf, K.; Zwillenberg, L.O. Epitheliocystis, a new infectious disease of the bluegill (Lepomis macrochirus). Antonie Van Leeuwenhoek 1969, 35, 146–158. [Google Scholar] [CrossRef]
- Nowak, B.F.; LaPatra, S.E. Epitheliocystis in fish. J. Fish. Dis. 2006, 29, 573–588. [Google Scholar] [CrossRef]
- Crespo, S.; Zarza, C.; Padrós, F.; Marín De Mateo, M. Epitheliocystis agents in sea bream Sparus aurata: Morphological evidence for two distinct chlamydia-like developmental cycles. Dis. Aquat. Org. 1999, 37, 61–72. [Google Scholar] [CrossRef]
- Draghi Ii, A.; Bebak, J.; Popov, V.; Noble, A.; Geary, S.; West, A.; Byrne, P.; Frasca, S.J. Characterization of a Neochlamydia-like bacterium associated with epitheliocystis in cultured Arctic charr Salvelinus alpinus. Dis. Aquat. Org. 2007, 76, 27–38. [Google Scholar] [CrossRef]
- Katharios, P.; Papadaki, M.; Papandroulakis, N.; Divanach, P. Severe mortality in mesocosm-reared sharpsnout sea bream Diplodus puntazzo larvae due to epitheliocystis infection. Dis. Aquat. Org. 2008, 82, 55–60. [Google Scholar] [CrossRef]
- Syasina, I.; Park, I.-S.; Kim, J.M. Gill Tissue Reactions to an Epitheliocystis Infection in Cultured Red Seabream, Pagrus major. J. Fish. Pathol. 2004, 17, 105–111. [Google Scholar]
- Grau, A.; Crespo, S. Epitheliocystis in the wild and cultured amberjack, Seriola dumerili Risso: Ultrastructural observations. Aquaculture 1991, 95, 1–6. [Google Scholar] [CrossRef]
- Draghi, A.; Popov, V.L.; Kahl, M.M.; Stanton, J.B.; Brown, C.C.; Tsongalis, G.J.; West, A.B.; Frasca, S. Characterization of “Candidatus Piscichlamydia salmonis” (Order Chlamydiales), a Chlamydia-like Bacterium Associated with Epitheliocystis in Farmed Atlantic Salmon (Salmo salar). J. Clin. Microbiol. 2004, 42, 5286–5297. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, S.O.; Rodger, H.D. A review of infectious gill disease in marine salmonid fish: Infectious gill disease in salmonids. J. Fish. Dis. 2011, 34, 411–432. [Google Scholar] [CrossRef]
- Corsaro, D.; Valassina, M.; Venditti, D. Increasing Diversity within Chlamydiae. Crit. Rev. Microbiol. 2003, 29, 37–78. [Google Scholar] [CrossRef] [PubMed]
- Everett, K.D.; Bush, R.M.; Andersen, A.A. Emended description of the order Chlamydiales, proposal of Parachlamydiaceae fam. nov. and Simkaniaceae fam. nov., each containing one monotypic genus, revised taxonomy of the family Chlamydiaceae, including a new genus and five new species, and standards for the identification of organisms. Int. J. Syst. Evol. Microbiol. 1999, 49, 415–440. [Google Scholar] [CrossRef]
- Corsaro, D.; Greub, G. Pathogenic potential of novel Chlamydiae and diagnostic approaches to infections due to these obligate intracellular bacteria. Clin. Microbiol. Rev. 2006, 19, 283–297. [Google Scholar] [CrossRef]
- Taylor-Brown, A.; Vaughan, L.; Greub, G.; Timms, P.; Polkinghorne, A. Twenty years of research into Chlamydia-like organisms: A revolution in our understanding of the biology and pathogenicity of members of the phylum Chlamydiae. FEMS Pathog. Dis. 2015, 73, 1–15. [Google Scholar] [CrossRef]
