Response of Rhizosphere Microenvironment of Mulberry (Morus alba L.) to Different Cultivars
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Soil Chemical Analysis
2.3. Soil Microbial Community Analysis
2.4. Data Analysis
3. Results
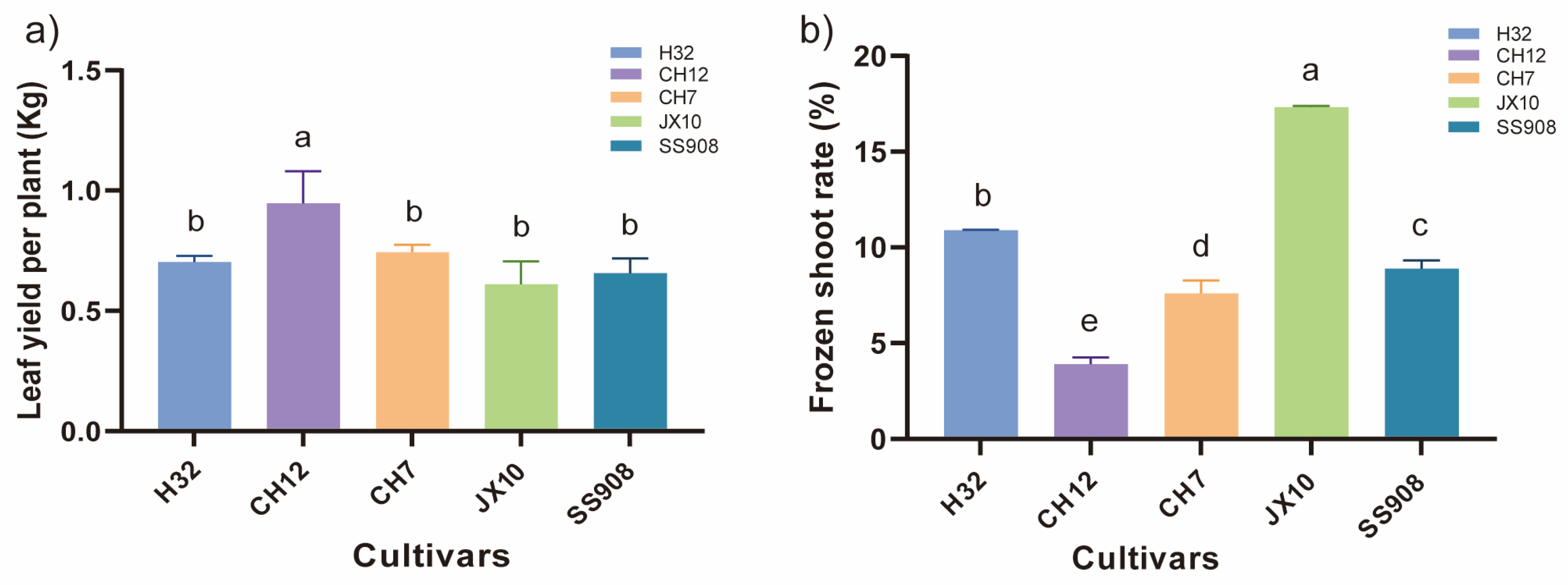
3.1. Agronomic Traits and Soil Chemical Properties
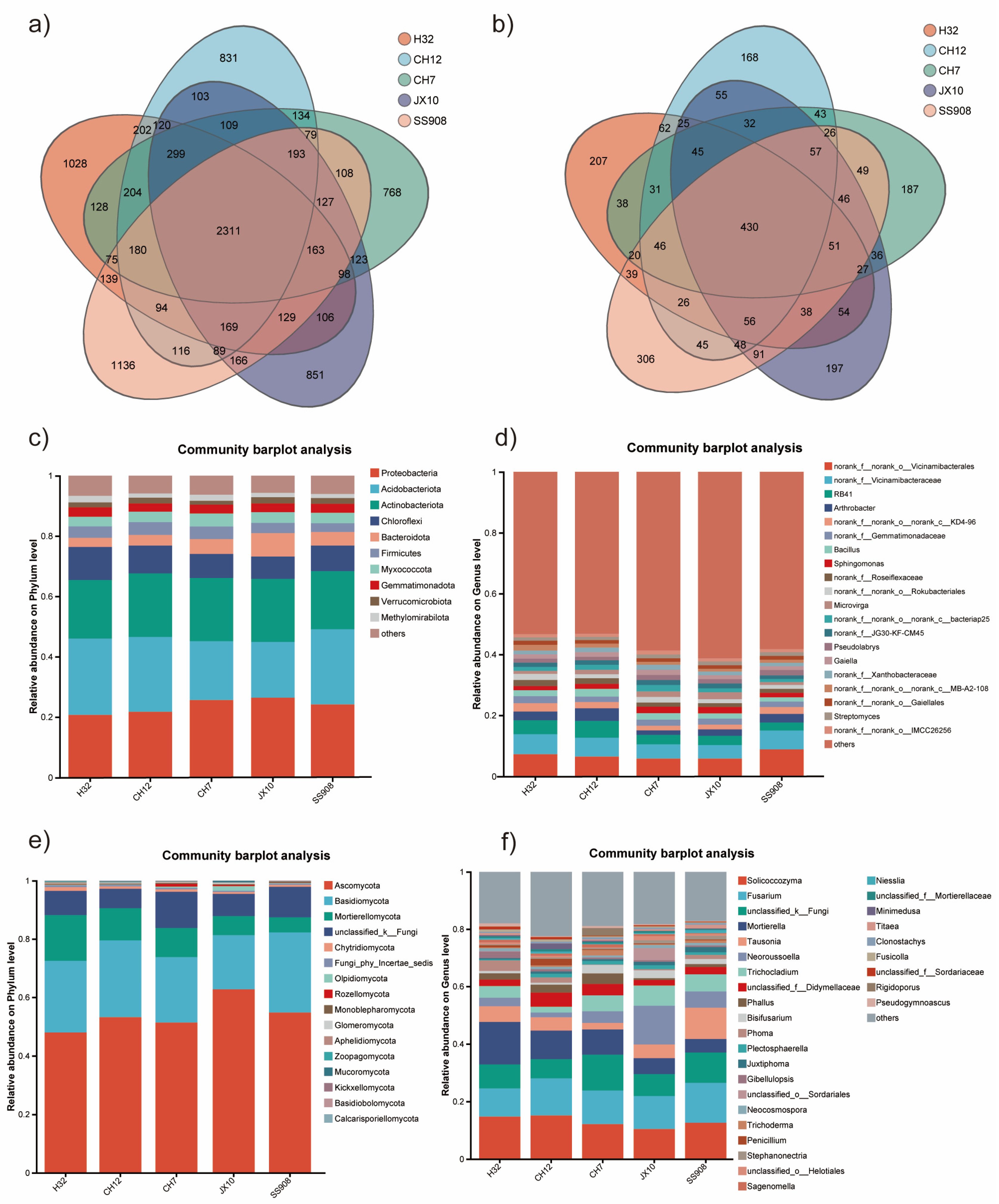
3.2. α-Diversity Index Analysis and Composition
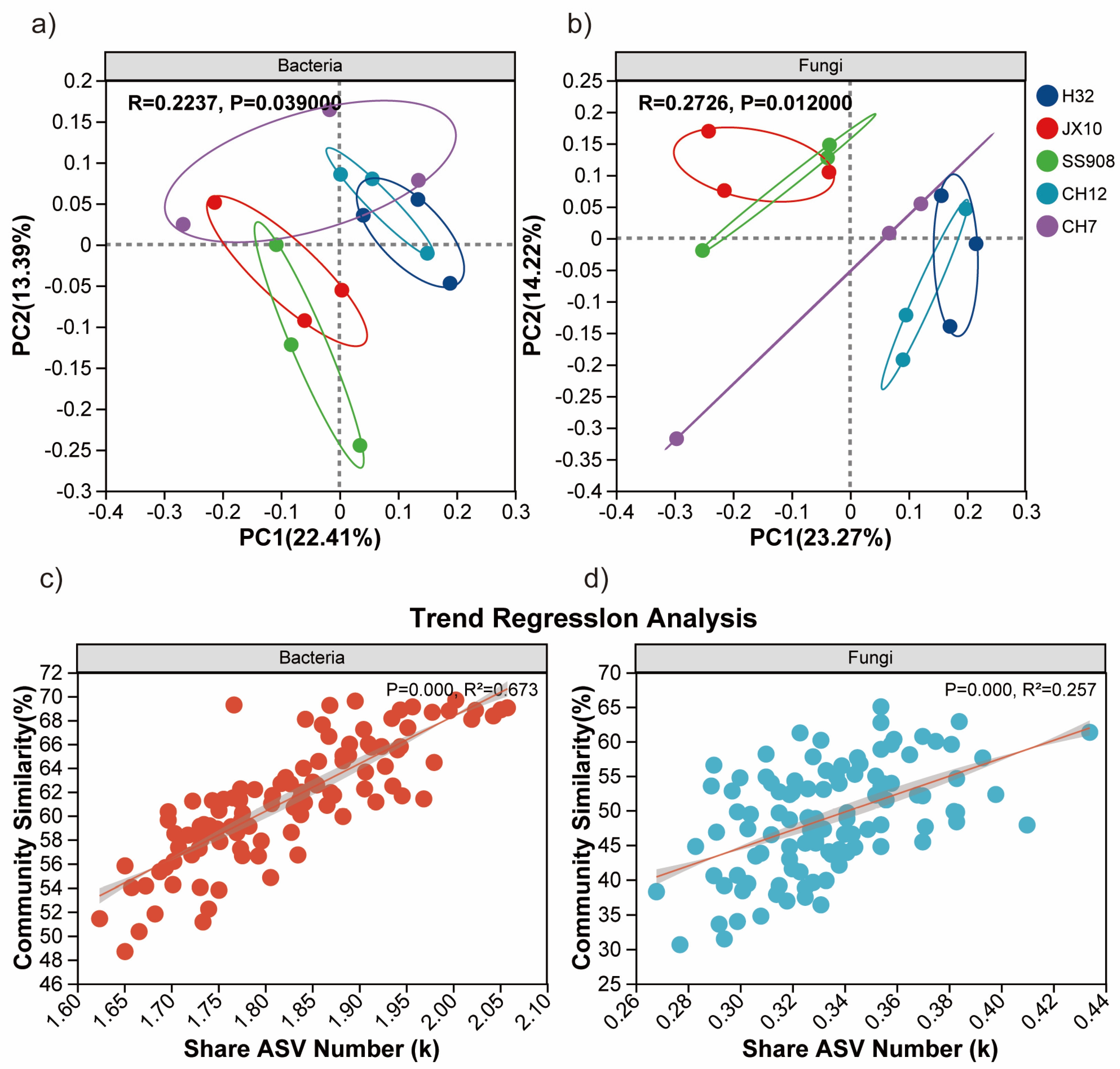
3.3. β-Diversity
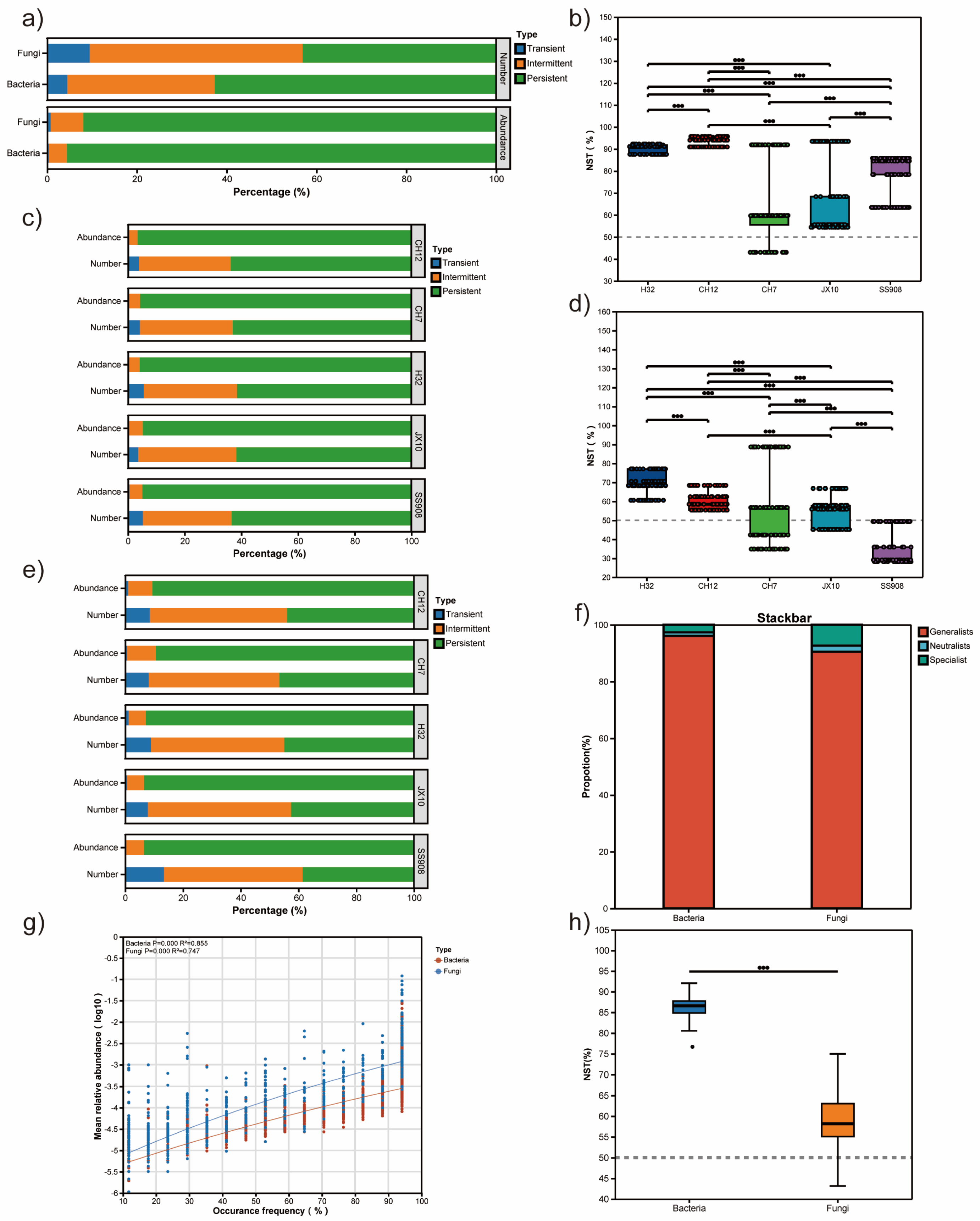
3.4. Mechanisms of Bacterial and Microbial Community Assembly
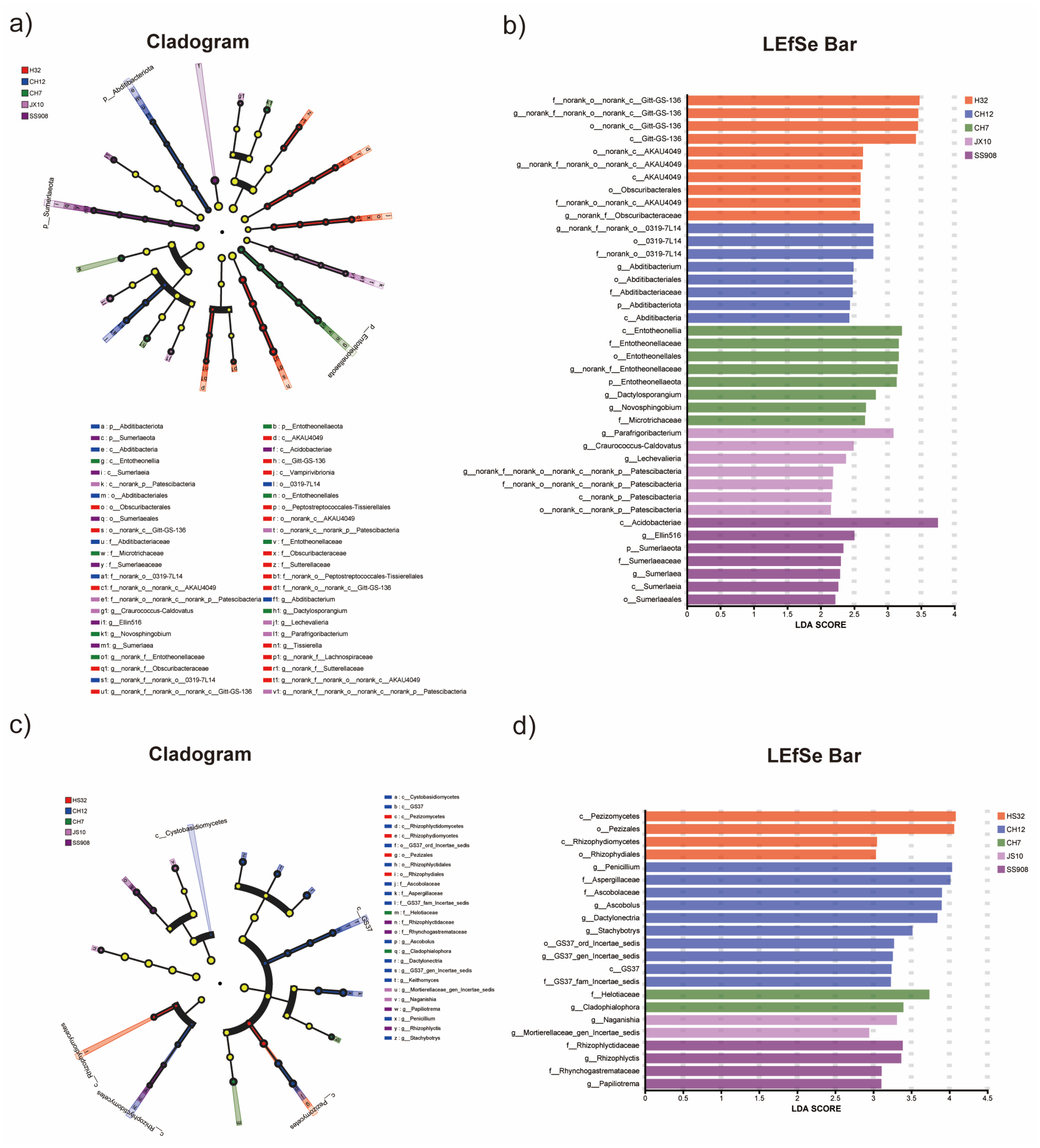
3.5. Difference Analysis
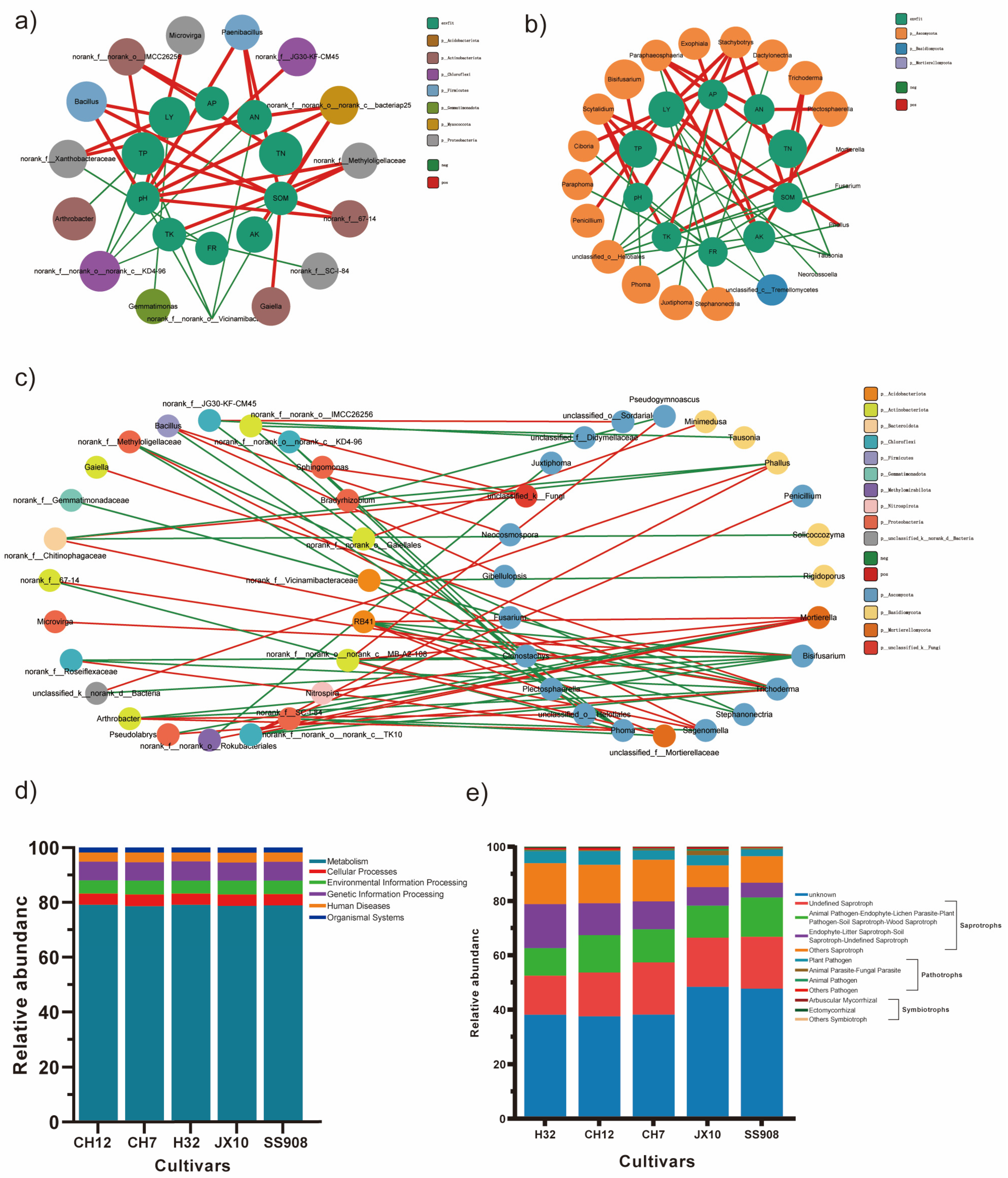
3.6. Relationship Between Environmental Parameters and Microbial Communities
3.7. Predicted Functional Consequences
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hussain, F.; Rana, Z.; Shafique, H.; Malik, A.; Hussain, Z. Phytopharmacological potential of different species of Morus alba and their bioactive phytochemicals: A review. Asian Pac. J. Trop. Biomed. 2017, 7, 950–956. [Google Scholar] [CrossRef]
- Lim, S.H.; Choi, C.I. Pharmacological Properties of Morus nigra L. (Black Mulberry) as A Promising Nutraceutical Resource. Nutrients 2019, 11, 437. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Q.X.; Zhao, L.Y. The Mulberry (Morus alba L.) Fruit—A Review of Characteristic Components and Health Benefits. J. Agric. Food Chem. 2017, 65, 10383–10394. [Google Scholar] [CrossRef] [PubMed]
- Venkatesh Kumar, R.; Chauhan, S. Mulberry: Life enhancer. J. Med. Plants Res. 2008, 2, 271–278. [Google Scholar]
- Kadam, R.A.; Dhumal, N.D.; Khyade, V.B. The mulberry, Morus alba (L.): The medicinal herbal source for human health. Int. J. Curr. Microbiol. App. Sci. 2019, 8, 2941–2964. [Google Scholar] [CrossRef]
- Qin, J.; He, N.; Wang, Y.; Xiang, Z.H. Ecological Issues of Mulberry and Sustainable Development. J. Resour. Ecol. 2012, 3, 330–339. [Google Scholar] [CrossRef]
- Jiang, Y.; Huang, R.; Yan, X.; Jia, C.; Jiang, S.; Long, T. Mulberry for environmental protection. Pak. J. Bot. 2017, 49, 781–788. [Google Scholar]
- Ghosh, A.; Gangopadhyay, D.; Chowdhury, T. Economical and environmental importance of mulberry: A review. Int. J. Plant Environ. 2017, 3, 51–58. [Google Scholar] [CrossRef]
- Jan, B.; Parveen, R.; Zahiruddin, S.; Khan, M.U.; Mohapatra SAhmad, S. Nutritional constituents of mulberry and their potential applications in food and pharmaceuticals: A review. Saudi J. Biol. Sci. 2021, 28, 3909–3921. [Google Scholar] [CrossRef]
