Mechanistic Insights into Eimeria tenella-Induced Host Cell Apoptosis Through Modulation of the Mitochondrial Permeability Transition Pore
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Animals
2.2. Parasites
2.3. Primary Culture of Chick Embryo Caecal Cells
2.4. Preparation of Sporozoites of E. tenella
2.5. Experimental Protocol
2.6. H&E Staining and Quantification of E. tenella Infection Rates
2.7. Dynamic Assessment of MPTP Opening in E. tenella-Infected Cells
2.8. Mitochondrial Isolation and Dynamic Detection of Apoptotic Factor Levels in E. tenella-Infected Host Cells
2.9. Statistical Analysis
3. Results
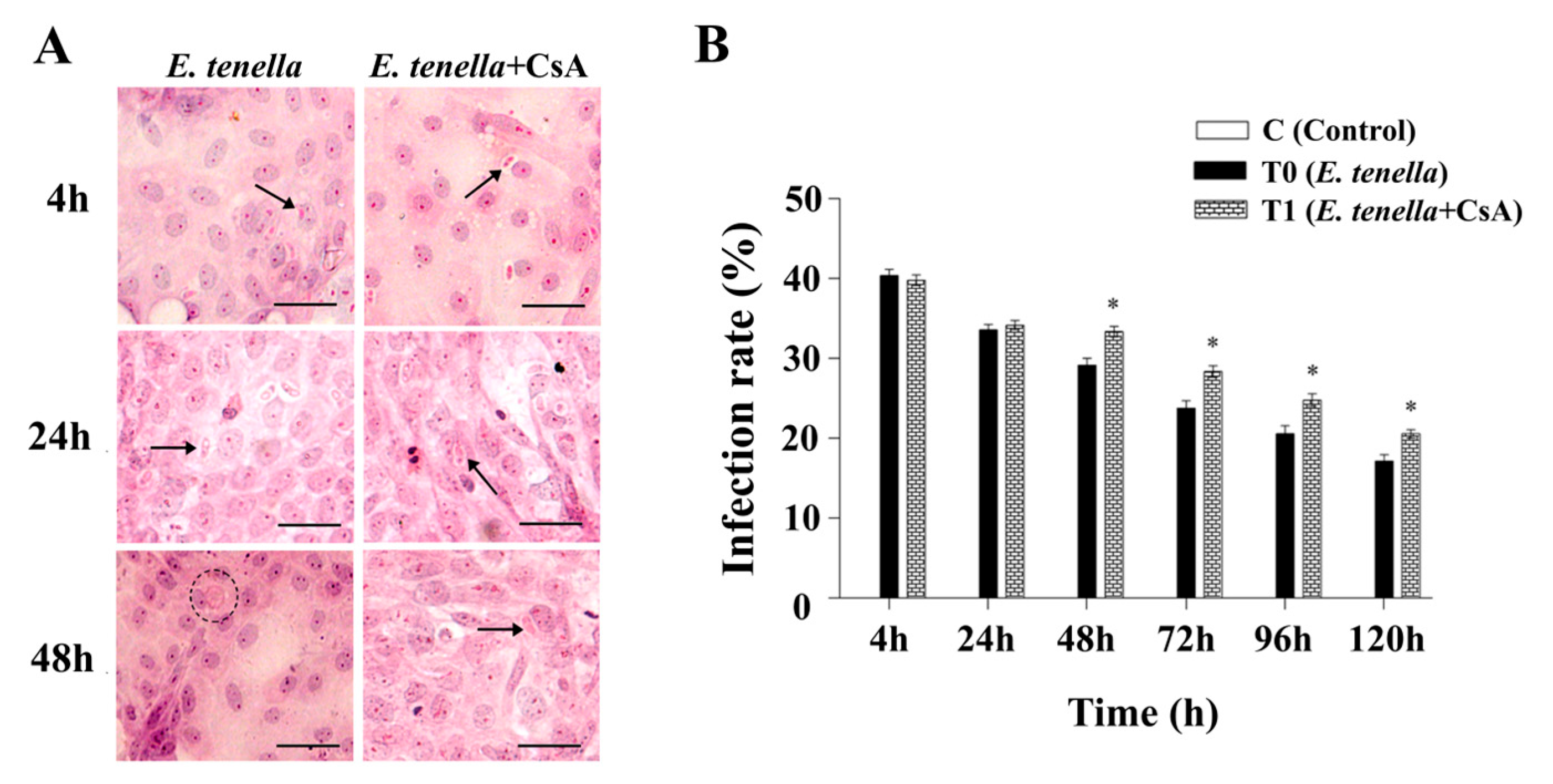
3.1. Inhibition of MPTP Selectively Enhances Intracellular Expansion of E. tenella
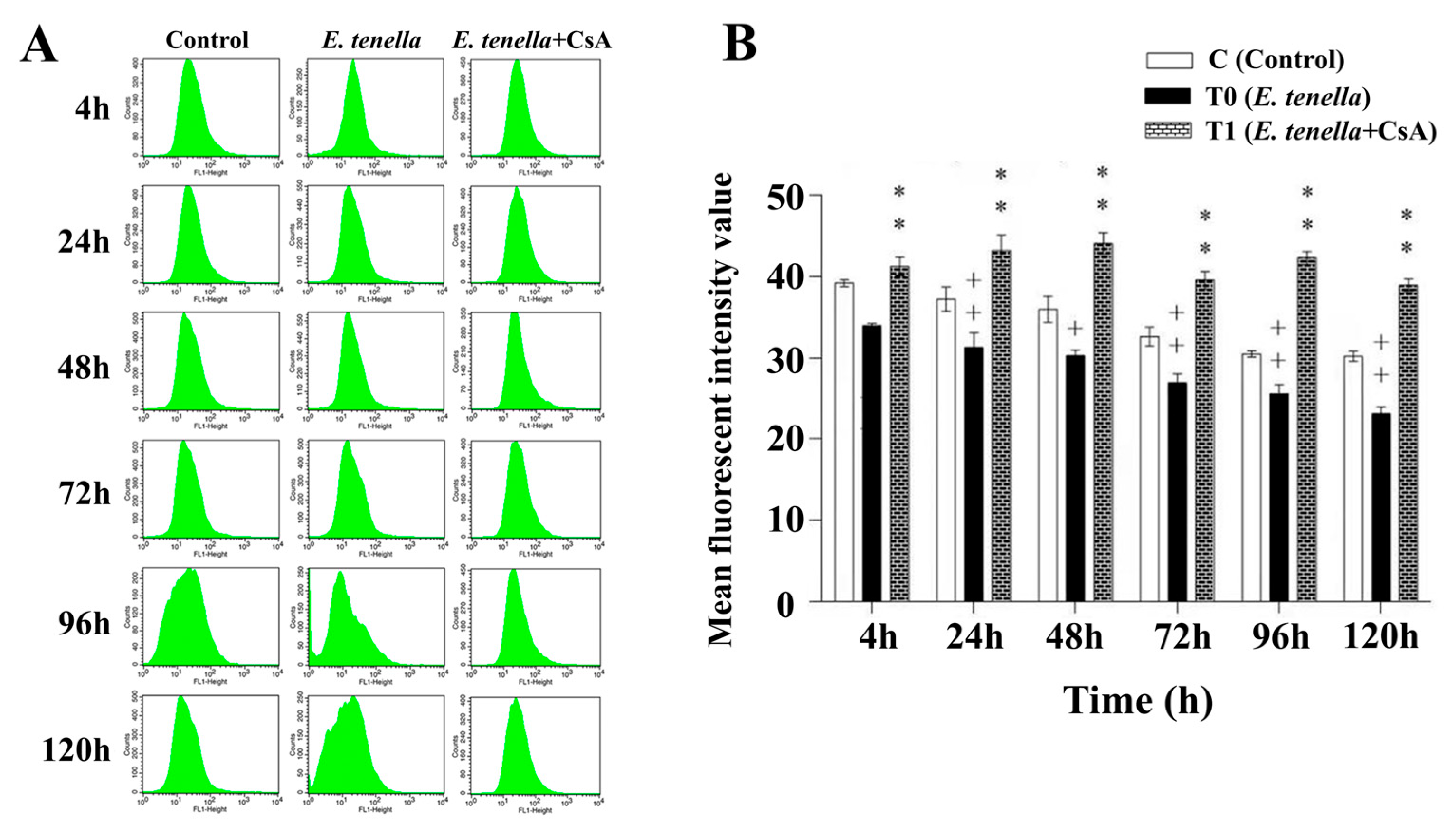
3.2. CsA Preserves Mitochondrial Integrity by Inhibiting E. tenella-Induced MPTP Opening
3.3. Temporal MPTP-Driven Smac Redistribution During E. tenella Infection
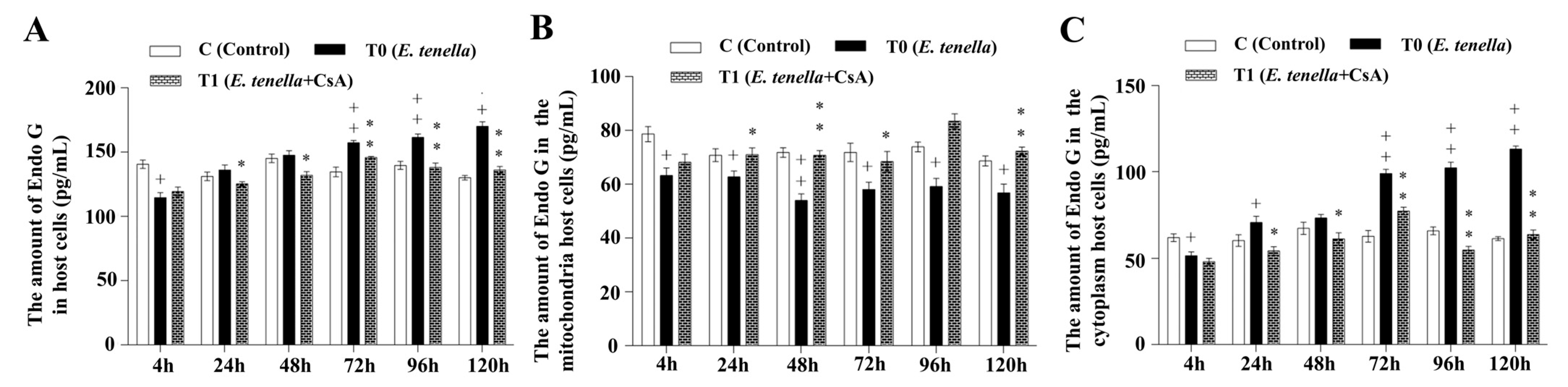
3.4. Temporal MPTP-Driven Endo G Redistribution During E. tenella Infection
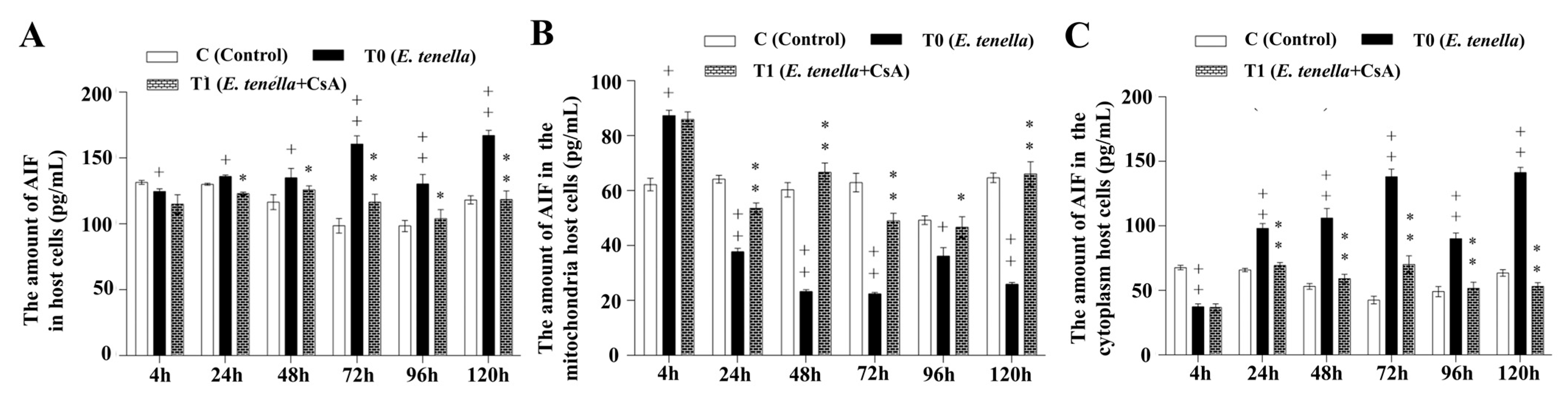
3.5. Temporal MPTP-Driven AIF Redistribution During E. tenella Infection
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xu, L.; Xiang, Q.; Li, M.; Sun, X.; Lu, M.; Yan, R.; Song, X.; Li, X. Pathogenic Effects of Single or Mixed Infections of Eimeria mitis, Eimeria necatrix, and Eimeria tenella in Chickens. Vet. Sci. 2022, 9, 657. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, S.; Abbas, R.Z.; Saeed, Z.; Baazaoui, N.; Khan, A.M.A. Use of Metallic Nanoparticles Against Eimeria-the Coccidiosis-Causing Agents: A Comprehensive Review. Biol. Trace Elem. Res. 2025, 203, 3412–3431. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Han, X.; Meng, J.; Yang, J.; Kang, S.; Lv, X.; Cui, X.; Li, J.; Liu, W.; Bai, R. Silymarin Effectively Prevents and Treats Eimeria tenella Infection in Chicks. Poult. Sci. 2024, 103, 103909. [Google Scholar] [CrossRef]