- Lienard, J.; Croxatto, A.; Prod’hom, G.; Greub, G. Estrella lausannensis, a new star in the Chlamydiales order. Microbes Infect. 2011, 13, 1232–1241. [Google Scholar] [CrossRef] [PubMed]
- Thomas, V.; Casson, N.; Greub, G. Criblamydia sequanensis, a new intracellular Chlamydiales isolated from Seine river water using amoebal co-culture. Environ. Microbiol. 2006, 8, 2125–2135. [Google Scholar] [CrossRef]
- Amann, R.; Springer, N.; Schönhuber, W.; Ludwig, W.; Schmid, E.N.; Müller, K.D.; Michel, R. Obligate intracellular bacterial parasites of acanthamoebae related to Chlamydia spp. Appl. Environ. Microbiol. 1997, 63, 115–121. [Google Scholar]
- Fritsche, T.R.; Horn, M.; Wagner, M.; Herwig, R.P.; Schleifer, K.-H.; Gautom, R.K. Phylogenetic diversity among geographically dispersed Chlamydiales endosymbionts recovered from clinical and environmental isolates of Acanthamoeba spp. Appl. Environ. Microbiol. 2000, 66, 2613–2619. [Google Scholar] [CrossRef]
- Corsaro, D.; Thomas, V.; Goy, G.; Venditti, D.; Radek, R.; Greub, G. ‘Candidatus Rhabdochlamydia crassificans’, an intracellular bacterial pathogen of the cockroach Blatta orientalis (Insecta: Blattodea). Syst. Appl. Microbiol. 2007, 30, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Croxatto, A.; Rieille, N.; Kernif, T.; Bitam, I.; Aeby, S.; Péter, O.; Greub, G. Presence of Chlamydiales DNA in ticks and fleas suggests that ticks are carriers of Chlamydiae. Ticks Tick-Borne Dis. 2014, 5, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Everett, K.D.; Thao, M.; Horn, M.; Dyszynski, G.E.; Baumann, P. Novel chlamydiae in whiteflies and scale insects: Endosymbionts ‘Candidatus Fritschea bemisiae’ strain Falk and ‘Candidatus Fritschea eriococci’ strain Elm. Int. J. Syst. Evol. Microbiol. 2005, 55, 1581–1587. [Google Scholar] [CrossRef]
- Kostanjšek, R.; Štrus, J.; Drobne, D.; Avguštin, G. ‘Candidatus Rhabdochlamydia porcellionis’, an intracellular bacterium from the hepatopancreas of the terrestrial isopod Porcellio scaber (Crustacea: Isopoda). Int. J. Syst. Evol. Microbiol. 2004, 54, 543–549. [Google Scholar] [CrossRef] [PubMed]
- Pilloux, L.; Aeby, S.; Gaümann, R.; Burri, C.; Beuret, C.; Greub, G. The high prevalence and diversity of Chlamydiales DNA within Ixodes ricinus ticks suggest a role for ticks as reservoirs and vectors of Chlamydia-related bacteria. Appl. Environ. Microbiol. 2015, 81, 8177–8182. [Google Scholar] [CrossRef]
- Blandford, M.I.; Taylor-Brown, A.; Schlacher, T.A.; Nowak, B.; Polkinghorne, A. Epitheliocystis in fish: An emerging aquaculture disease with a global impact. Transbound. Emerg. Dis. 2018, 65, 1436–1446. [Google Scholar] [CrossRef]
- Sood, N.; Pradhan, P.K.; Verma, D.K.; Gupta, S.; Ravindra; Dev, A.K.; Yadav, M.K.; Swaminathan, T.R.; Rathore, G. Epitheliocystis in rohu Labeo rohita (Hamilton, 1822) is caused by novel Chlamydiales. Aquaculture 2019, 505, 539–543. [Google Scholar] [CrossRef]