- Sun, C.Z.; Wu, W.J.; Ma, Y.R.; Min, T.; Lai, F.R.; Wu, H. Physicochemical, functional properties, and antioxidant activities of protein fractions obtained from mulberry (Morus atropurpurea roxb.) leaf. Int. J. Food Prop. 2017, 20 (Suppl. S3), S3311–S3325. [Google Scholar] [CrossRef]
- Ding, N.; Lin, H.; Zhang, X.H.; He, Y.; Yu, G. Interaction mechanism between root secretion and rhizosphere microorganisms: A review. Chin. J. Soil Sci. 2022, 53, 1212–1219. [Google Scholar] [CrossRef]
- Thepbandit, W.; Athinuwat, D. Rhizosphere microorganisms supply availability of soil nutrients and induce plant defense. Microorganisms 2024, 12, 558. [Google Scholar] [CrossRef]
- Hakim, S.; Naqqash, T.; Nawaz, M.S.; Laraib, I.; Siddique, M.J.; Zia, R.; Mirza, M.S.; Imran, A. Rhizosphere engineering with plant growth-promoting microorganisms for agriculture and ecological sustainability. Front. Sustain. Food Syst. 2021, 5, 617157. [Google Scholar] [CrossRef]
- Beevi, N.D.; Qadri, S.M.H. Biological control of mulberry root rot disease (Fusarium spp.) with antagonistic microorganisms. J. Biopestic. 2010, 3, 90. [Google Scholar]
- Ou, T.; Zhang, M.; Gao, H.; Wang, F.; Xu, W.; Liu, X.; Wang, L.; Wang, R.; Xie, J. Study on the potential for stimulating mulberry growth and drought tolerance of plant growth-promoting fungi. Int. J. Mol. Sci. 2023, 24, 4090. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Guo, Y.; Yu, C.; Zhixian, Z.; Rongli, M.; Deng, W.; Li, Y.; Hu, X. The dynamics in rhizosphere microbial communities under bacterial wilt resistance by mulberry genotypes. Arch. Microbiol. 2021, 203, 1107–1121. [Google Scholar] [CrossRef]
- Yu, C.; Hu, X.; Deng, W.; Li, Y.; Han, G.; Ye, C. Soil fungal community comparison of different mulberry genotypes and the relationship with mulberry fruit sclerotiniosis. Sci. Rep. 2016, 6, 28365. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Tang, M.; Tan, Y.; Shi, J.; Zhang, Q.; Zhou, W. Pathogen susceptibility drives rhizosphere microbiota across mulberry genotypes. Rhizosphere 2023, 27, 100765. [Google Scholar] [CrossRef]
- Bin, Z.; Van der Putten, W.H.; Wang, G.; Zhang, J.; Veen, G.F. Effects of crop species on soil functions and soil multifunctionality are species-specific. Funct. Ecol. 2025, 39, 2354–2369. [Google Scholar] [CrossRef]
- Acharya, R.; Gangopadhyay, D.; Bhattacharyya, P.; Ghosh, A. Evaluation of salt-tolerant germplasm of mulberry (Morus L.) through in vitro and field experiments under different salinity stresses. Heliyon 2024, 10, e35868. [Google Scholar] [CrossRef]
- Peng, F.; Li, X.; Wei, Z.; Luo, Y.; Wang, W.; Han, G. Structure and Ecological Function of Fungal Endophytes from Stems of Different Mulberry Cultivars. Curr Microbiol. 2023, 80, 401. [Google Scholar] [CrossRef]