- Zhang, X.-S.; Zhao, Y.-J.; Zhang, Y.; Xu, T.; Cui, K.-L.; Duan, B.-T.; Lv, X.-L.; Zhang, L.; Xu, Z.-Y.; Bai, R.; et al. Role of EtMIC4 EGF-like in Regulating the Apoptosis of Eimeria tenella Host Cells via the EGFR Pathway. Poult. Sci. 2022, 101, 102075. [Google Scholar] [CrossRef]
- Xu, Z.-Y.; Zheng, M.-X.; Zhang, Y.; Cui, X.-Z.; Yang, S.-S.; Liu, R.-L.; Li, S.; Xi, R.; Gong, X.; Bai, R. Dynamic Changes in the Main Regulatory Genes of Mitochondrial Permeability Transition Pore in Eimeria tenella Host Cells. Exp. Parasitol. 2016, 171, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Sunanda, T.; Ray, B.; Mahalakshmi, A.M.; Bhat, A.; Rashan, L.; Rungratanawanich, W.; Song, B.-J.; Essa, M.M.; Sakharkar, M.K.; Chidambaram, S.B. Mitochondria-Endoplasmic Reticulum Crosstalk in Parkinson’s Disease: The Role of Brain Renin Angiotensin System Components. Biomolecules 2021, 11, 1669. [Google Scholar] [CrossRef]
- Zong, Y.; Li, H.; Liao, P.; Chen, L.; Pan, Y.; Zheng, Y.; Zhang, C.; Liu, D.; Zheng, M.; Gao, J. Mitochondrial Dysfunction: Mechanisms and Advances in Therapy. Signal Transduct. Target. Ther. 2024, 9, 124. [Google Scholar] [CrossRef]
- Morciano, G.; Naumova, N.; Koprowski, P.; Valente, S.; Sardão, V.A.; Potes, Y.; Rimessi, A.; Wieckowski, M.R.; Oliveira, P.J. The Mitochondrial Permeability Transition Pore: An Evolving Concept Critical for Cell Life and Death. Biol. Rev. Camb. Philos. Soc. 2021, 96, 2489–2521. [Google Scholar] [CrossRef]