- Dinh-Hung, N.; Dong, H.T.; Soontara, C.; Rodkhum, C.; Nimitkul, S.; Srisapoome, P.; Kayansamruaj, P.; Chatchaiphan, S. Co-infection of Candidatus Piscichlamydia Trichopodus (Order Chlamydiales) and Henneguya sp. (Myxosporea, Myxobolidae) in Snakeskin Gourami Trichopodus pectoralis (Regan 1910). Front. Vet. Sci. 2022, 9, 847977. [Google Scholar] [CrossRef]
- Pillonel, T.; Bertelli, C.; Salamin, N.; Greub, G. Taxogenomics of the order Chlamydiales. Int. J. Syst. Evol. Microbiol. 2015, 65, 1381–1393. [Google Scholar] [CrossRef]
- Liu, F.; Feng, Y.; Geng, Y.; Chen, D.; Ou Yang, P.; Huang, X.; Guo, H.; Zuo, Z.; Deng, H.; Fang, J. Epitheliocystis caused by a novel Chlamydia emerging in Spinibarbus denticulatus in China. Dis. Aquat. Org. 2022, 150, 31–36. [Google Scholar] [CrossRef]
- Marquis, B.; Kebbi-Beghdadi, C.; Pillonel, T.; Ruetten, M.; Marques-Vidal, P.; Aeby, S.; Greub, G. Chlamydia vaughanii sp. nov., a novel Chlamydia isolated from a tropical fish (bushymouth catfish). Int. J. Syst. Evol. Microbiol. 2025, 75, 006753. [Google Scholar] [CrossRef]
- Borel, N.; Polkinghorne, A.; Pospischil, A. A Review on Chlamydial Diseases in Animals: Still a Challenge for Pathologists? Vet. Pathol. 2018, 55, 374–390. [Google Scholar] [CrossRef]
- Meijer, A.; Roholl, P.J.M.; Ossewaarde, J.M.; Jones, B.; Nowak, B.F. Molecular Evidence for Association of Chlamydiales Bacteria with Epitheliocystis in Leafy Seadragon (Phycodurus eques), Silver Perch (Bidyanus bidyanus), and Barramundi (Lates calcarifer). Appl. Environ. Microbiol. 2006, 72, 284–290. [Google Scholar] [CrossRef] [PubMed]
- Stride, M.C.; Polkinghorne, A.; Nowak, B.F. Correction: Chlamydial infections of fish: Diverse pathogens and emerging causes of disease in aquaculture species. Vet. Microbiol. 2014, 171, 257. [Google Scholar] [CrossRef] [PubMed]
- Steigen, A.; Nylund, A.; Karlsbakk, E.; Akoll, P.; Fiksdal, I.U.; Nylund, S.; Odong, R.; Plarre, H.; Semyalo, R.; Skår, C.; et al. ‘Cand. Actinochlamydia clariae’ gen. nov., sp. nov., a Unique Intracellular Bacterium Causing Epitheliocystis in Catfish (Clarias gariepinus) in Uganda. PLoS ONE 2013, 8, e66840. [Google Scholar] [CrossRef]
- Draghi, A.; Bebak, J.; Daniels, S.; Tulman, E.; Geary, S.; West, A.; Popov, V.; Frasca, S. Identification of ‘Candidatus Piscichlamydia salmonis’ in Arctic charr Salvelinus alpinus during a survey of charr production facilities in North America. Dis. Aquat. Org. 2010, 89, 39–49. [Google Scholar] [CrossRef]
- Karlsen, M.; Nylund, A.; Watanabe, K.; Helvik, J.V.; Nylund, S.; Plarre, H. Characterization of ‘Candidatus Clavochlamydia salmonicola’: An intracellular bacterium infecting salmonid fish. Environ. Microbiol. 2008, 10, 208–218. [Google Scholar] [CrossRef]
- Mitchell, S.O.; Steinum, T.; Rodger, H.; Holland, C.; Falk, K.; Colquhoun, D.J. Epitheliocystis in Atlantic salmon, Salmo salar L., farmed in fresh water in Ireland is associated with ‘Candidatus Clavochlamydia salmonicola’ infection. J. Fish. Dis. 2010, 33, 665–673. [Google Scholar] [CrossRef]