- Li, X.; Sun, M.; Zhang, H.; Xu, N.; Sun, G. Use of mulberry–soybean intercropping in salt–alkali soil impacts the diversity of the soil bacterial community. Microb. Biotechnol. 2016, 9, 293–304. [Google Scholar] [CrossRef]
- Li, M.; Wei, Y.; Yin, Y.; Ding, H.; Zhu, W.; Zhou, Y. The effect of intercropping mulberry (Morus alba L.) with peanut (Arachis hypogaea L.), on the soil rhizosphere microbial community. Forests 2022, 13, 1757. [Google Scholar] [CrossRef]
- Han, Y.; Dong, Q.; Zhang, K.; Sha, D.; Jiang, C.; Yang, X.; Liu, X.; Zhang, H.; Wang, X.; Guo, F.; et al. Maize-peanut rotational strip intercropping improves peanut growth and soil properties by optimizing microbial community diversity. PeerJ 2022, 10, e13777. [Google Scholar] [CrossRef] [PubMed]
- Dang, K.; Gong, X.; Zhao, G.; Wang, H.; Ivanistau, A.; Feng, B. Intercropping alters the soil microbial diversity and community to facilitate nitrogen assimilation: A potential mechanism for increasing proso millet grain yield. Front. Microbiol. 2020, 11, 601054. [Google Scholar] [CrossRef] [PubMed]
- Eichorst, S.A.; Kuske, C.R.; Schmidt, T.M. Influence of plant polymers on the distribution and cultivation of bacteria in the phylum Acidobacteria. Appl. Environ. Microbiol. 2011, 77, 586–596. [Google Scholar] [CrossRef]
- Zhou, J.; Jiang, X.; Zhou, B.; Zhao, B.; Ma, M.; Guan, D.; Li, J.; Chen, S.; Cao, F.; Shen, D.; et al. Thirty four years of nitrogen fertilization decreases fungal diversity and alters fungal community composition in black soil in northeast China. Soil Biol. Biochem. 2016, 95, 135–143. [Google Scholar] [CrossRef]
- Ma, R.; Wu, X.; Liu, Z.; Yi, Q.; Xu, M.; Zheng, J.; Bian, R.; Zhang, X.; Pan, G. Biochar improves soil organic carbon stability by shaping the microbial community structures at different soil depths four years after an incorporation in a farmland soil. Curr. Res. Environ. Sustain. 2023, 5, 100214. [Google Scholar] [CrossRef]
- Cao, Y.; Zhang, Z.; Ling, N.; Yuan, Y.; Zheng, X.; Shen, B.; Shen, Q. Bacillus subtilis SQR 9 can control Fusarium wilt in cucumber by colonizing plant roots. Biol. Fertil. Soils 2011, 47, 495–506. [Google Scholar] [CrossRef]
- Li, H.; Li, C.; Song, X.; Li, J.; Zhang, P.; Sun, F.; Geng, Z.; Liu, X. Isolation and identification of antagonistic Bacillus amyloliquefaciens HSE-12 and its effects on peanut growth and rhizosphere microbial community. Front. Microbiol. 2023, 14, 1274346. [Google Scholar] [CrossRef]
