New Marine Actinobacteria Strain, Micromonospora sp. SH-82: Characterization, Specialized Metabolites and Biological Activities
Abstract
1. Introduction
2. Materials and Methods
2.1. General Experimental Procedures
2.2. Biological Material and Strain Culture
2.3. Genomic Analysis, Observation, and Identification
2.4. Extract Preparation and Purification
2.5. Description of the Isolated Compounds
2.6. UHPLC-ESI-HRMS/MS Analyses
2.7. Raw Data Processing and Ion Identity Molecular Network
2.8. Biological Activities
3. Results and Discussion
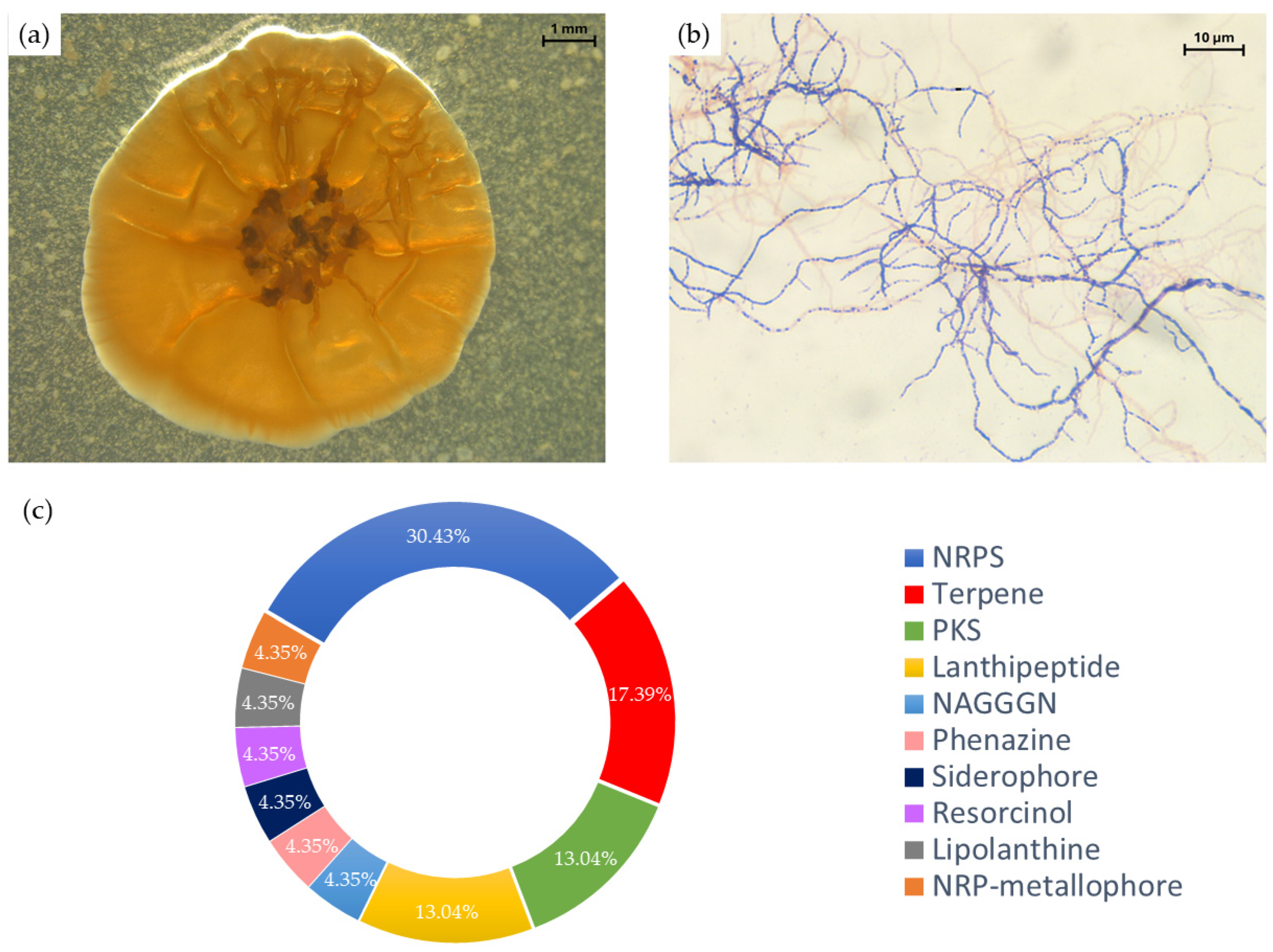
3.1. Strain Characterization, Identification, and Genomic Analysis