- Schmidt-Posthaus, H.; Polkinghorne, A.; Nufer, L.; Schifferli, A.; Zimmermann, D.R.; Segner, H.; Steiner, P.; Vaughan, L. A natural freshwater origin for two chlamydial species, Candidatus Piscichlamydia salmonis and Candidatus Clavochlamydia salmonicola, causing mixed infections in wild brown trout (Salmo trutta). Environ. Microbiol. 2012, 14, 2048–2057. [Google Scholar] [CrossRef]
- Nylund, S.; Steigen, A.; Karlsbakk, E.; Plarre, H.; Andersen, L.; Karlsen, M.; Watanabe, K.; Nylund, A. Characterization of ‘Candidatus Syngnamydia salmonis’ (Chlamydiales, Simkaniaceae), a bacterium associated with epitheliocystis in Atlantic salmon (Salmo salar L.). Arch. Microbiol. 2015, 197, 17–25. [Google Scholar] [CrossRef]
- Nylund, S.; Andersen, L.; Sævareid, I.; Plarre, H.; Watanabe, K.; Arnesen, C.; Karlsbakk, E.; Nylund, A. Diseases of farmed Atlantic salmon Salmo salar associated with infections by the microsporidian Paranucleospora theridion. Dis. Aquat. Org. 2011, 94, 41–57. [Google Scholar] [CrossRef]
- Steinum, T.; Kvellestad, A.; Colquhoun, D.; Heum, M.; Mohammad, S.; Grøntvedt, R.; Falk, K. Microbial and pathological findings in farmed Atlantic salmon Salmo salar with proliferative gill inflammation. Dis. Aquat. Org. 2010, 91, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Steigen, A.; Karlsbakk, E.; Plarre, H.; Watanabe, K.; Øvergård, A.-C.; Brevik, Ø.; Nylund, A. A new intracellular bacterium, Candidatus Similichlamydia labri sp. nov. (Chlamydiaceae) producing epitheliocysts in ballan wrasse, Labrus bergylta (Pisces, Labridae). Arch. Microbiol. 2015, 197, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Stride, M.C.; Polkinghorne, A.; Powell, M.D.; Nowak, B.F. “Candidatus Similichlamydia laticola”, a Novel Chlamydia-like Agent of epitheliocystis in Seven Consecutive Cohorts of Farmed Australian Barramundi, Lates calcarifer (Bloch). PLoS ONE 2013, 8, e82889. [Google Scholar] [CrossRef] [PubMed]
- Corsaro, D.; Work, T. Candidatus Renichlamydia lutjani, a Gram-negative bacterium in internal organs of blue-striped snapper Lutjanus kasmira from Hawaii. Dis. Aquat. Org. 2012, 98, 249–254. [Google Scholar] [CrossRef]
- Fehr, A.; Walther, E.; Schmidt-Posthaus, H.; Nufer, L.; Wilson, A.; Svercel, M.; Richter, D.; Segner, H.; Pospischil, A.; Vaughan, L. Candidatus Syngnamydia Venezia, a Novel Member of the Phylum Chlamydiae from the Broad Nosed Pipefish, Syngnathus typhle. PLoS ONE 2013, 8, e70853. [Google Scholar] [CrossRef]
- Guevara Soto, M.; Vaughan, L.; Segner, H.; Wahli, T.; Vidondo, B.; Schmidt-Posthaus, H. Epitheliocystis Distribution and Characterization in Brown Trout (Salmo trutta) from the Headwaters of Two Major European Rivers, the Rhine and Rhone. Front. Physiol. 2016, 7, 131. [Google Scholar] [CrossRef]