- Bigatton, E.D.; Verdenelli, R.A.; Haro, R.J.; Ayoub, I.; Barbero, F.M.; Martín, M.P.; Dubini, L.E.; Novo, J.V.J.; Lucini, E.I.; Castillejo, M.Á. Metagenomic Analysis to Assess the Impact of Plant Growth-Promoting Rhizobacteria on Peanut (Arachis hypogaea L.) Crop Production and Soil Enzymes and Microbial Diversity. J. Agric. Food Chem. 2024, 72, 22385–22397. [Google Scholar] [CrossRef]
- Xu, Y.; Ding, H.; Zhang, G.; Li, Z.; Guo, Q.; Feng, H.; Qin, F.; Dai, L.; Zhang, Z. Green manure increases peanut production by shaping the rhizosphere bacterial community and regulating soil metabolites under continuous peanut production systems. BMC Plant Biol. 2023, 23, 69. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhou, X.; Li, J.; Feng, J.; Huang, Z.; Chen, B.; Liu, W.; Yang, S. Can Functional Micro-organisms Associated with Pumpkin Sizes Be Sought Out from the Soil?—A Comparison of Soil Microbial Community Structures in Rhizospheres between Giant- and Small-Sized Pumpkin Varieties. Plants 2024, 13, 2258. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.; Li, Y.; Wang, Q.; Zhang, X.; Xiong, J.; Li, Y.; Lei, Y.; Sun, Y. Analysis of microbial diversity and community structure of rhizosphere soil of Cistanche salsa from different host plants. Front. Microbiol. 2022, 13, 971228. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Li, J.; Huang, Y.; Gao, S.; Zhang, J. Root-soil facilitation in mixed Eucalyptus grandis plantations including nitrogen-fixing species. For. Ecol. Manag. 2022, 516, 120215. [Google Scholar] [CrossRef]
- Obermeier, M.M.; Minarsch, E.M.L.; Durai Raj, A.C.; Rineau, F.; Schröder, P. Changes of soil-rhizosphere microbiota after organic amendment application in a Hordeum vulgare L. short-term greenhouse experiment. Plant Soil 2020, 455, 489–506. [Google Scholar] [CrossRef]
- Yang, M.; Yang, R.; Li, Y.; Pan, Y.; Zhang, Z. Effects of different biomass materials as a salt-isolation layer on water and salt migration in coastal saline soil. PeerJ 2021, 9, e11766. [Google Scholar] [CrossRef]
- Manrique-de-la-Cuba, M.F.; Parada-Pozo, G.; Rodríguez-Marconi, S.; López-Rodríguez, M.R.; Abades, S.; Trefault, N. Evidence of habitat specificity in sponge microbiomes from Antarctica. Environ. Microbiome 2024, 19, 100. [Google Scholar] [CrossRef]
- Kong, L.; Zhang, L.; Wang, Y.; Huang, Z. Impact of Ecological Restoration on the Physicochemical Properties and Bacterial Communities in Alpine Mining Area Soils. Microorganisms 2024, 12, 41. [Google Scholar] [CrossRef]
- Zhang, K.; Bonito, G.; Hsu, C.-M.; Hameed, K.; Vilgalys, R.; Liao, H.-L. Mortierella elongata increases plant biomass among non-leguminous crop species. Agronomy 2020, 10, 754. [Google Scholar] [CrossRef]