- Song, X.; Zhang, L.; Hui, X.; Sun, X.; Yang, J.; Wang, J.; Wu, H.; Wang, X.; Zheng, Z.; Che, F.; et al. Selenium-Containing Protein from Selenium-Enriched Spirulina Platensis Antagonizes Oxygen Glucose Deprivation-Induced Neurotoxicity by Inhibiting ROS-Mediated Oxidative Damage through Regulating MPTP Opening. Pharm. Biol. 2021, 59, 629–638. [Google Scholar] [CrossRef]
- Yao, H.; Xie, Q.; He, Q.; Zeng, L.; Long, J.; Gong, Y.; Li, X.; Li, X.; Liu, W.; Xu, Z.; et al. Pretreatment with Panaxatriol Saponin Attenuates Mitochondrial Apoptosis and Oxidative Stress to Facilitate Treatment of Myocardial Ischemia-Reperfusion Injury via the Regulation of Keap1/Nrf2 Activity. Oxid. Med. Cell. Longev. 2022, 2022, 9626703. [Google Scholar] [CrossRef]
- Li, J.-Y.; Huang, H.-B.; Pan, T.-X.; Wang, N.; Shi, C.-W.; Zhang, B.; Wang, C.-F.; Yang, G.-L. Sanguinarine Induces Apoptosis in Eimeria tenella Sporozoites via the Generation of Reactive Oxygen Species. Poult. Sci. 2022, 101, 101771. [Google Scholar] [CrossRef]
- Bai, R.; Wang, H.; Yang, T.; Yan, Y.; Zhu, S.; Lv, C.; Pei, Y.; Guo, J.; Li, J.; Cui, X.; et al. Mechanisms of Mitochondria-Mediated Apoptosis During Eimeria tenella Infection. Animals 2025, 15, 577. [Google Scholar] [CrossRef] [PubMed]
- Protasoni, M.; López-Polo, V.; Stephan-Otto Attolini, C.; Brandariz, J.; Herranz, N.; Mateo, J.; Ruiz, S.; Fernandez-Capetillo, O.; Kovatcheva, M.; Serrano, M. Cyclophilin D Plays a Critical Role in the Survival of Senescent Cells. EMBO J. 2024, 43, 5972–6000. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Arroum, T.; Luo, X.; Kang, R.; Lee, Y.J.; Tang, D.; Hüttemann, M.; Song, X. Diverse Functions of Cytochrome c in Cell Death and Disease. Cell Death Differ. 2024, 31, 387–404. [Google Scholar] [CrossRef]