- Mitchell, S.; Steinum, T.; Toenshoff, E.; Kvellestad, A.; Falk, K.; Horn, M.; Colquhoun, D. ‘Candidatus Branchiomonas cysticola’ is a common agent of epitheliocysts in seawater-farmed Atlantic salmon Salmo salar in Norway and Ireland. Dis. Aquat. Org. 2013, 103, 35–43. [Google Scholar] [CrossRef]
- Sellyei, B.; Molnár, K.; Székely, C. Diverse Chlamydia-like agents associated with epitheliocystis infection in two cyprinid fish species, the common carp (Cyprinus carpio L.) and the gibel carp (Carassius auratus gibelio L.). Acta Vet. Hung. 2017, 65, 29–40. [Google Scholar] [CrossRef]
- Seth-Smith, H.M.B.; Katharios, P.; Dourala, N.; Mateos, J.M.; Fehr, A.G.J.; Nufer, L.; Ruetten, M.; Guevara Soto, M.; Vaughan, L. Ca. Similichlamydia in Epitheliocystis Co-infection of Gilthead Seabream Gills: Unique Morphological Features of a Deep Branching Chlamydial Family. Front. Microbiol. 2017, 8, 508. [Google Scholar] [CrossRef] [PubMed]
- Kumar, G.; Mayrhofer, R.; Soliman, H.; El-Matbouli, M. Novel Chlamydiales associated with epitheliocystis in grass carp (Ctenopharyngodon idella). Vet. Rec. 2013, 172, 47. [Google Scholar] [CrossRef]
- Polkinghorne, A.; Schmidt-Posthaus, H.; Meijer, A.; Lehner, A.; Vaughan, L. Novel Chlamydiales associated with epitheliocystis in a leopard shark Triakis semifasciata. Dis. Aquat. Org. 2010, 91, 75–81. [Google Scholar] [CrossRef]
- Taylor-Brown, A.; Pillonel, T.; Bridle, A.; Qi, W.; Bachmann, N.L.; Miller, T.L.; Greub, G.; Nowak, B.; Seth-Smith, H.M.B.; Vaughan, L.; et al. Culture-independent genomics of a novel chlamydial pathogen of fish provides new insight into host-specific adaptations utilized by these intracellular bacteria. Environ. Microbiol. 2017, 19, 1899–1913. [Google Scholar] [CrossRef]
- Camus, A.; Soto, E.; Berliner, A.; Clauss, T.; Sanchez, S. Epitheliocystis hyperinfection in captive spotted eagle rays Aetobatus narinari associated with a novel Chlamydiales 16S rDNA signature sequence. Dis. Aquat. Org. 2013, 104, 13–21. [Google Scholar] [CrossRef]
- Sood, N.; Pradhan, P.K.; Verma, D.K.; Yadav, M.K.; Ravindra; Dev, A.K.; Swaminathan, T.R.; Sood, N.K. Candidatus Actinochlamydia pangasiae sp. nov. (Chlamydiales, Actinochlamydiaceae), a bacterium associated with epitheliocystis in Pangasianodon hypophthalmus. J. Fish. Dis. 2018, 41, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Stride, M.C.; Polkinghorne, A.; Miller, T.L.; Nowak, B.F. Molecular Characterization of “Candidatus Similichlamydia latridicola” gen. nov., sp. nov. (Chlamydiales: “Candidatus Parilichlamydiaceae”), a Novel Chlamydia-Like Epitheliocystis Agent in the Striped Trumpeter, Latris lineata (Forster). Appl. Environ. Microbiol. 2013, 79, 4914–4920. [Google Scholar] [CrossRef] [PubMed]
- Stride, M.C.; Polkinghorne, A.; Miller, T.L.; Groff, J.M.; LaPatra, S.E.; Nowak, B.F. Molecular characterization of “Candidatus Parilichlamydia carangidicola,” a novel Chlamydia-like epitheliocystis agent in yellowtail kingfish, Seriola lalandi (Valenciennes), and the proposal of a new family “Candidatus Parilichlamydiaceae” fam. nov. (order Chlamydiales). Appl. Environ. Microbiol. 2013, 79, 1590–1597. [Google Scholar] [CrossRef]