- Yu, S.; Wang, T.; Meng, Y.; Yao, S.; Wang, L.; Zheng, H.; Zhou, Y.; Song, Z.; Zhang, B. Leguminous cover crops and soya increased soil fungal diversity and suppressed pathotrophs caused by continuous cereal cropping. Front. Microbiol. 2022, 13, 993214. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Zhang, S.; Wang, Y.; Li, Y.; Li, P.; Chen, L.; Jie, X.; Hu, D.; Feng, B.; Yue, K.; et al. Rare fungus, Mortierella capitata, promotes crop growth by stimulating primary metabolisms related genes and reshaping rhizosphere bacterial community. Soil Biol. Biochem. 2020, 151, 108017. [Google Scholar] [CrossRef]
- Osorio, N.W.; Habte, M. Soil phosphate desorption induced by a phosphate-solubilizing fungus. Commun. Soil Sci. Plant Anal. 2014, 45, 451–460. [Google Scholar] [CrossRef]
- Wang, W.; Li, Y.; Wang, H.; Zu, Y. Differences in the activities of eight enzymes from ten soil fungi and their possible influences on the surface structure, functional groups, and element composition of soil colloids. PLoS ONE 2014, 9, e111740. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, J.; Yuan, Q.; Guo, L.; Zheng, G.; Xiao, C.; Yang, C.; Jiang, W.; Zhou, T. Composition and diversity of soil microbial communities change by introducing Phallus impudicus into a Gastrodia elata Bl.-based soil. BMC Microbiol. 2024, 24, 204. [Google Scholar] [CrossRef]
- Hong, S.K.; Kim, W.G.; Sung, G.B.; Nam, S.H. Identification and distribution of two fungal species causing sclerotial disease on mulberry fruits in Korea. Mycobiology 2007, 35, 87–90. [Google Scholar] [CrossRef][Green Version]
- Becarelli, S.; Chicca, I.; La China, S.; Siracusa, G.; Bardi, A.; Gullo, M.; Petroni, G.; Levin, D.B.; Di Gregorio, S. A New Ciboria sp. for Soil Mycoremediation and the Bacterial Contribution to the Depletion of Total Petroleum Hydrocarbons. Front. Microbiol. 2021, 12, 647373. [Google Scholar] [CrossRef]
- Wang, Y.; Gong, H.; Zhang, Z.; Sun, Z.; Liu, S.; Ma, C.; Wang, X.; Liu, Z. Effects of microbial communities during the cultivation of three salt-tolerant plants in saline-alkali land improvement. Front. Microbiol. 2024, 15, 1470081. [Google Scholar] [CrossRef]
- Qin, J.; Bian, C.; Duan, S.; Wang, W.; Li, G.; Jin, L. Effects of different rotation cropping systems on potato yield, rhizosphere microbial community and soil biochemical properties. Front. Plant Sci. 2022, 13, 999730. [Google Scholar] [CrossRef]
- Zhu, W.; Han, S.; Cheng, Y.; Yu, Z.; Zhao, G.; He, X. Root zone microbial communities of Artemisia ordosica Krasch. at different successional stages in Mu US Sandy Land: A metagenomic perspective with culturomics insights. Front. Microbiol. 2025, 16, 1585700. [Google Scholar] [CrossRef]