- Aghaei, M.; Aghaei, S.; Shahmoradi, Z.; Hejazi, S.H. The Role of Metacaspases and Other Proteins Involved in the Apoptosis of Leishmania: Review Article. Iran. J. Parasitol. 2025, 20, 1–12. [Google Scholar] [CrossRef]
- Ehrmann, J.F.; Grabarczyk, D.B.; Heinke, M.; Deszcz, L.; Kurzbauer, R.; Hudecz, O.; Shulkina, A.; Gogova, R.; Meinhart, A.; Versteeg, G.A.; et al. Structural Basis for Regulation of Apoptosis and Autophagy by the BIRC6/SMAC Complex. Science 2023, 379, 1117–1123. [Google Scholar] [CrossRef]
- Wang, W.; Li, J.; Zhou, Q. The Biological Function of Cytoplasm-Translocated ENDOG (Endonuclease G). Autophagy 2024, 20, 445–447. [Google Scholar] [CrossRef]
- Kominsky, D.J.; Bickel, R.J.; Tyler, K.L. Reovirus-Induced Apoptosis Requires Mitochondrial Release of Smac/DIABLO and Involves Reduction of Cellular Inhibitor of Apoptosis Protein Levels. J. Virol. 2002, 76, 11414–11424. [Google Scholar] [CrossRef]
- Benítez-Guzmán, A.; Arriaga-Pizano, L.; Morán, J.; Gutiérrez-Pabello, J.A. Endonuclease G Takes Part in AIF-Mediated Caspase-Independent Apoptosis in Mycobacterium bovis-Infected Bovine Macrophages. Vet. Res. 2018, 49, 69. [Google Scholar] [CrossRef] [PubMed]
- Azami, M.; Ranjkesh Adermanabadi, V.; Khanahmad, H.; Mohaghegh, M.A.; Zaherinejad, E.; Aghaei, M.; Jalali, A.; Hejazi, S.H. Immunology and Genetic of Leishmania infantum: The Role of Endonuclease G in the Apoptosis. J. Res. Med. Sci. Off. J. Isfahan Univ. Med. Sci. 2018, 23, 36. [Google Scholar] [CrossRef]
- Graumann, K.; Schaumburg, F.; Reubold, T.F.; Hippe, D.; Eschenburg, S.; Lüder, C.G.K. Toxoplasma gondii Inhibits Cytochrome C-Induced Caspase Activation in Its Host Cell by Interference with Holo-Apoptosome Assembly. Microb. Cell 2015, 2, 150–162. [Google Scholar] [CrossRef]