- Downes, J.K.; Yatabe, T.; Marcos-Lopez, M.; Rodger, H.D.; MacCarthy, E.; O’Connor, I.; Collins, E.; Ruane, N.M. Investigation of co-infections with pathogens associated with gill disease in Atlantic salmon during an amoebic gill disease outbreak. J. Fish. Dis. 2018, 41, 1217–1227. [Google Scholar] [CrossRef]
- Nylund, A.; Watanabe, K.; Nylund, S.; Karlsen, M.; Saether, P.A.; Arnesen, C.E.; Karlsbakk, E. Morphogenesis of salmonid gill poxvirus associated with proliferative gill disease in farmed Atlantic salmon (Salmo salar) in Norway. Arch. Virol. 2008, 153, 1299–1309. [Google Scholar] [CrossRef]
- Glover, C.N.; Bucking, C.; Wood, C.M. The skin of fish as a transport epithelium: A review. J. Comp. Physiol. B 2013, 183, 877–891. [Google Scholar] [CrossRef]
- Salinas, I.; Fernández-Montero, Á.; Ding, Y.; Sunyer, J.O. Mucosal immunoglobulins of teleost fish: A decade of advances. Dev. Comp. Immunol. 2021, 121, 104079. [Google Scholar] [CrossRef]
- Slinger, J.; Adams, M.B.; Wynne, J.W. Bacteriomic Profiling of Branchial Lesions Induced by Neoparamoeba perurans Challenge Reveals Commensal Dysbiosis and an Association with Tenacibaculum dicentrarchi in AGD-Affected Atlantic Salmon (Salmo salar L.). Microorganisms 2020, 8, 1189. [Google Scholar] [CrossRef]
- Gomez, D.; Sunyer, J.O.; Salinas, I. The mucosal immune system of fish: The evolution of tolerating commensals while fighting pathogens. Fish Shellfish Immunol. 2013, 35, 1729–1739. [Google Scholar] [CrossRef]
- Xu, Z.; Takizawa, F.; Parra, D.; Gómez, D.; Von Gersdorff Jørgensen, L.; LaPatra, S.E.; Sunyer, J.O. Mucosal immunoglobulins at respiratory surfaces mark an ancient association that predates the emergence of tetrapods. Nat. Commun. 2016, 7, 10728. [Google Scholar] [CrossRef]
- Horn, M. Chlamydiae as Symbionts in Eukaryotes. Annu. Rev. Microbiol. 2008, 62, 113–131. [Google Scholar] [CrossRef] [PubMed]
- Guivier, E.; Pech, N.; Chappaz, R.; Gilles, A. Microbiota associated with the skin, gills, and gut of the fish Parachondrostoma toxostoma from the Rhône basin. Freshw. Biol. 2020, 65, 446–459. [Google Scholar] [CrossRef]
- Lorgen-Ritchie, M.; Chalmers, L.; Clarkson, M.; Taylor, J.F.; Migaud, H.; Martin, S.A.M. Time is a stronger predictor of microbiome community composition than tissue in external mucosal surfaces of Atlantic salmon (Salmo salar) reared in a semi-natural freshwater environment. Aquaculture 2023, 566, 739211. [Google Scholar] [CrossRef]
- Kebbi-Beghdadi, C.; Batista, C.; Greub, G. Permissivity of fish cell lines to three Chlamydia-related bacteria: Waddlia chondrophila, Estrella lausannensis and Parachlamydia acanthamoebae. FEMS Immunol. Med. Microbiol. 2011, 63, 339–345. [Google Scholar] [CrossRef]
- Lieschke, G.J.; Currie, P.D. Animal models of human disease: Zebrafish swim into view. Nat. Rev. Genet. 2007, 8, 353–367. [Google Scholar] [CrossRef]