- Hermosa, R.; Viterbo, A.; Chet, I.; Monte, E. Plant-beneficial effects of Trichoderma and of its genes. Microbiology 2012, 158, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.R.; Mohiddin, F.A. Trichoderma: Its multifarious utility in crop improvement. In Crop Improvement through Microbial Biotechnology; Prasad, R., Gill, S.S., Tuteja, N., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; Chapter 13; pp. 263–291. [Google Scholar] [CrossRef]
- Wei, T.-J.; Li, G.; Cui, Y.-R.; Xie, J.; Liang, Z.-W.; Guan, F.-C.; Li, Z.-H. Response of Alfalfa Leaf Traits and Rhizosphere Fungal Communities to Compost Application in Saline-Sodic Soil. Microorganisms 2024, 12, 2287. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xiong, X.; Li, P.; Wan, H.F.; Yang, Y.H. Characteristics and Influencing Factors of Rhizosphere Microbial Communities of Tuber himalayense-Corylus heterophylla Ectomycorrhizosphere. Pol. J. Microbiol. 2025, 74, 177–191. [Google Scholar] [CrossRef] [PubMed]
- Hao, X.L.; Zhu, Y.G.; Nybroe, O.; Nicolaisen, M.H. The composition and phosphorus cycling potential of bacterial communities associated with hyphae of penicillium in soil are strongly affected by soil origin. Front. Microbiol. 2020, 10, 2951. [Google Scholar] [CrossRef]
- Shi, M.; Hao, S.; Wang, Y.; Zhang, S.; Cui, G.; Zhang, B.; Zhou, W.; Chen, H.; Wang, M. Plant growth-promoting fungi improve tobacco yield and chemical components by reassembling rhizosphere fungal microbiome and recruiting probiotic taxa. Environ. Microbiome 2024, 19, 83. [Google Scholar] [CrossRef]
- Zhang, Y.; Hao, X.; Garcia-Lemos, A.M.; Nunes, I.; Nicolaisen, M.H.; Nybroe, O. Different effects of soil fertilization on bacterial community composition in the Penicillium canescens hyphosphere and in bulk soil. Appl. Environ. Microbiol. 2020, 86, e02969-19. [Google Scholar] [CrossRef]
- Nunes, I.; Hansen, V.; Bak, F.; Bonnichsen, L.; Su, J.; Hao, X.; Raymond, N.S.; Nicolaisen, M.H.; Jensen, L.S.; Nybroe, O. Succession of the wheat seed-associated microbiome as affected by soil fertility level and introduction of Penicillium and Bacillus inoculants in the field. FEMS Microbiol. Ecol. 2022, 98, fiac028. [Google Scholar] [CrossRef]
- Tkacz, A.; Bestion, E.; Bo, Z.Y.; Hortala, M.; Poole, P.S. Influence of Plant Fraction, Soil, and Plant Species on Microbiota: A Multikingdom Comparison. mBio 2020, 11, 10–128. [Google Scholar] [CrossRef]