3.2. Chemical Investigation
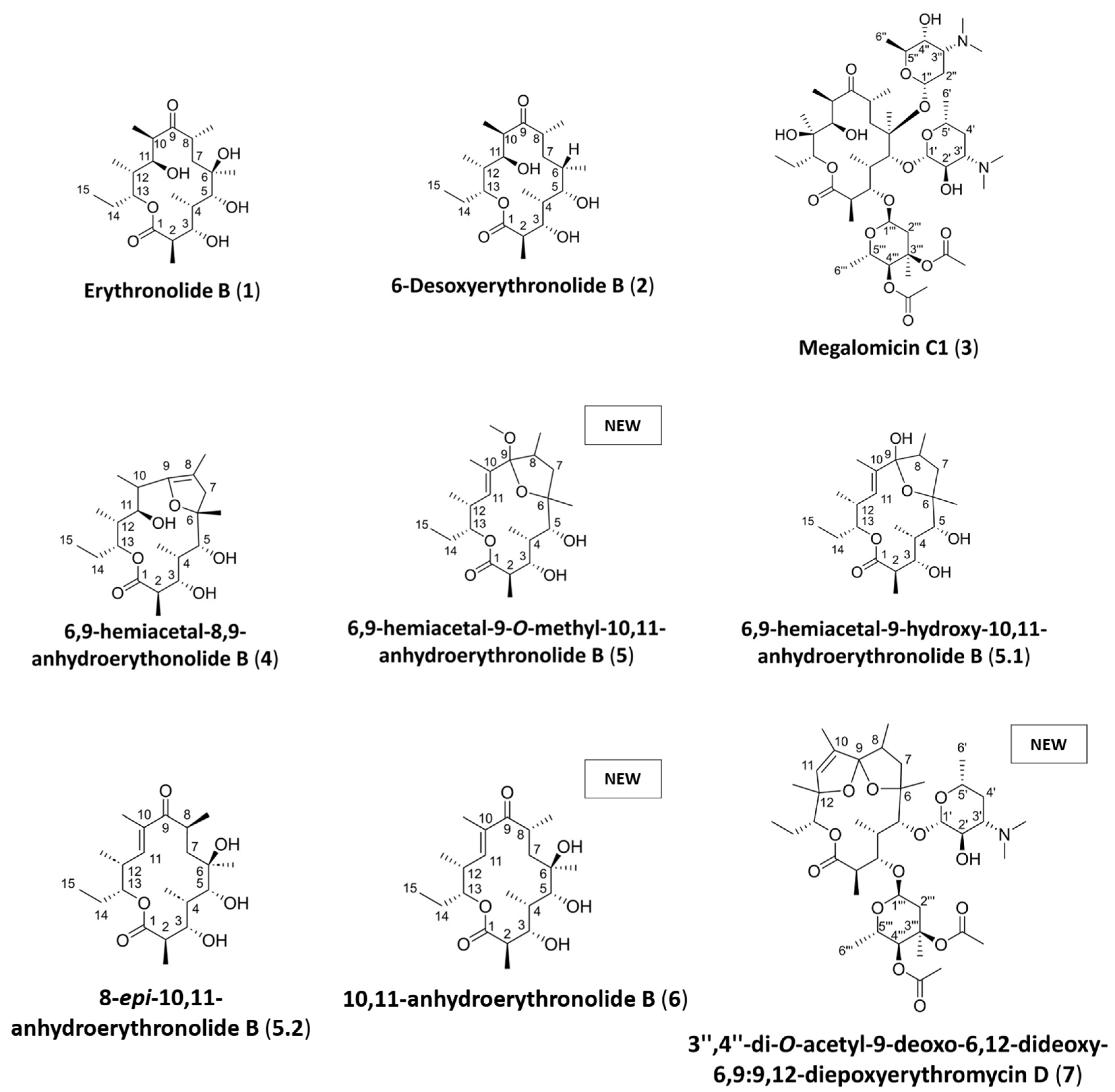
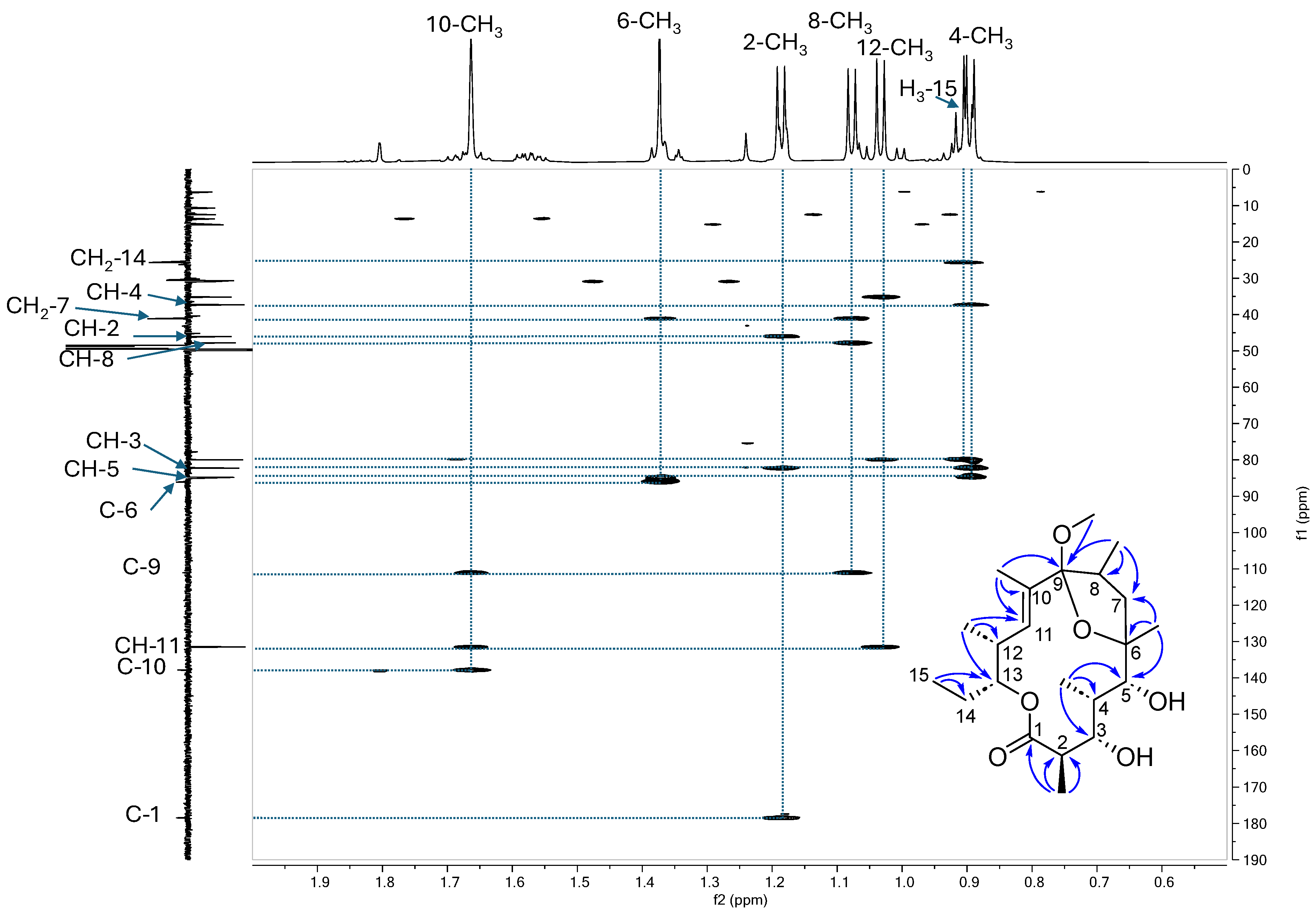
3.2.1. Purification and Structural Elucidation
| 5 | Erythronolide B (1) | 5.1 | ||||
|---|---|---|---|---|---|---|
| No | δH (Multiplicity, J) | δC | δH (Multiplicity, J) | δC | δH (Multiplicity, J) | δC |
| 1 | - | 178.4 | - | 177.6 | - | 178.7 |
| 2 | 2.58 (dq, 10.4, 6.9 Hz) | 46.1 | 2.69 (dq, 10.5, 6.7 Hz) | 45.1 | 2.58 (dq, 10.4, 6.9 Hz) | 46.1 |
| 2-CH3 | 1.19 (d, 6.9 Hz) | 15.3 | 1.19 (d, 6.7 Hz) | 15.5 | 1.19 (d, 6.9 Hz) | 13.2 |
| 3 | 3.74 (d, 10.4 Hz) | 82.3 | 3.58 (dd, 10.5, 1.5 Hz) | 80.3 | 3.75 (d, 10.4 Hz) | 82.3 |