- Ronco, M.T.; Francés, D.E.; Ingaramo, P.I.; Quiroga, A.D.; Alvarez, M.L.; Pisani, G.B.; Revelli, S.S.; Carnovale, C.E. Tumor Necrosis Factor Alpha Induced by Trypanosoma cruzi Infection Mediates Inflammation and Cell Death in the Liver of Infected Mice. Cytokine 2010, 49, 64–72. [Google Scholar] [CrossRef]
- Lian, L.; Sun, Q.; Huang, X.; Li, W.; Cui, Y.; Pan, Y.; Yang, X.; Wang, P. Inhibition of Cell Apoptosis by Apicomplexan Protozoa-Host Interaction in the Early Stage of Infection. Animals 2023, 13, 3817. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Li, Y.; Yang, X.; Li, R.; Zhu, C.; He, X.; Jin, X.; Zheng, G.; Mehmood, N.; Cho, W.C.; et al. PI3K/AKT Signaling in Parasites and Parasite Diseases: Role and Therapeutic Potential. Virulence 2025, 16, 2532803. [Google Scholar] [CrossRef] [PubMed]
- Duan, B.-T.; Zhang, H.-Y.; Song, Z.-H.; Han, X.-Y.; Cui, K.-L.; Xu, T.; Zhang, Y.; Zhao, Y.-J.; Lei, X.; Tan, F.; et al. EtROP38 Suppresses Apoptosis of Host Cells Infected with Eimeria tenella by Inhibition of the P38MAPK Pathway. Vet. Parasitol. 2024, 331, 110296. [Google Scholar] [CrossRef] [PubMed]
- Kummer, E.; Ban, N. Mechanisms and Regulation of Protein Synthesis in Mitochondria. Nat. Rev. Mol. Cell Biol. 2021, 22, 307–325. [Google Scholar] [CrossRef]
- Zaib, S.; Hayyat, A.; Ali, N.; Gul, A.; Naveed, M.; Khan, I. Role of Mitochondrial Membrane Potential and Lactate Dehydrogenase A in Apoptosis. Anticancer. Agents Med. Chem. 2022, 22, 2048–2062. [Google Scholar] [CrossRef]
- Zhou, X.; Chen, H.; Wang, L.; Lenahan, C.; Lian, L.; Ou, Y.; He, Y. Mitochondrial Dynamics: A Potential Therapeutic Target for Ischemic Stroke. Front. Aging Neurosci. 2021, 13, 721428. [Google Scholar] [CrossRef]