- Ren, X.; Lin, M.; Liu, J.; Khan, W.; Zhao, H.; Sun, B.; Liu, S.; Zheng, P. Effects of Altitude on Tea Composition: Dual Regulation by Soil Physicochemical Properties and Microbial Communities. Plants 2025, 14, 1642. [Google Scholar] [CrossRef]
- Ali, A.; Elrys, A.S.; Liu, L.; Iqbal, M.; Zhao, J.; Huang, X.; Cai, Z. Cover Plants-Mediated Suppression of Fusarium Wilt and Root-Knot Incidence of Cucumber is Associated with the Changes of Rhizosphere Fungal Microbiome Structure-Under Plastic Shed System of North China. Front. Microbiol. 2022, 13, 697815. [Google Scholar] [CrossRef]
- Duan, Y.; Lian, J.; Wang, L.; Wang, X.; Luo, Y.; Wang, W.; Wu, F.; Zhao, J.; Ding, Y.; Ma, J.; et al. Variation in Soil Microbial Communities Along an Elevational Gradient in Alpine Meadows of the Qilian Mountains, China. Front. Microbiol. 2021, 12, 684386. [Google Scholar] [CrossRef]
- Zhu, P.; Yang, S.; Wu, Y.; Ru, Y.; Yu, X.; Wang, L.; Guo, W. Shifts in Soil Microbial Community Composition, Function, and Co-occurrence Network of Phragmites australis in the Yellow River Delta. Front. Microbiol. 2022, 13, 858125. [Google Scholar] [CrossRef]
- Banerjee, S.; Schlaeppi, K.; van der Heijden, M.G.A. Keystone taxa as drivers of microbiome structure and functioning. Nat. Rev. Microbiol. 2018, 16, 567–576. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Hewezi, T.; Lebeis, S.L.; Pantalone, V.; Grewal, P.S.; Staton, M.E. Soil indigenous microbiome and plant genotypes cooperatively modify soybean rhizosphere microbiome assembly. BMC Microbiol. 2019, 19, 201. [Google Scholar] [CrossRef]
- Jiao, S.; Lu, Y.H. Soil pH and temperature regulate assembly processes of abundant and rare bacterial communities in agricultural ecosystems. Environ. Microbiol. 2020, 22, 1052–1065. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, Q.; Li, W.; Zhang, D.; Fang, W.; Li, Y.; Wang, Q.; Cao, A.; Yan, D. Long-term effects of chloropicrin fumigation on soil microbe recovery and growth promotion of Panax notoginseng. Front. Microbiol. 2023, 14, 1225944. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Xiong, C.; Gao, C.; Tsui, C.K.M.; Wang, M.-M.; Zhou, X.; Zhang, A.-M.; Cai, L. Disease-induced changes in plant microbiome assembly and functional adaptation. Microbiome 2021, 9, 187. [Google Scholar] [CrossRef]
- De Vries, F.T.; Griffiths, R.I.; Bailey, M.; Craig, H.; Girlanda, M.; Gweon, H.S.; Hallin, S.; Kaisermann, A.; Keith, A.M.; Kretzschmar, M.; et al. Soil bacterial networks are less stable under drought than fungal networks. Nat. Commun. 2018, 9, 3033. [Google Scholar] [CrossRef]
- Falkowski, P.G.; Fenchel, T.; Delong, E.F. The microbial engines that drive Earth’s biogeochemical cycles. Science 2008, 320, 1034–1039. [Google Scholar] [CrossRef]