| 4 | 2.33 (q, 7.0 Hz) | 37.3 | 2.16 (overlapped) | 37.3 | 2.30 (q, 7.3 Hz) | 37.3 |
| 4-CH3 | 0.89 (d, 7.0 Hz) | 6.3 | 1.01 (d, 7.2 Hz) | 8.1 | 0.90 (d, 7.3 Hz) | 6.4 |
| 5 | 3.59 (s) | 84.8 | 3.52 (d, 2.9 Hz) | 82.6 | 3.60 (s) | 84.7 |
| 6 | - | 86.1 | - | 75.8 | - | 86.2 |
| 6-CH3 | 1.37 (s) | 31.0 | 1.30 (s) | 26.4 | 1.37 (s) | 30.9 |
| 7 | 1.37 (dd, 13.1, 12.0 Hz) 2.44 (dd, 13.1, 9.0 Hz) | 41.2 | 1.98 (dd, 14.7, 9.3 Hz) 1.39 (dd, 14.7, 4.2 Hz) | 38.8 | 1.37 (dd, 13.2, 12.0 Hz) 2.43 (dd, 13.2, 9.0 Hz) | 41.1 |
| 8 | 2.06 (ddq, 12.0, 9.0, 6.7 Hz) | 47.8 | 2.72 (dqd, 9.3, 7.0, 4.2 Hz) | 45.5 | 2.06 (ddq, 12.0, 9.0, 6.8 Hz) | 47.8 |