- Howe, K.; Clark, M.D.; Torroja, C.F.; Torrance, J.; Berthelot, C.; Muffato, M.; Collins, J.E.; Humphray, S.; McLaren, K.; Matthews, L.; et al. The zebrafish reference genome sequence and its relationship to the human genome. Nature 2013, 496, 498–503. [Google Scholar] [CrossRef]
- Streisinger, G.; Walker, C.; Dower, N.; Knauber, D.; Singer, F. Production of clones of homozygous diploid zebra fish (Brachydanio rerio). Nature 1981, 291, 293–296. [Google Scholar] [CrossRef] [PubMed]
- Meeker, N.D.; Trede, N.S. Immunology and zebrafish: Spawning new models of human disease. Dev. Comp. Immunol. 2008, 32, 745–757. [Google Scholar] [CrossRef] [PubMed]
- Polymenakou, P.N.; Bertilsson, S.; Tselepides, A.; Stephanou, E.G. Bacterial Community Composition in Different Sediments from the Eastern Mediterranean Sea: A Comparison of Four 16S Ribosomal DNA Clone Libraries. Microb. Ecol. 2005, 50, 447–462. [Google Scholar] [CrossRef]
- Agustí, G.; Le Calvez, T.; Trouilhé, M.-C.; Humeau, P.; Codony, F. Presence of Waddlia chondrophila in hot water systems from non-domestic buildings in France. J. Water Health 2018, 16, 44–48. [Google Scholar] [CrossRef] [PubMed]
- Codony, F.; Fittipaldi, M.; López, E.; Morató, J.; Agustí, G. Well Water as a Possible Source of Waddlia chondrophila Infections. Microbes Environ. 2012, 27, 529–532. [Google Scholar] [CrossRef]
- Van Dooremalen, W.T.M.; Learbuch, K.L.G.; Morré, S.A.; Van Der Wielen, P.W.J.J.; Ammerdorffer, A. Limited presence of Waddlia chondrophila in drinking water systems in the Netherlands. New Microbes New Infect. 2020, 34, 100635. [Google Scholar] [CrossRef]
- Fehr, A.G.J.; Ruetten, M.; Seth-Smith, H.M.B.; Nufer, L.; Voegtlin, A.; Lehner, A.; Greub, G.; Crosier, P.S.; Neuhauss, S.C.F.; Vaughan, L. A Zebrafish Model for Chlamydia Infection with the Obligate Intracellular Pathogen Waddlia chondrophila. Front. Microbiol. 2016, 7, 1829. [Google Scholar] [CrossRef]
- Morin, M. Evaluating a Zebrafish Model to Determine The Etiology of Epitheliocystis; University of Massachusetts Amherst: Amherst, MA, USA, 2023. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahmoud-Elkamouny, B.; Kebbi-Beghdadi, C.; Greub, G. Aquatic Chlamydiae: A Review of Their Roles in Fish Health. Microorganisms 2025, 13, 2166. https://doi.org/10.3390/microorganisms13092166
Mahmoud-Elkamouny B, Kebbi-Beghdadi C, Greub G. Aquatic Chlamydiae: A Review of Their Roles in Fish Health. Microorganisms. 2025; 13(9):2166. https://doi.org/10.3390/microorganisms13092166
Chicago/Turabian StyleMahmoud-Elkamouny, Basma, Carole Kebbi-Beghdadi, and Gilbert Greub. 2025. "Aquatic Chlamydiae: A Review of Their Roles in Fish Health" Microorganisms 13, no. 9: 2166. https://doi.org/10.3390/microorganisms13092166
APA StyleMahmoud-Elkamouny, B., Kebbi-Beghdadi, C., & Greub, G. (2025). Aquatic Chlamydiae: A Review of Their Roles in Fish Health. Microorganisms, 13(9), 2166. https://doi.org/10.3390/microorganisms13092166