- Li, B.; Chen, X.; Yang, W.; He, J.; He, K.; Xia, Z.; Zhang, J.; Xiang, G. Single-Walled Carbon Nanohorn Aggregates Promotes Mitochondrial Dysfunction-Induced Apoptosis in Hepatoblastoma Cells by Targeting SIRT3. Int. J. Oncol. 2018, 53, 1129–1137. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Wei, S.; Nguyen, T.H.; Jo, Y.; Zhang, Y.; Park, W.; Gariani, K.; Oh, C.-M.; Kim, H.H.; Ha, K.-T.; et al. Mitochondria-Associated Programmed Cell Death as a Therapeutic Target for Age-Related Disease. Exp. Mol. Med. 2023, 55, 1595–1619. [Google Scholar] [CrossRef]
- Zong, L.; Liang, Z. Apoptosis-Inducing Factor: A Mitochondrial Protein Associated with Metabolic Diseases-a Narrative Review. Cardiovasc. Diagn. Ther. 2023, 13, 609–622. [Google Scholar] [CrossRef] [PubMed]
- Frolova, A.S.; Chepikova, O.E.; Deviataikina, A.S.; Solonkina, A.D.; Zamyatnin, A.A.J. New Perspectives on the Role of Nuclear Proteases in Cell Death Pathways. Biology 2023, 12, 797. [Google Scholar] [CrossRef]
- Mammari, N.; Halabi, M.A.; Yaacoub, S.; Chlala, H.; Dardé, M.-L.; Courtioux, B. Toxoplasma gondii Modulates the Host Cell Responses: An Overview of Apoptosis Pathways. Biomed Res. Int. 2019, 2019, 6152489. [Google Scholar] [CrossRef]
- Zhang, X.; Li, S.; Zheng, M.; Zhang, L.; Bai, R.; Li, R.; Hao, S.; Bai, B.; Kang, H. Effects of the PI3K/Akt Signaling Pathway on the Apoptosis of Early Host Cells Infected with Eimeria tenella. Parasitol. Res. 2020, 119, 2549–2561. [Google Scholar] [CrossRef]
- Hu, W.-L.; Dong, H.-Y.; Li, Y.; Ojcius, D.M.; Li, S.-J.; Yan, J. Bid-Induced Release of AIF/EndoG from Mitochondria Causes Apoptosis of Macrophages during Infection with Leptospira interrogans. Front. Cell. Infect. Microbiol. 2017, 7, 471. [Google Scholar] [CrossRef]