- Jia, X.; Lin, S.; Wang, Y.; Zhang, Q.; Jia, M.; Li, M.; Chen, Y.; Cheng, P.; Hong, L.; Zhang, Y.; et al. Recruitment and Aggregation Capacity of Tea Trees to Rhizosphere Soil Characteristic Bacteria Affects the Quality of Tea Leaves. Plants 2024, 13, 1686. [Google Scholar] [CrossRef]
- Schmidt, R.; Mitchell, J.; Scow, K. Cover cropping and no-till increase diversity and symbiotroph: Saprotroph ratios of soil fungal communities. Soil Biol. Biochem. 2019, 129, 99–109. [Google Scholar] [CrossRef]
- Nguyen, N.H.; Song, Z.; Bates, S.T.; Branco, S.; Tedersoo, L.; Menke, J.; Schilling, J.S.; Kennedy, P.G. FUNGuild: An open annotation tool for parsing fungal com-munity datasets by ecological guild. Fungal Ecol. 2016, 20, 241–248. [Google Scholar] [CrossRef]


| H32 | CH12 | CH7 | JX10 | SS908 | |
|---|---|---|---|---|---|
| TN (mg/kg) | 883.4 ± 31.2 b | 896.5 ± 17.8 b | 1375.5 ± 57.4 a | 819.8 ± 60.5 b | 887.2 ± 53.5 b |
| TP (mg/kg) | 859.8 ± 46.4 b | 812.5 ± 53.5 b | 1057.3 ± 10.2 a | 883.6 ± 4.3 b | 834.4 ± 80.3 b |
| TK (g/kg) | 22.5 ± 1 a | 20.7 ± 0.4 b | 22.6 ± 0.6 a | 20.7 ± 0.4 b | 20.3 ± 0.5 b |
| SOM (g/kg) | 17.2 ± 2.2 b | 18.3 ± 1.6 b | 24.9 ± 1.1 a | 17.7 ± 1 b | 16.2 ± 1.6 b |
| AN (mg/kg) | 110.8 ± 11.1 c | 149.8 ± 6 b | 167.2 ± 9.4 a | 123.1 ± 3 c | 117.2 ± 5.7 c |
| AP (mg/kg) | 60.5 ± 5.7 ab | 54.3 ± 3 ab | 62.6 ± 5.2 a | 52.8 ± 2.3 b | 57.7 ± 5.2 ab |
| AK (mg/kg) | 210.2 ± 2.6 c | 323.6 ± 19.1 ab | 376.9 ± 48.8 a | 212.7 ± 25.1 c | 276.9 ± 49.2 bc |
| pH | 6.9 ± 0.1 b | 6.9 ± 0.1 b | 7 ± 0.1 a | 6.9 ± 0 b | 6.7 ± 0.1 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, C.; Zhang, H.; Liang, X.; Li, M.; Gu, Y. Response of Rhizosphere Microenvironment of Mulberry (Morus alba L.) to Different Cultivars. Microorganisms 2025, 13, 2157. https://doi.org/10.3390/microorganisms13092157
Chen C, Zhang H, Liang X, Li M, Gu Y. Response of Rhizosphere Microenvironment of Mulberry (Morus alba L.) to Different Cultivars. Microorganisms. 2025; 13(9):2157. https://doi.org/10.3390/microorganisms13092157
Chicago/Turabian StyleChen, Chuanjie, Haiyang Zhang, Xiaoyan Liang, Meng Li, and Yinyu Gu. 2025. "Response of Rhizosphere Microenvironment of Mulberry (Morus alba L.) to Different Cultivars" Microorganisms 13, no. 9: 2157. https://doi.org/10.3390/microorganisms13092157
APA StyleChen, C., Zhang, H., Liang, X., Li, M., & Gu, Y. (2025). Response of Rhizosphere Microenvironment of Mulberry (Morus alba L.) to Different Cultivars. Microorganisms, 13(9), 2157. https://doi.org/10.3390/microorganisms13092157