- Bai, L.; Xu, S.; Chen, W.; Li, Z.; Wang, X.; Tang, H.; Lin, Y. Blocking NF-ΚB and Akt by Hsp90 Inhibition Sensitizes Smac Mimetic Compound 3-Induced Extrinsic Apoptosis Pathway and Results in Synergistic Cancer Cell Death. Apoptosis 2011, 16, 45–54. [Google Scholar] [CrossRef]
- Song, X.-B.; Liu, G.; Wang, Z.-Y.; Wang, L. Puerarin Protects against Cadmium-Induced Proximal Tubular Cell Apoptosis by Restoring Mitochondrial Function. Chem. Biol. Interact. 2016, 260, 219–231. [Google Scholar] [CrossRef]
- Saita, S.; Nolte, H.; Fiedler, K.U.; Kashkar, H.; Venne, A.S.; Zahedi, R.P.; Krüger, M.; Langer, T. PARL Mediates Smac Proteolytic Maturation in Mitochondria to Promote Apoptosis. Nat. Cell Biol. 2017, 19, 318–328. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.; Pan, J.; Shen, M.; Xing, C. Apoptotic Effect of Pyrroloquinoline Quinone on Chondrosarcoma Cells through Activation of the Mitochondrial Caspase-dependent and Caspase-independent Pathways. Oncol. Rep. 2018, 40, 1614–1620. [Google Scholar] [CrossRef]
- Sun, Y.; Ge, X.; Li, X.; He, J.; Wei, X.; Du, J.; Sun, J.; Li, X.; Xun, Z.; Liu, W.; et al. High-Fat Diet Promotes Renal Injury by Inducing Oxidative Stress and Mitochondrial Dysfunction. Cell Death Dis. 2020, 11, 914. [Google Scholar] [CrossRef] [PubMed]
- Flores-Romero, H.; Dadsena, S.; García-Sáez, A.J. Mitochondrial Pores at the Crossroad between Cell Death and Inflammatory Signaling. Mol. Cell 2023, 83, 843–856. [Google Scholar] [CrossRef] [PubMed]
- Schmuckli-Maurer, J.; Bindschedler, A.F.; Wacker, R.; Würgler, O.M.; Rehmann, R.; Lehmberg, T.; Murphy, L.O.; Nguyen, T.N.; Lazarou, M.; Monfregola, J.; et al. Plasmodium Berghei Liver Stage Parasites Exploit Host GABARAP Proteins for TFEB Activation. Commun. Biol. 2024, 7, 1554. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.M.; Levine, S.A.; Splinter, P.L.; Tietz, P.S.; Ganong, A.L.; Jobin, C.; Gores, G.J.; Paya, C.V.; LaRusso, N.F. Cryptosporidium Parvum Activates Nuclear Factor KappaB in Biliary Epithelia Preventing Epithelial Cell Apoptosis. Gastroenterology 2001, 120, 1774–1783. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bai, R.; Zhu, S.; Wang, H.; Lv, C.; Zhao, W.; Zhang, L.; Liu, Y.; Gao, H.; Lv, X.; Li, J.; et al. Mechanistic Insights into Eimeria tenella-Induced Host Cell Apoptosis Through Modulation of the Mitochondrial Permeability Transition Pore. Microorganisms 2025, 13, 2139. https://doi.org/10.3390/microorganisms13092139
Bai R, Zhu S, Wang H, Lv C, Zhao W, Zhang L, Liu Y, Gao H, Lv X, Li J, et al. Mechanistic Insights into Eimeria tenella-Induced Host Cell Apoptosis Through Modulation of the Mitochondrial Permeability Transition Pore. Microorganisms. 2025; 13(9):2139. https://doi.org/10.3390/microorganisms13092139
Chicago/Turabian StyleBai, Rui, Shuying Zhu, Hui Wang, Chenyang Lv, Wenlong Zhao, Li Zhang, Yao Liu, Hanze Gao, Xiaoling Lv, Jianhui Li, and et al. 2025. "Mechanistic Insights into Eimeria tenella-Induced Host Cell Apoptosis Through Modulation of the Mitochondrial Permeability Transition Pore" Microorganisms 13, no. 9: 2139. https://doi.org/10.3390/microorganisms13092139
APA StyleBai, R., Zhu, S., Wang, H., Lv, C., Zhao, W., Zhang, L., Liu, Y., Gao, H., Lv, X., Li, J., & Cui, X. (2025). Mechanistic Insights into Eimeria tenella-Induced Host Cell Apoptosis Through Modulation of the Mitochondrial Permeability Transition Pore. Microorganisms, 13(9), 2139. https://doi.org/10.3390/microorganisms13092139