| 8-CH3 | 1.08 (d, 6.7 Hz) | 15.2 | 1.14 (d, 7.0 Hz) | 18.3 | 1.07 (d, 6.8 Hz) | 15.1 |
| 9 | - | 111.1 | - | 220.5 | - | 111.1 |
| 10 | - | 137.8 | 3.03 (qd, 6.9, 1.8 Hz) | 41.3 | - | 137.8 |
| 10-CH3 | 1.66 (t, 1.3 Hz) | 13.7 | 0.96 (d, 6.9 Hz) | 9.7 | 1.66 (d, 1.2 Hz) | 13.7 |
| 11 | 5.86 (dq, 6.3, 1.3 Hz) | 131.5 | 3.95 (dd, 10.1, 1.8 Hz) | 71.3 | 5.84 (dq, 6.4, 1.2 Hz) | 131.5 |
| 12 | 2.62 (pt, 6.9, 6.3, 2.5, 1.3 Hz) | 35.2 | 1.66 (dq, 10.1, 6.5 Hz) | 41.5 | 2.63 (overlapped) | 35.2 |
| 12-CH3 | 1.03 (d, 6.9 Hz) | 12.5 | 0.95 (d, 6.5 Hz) | 9.7 | 1.03 (d, 6.9 Hz) | 12.5 |
| 13 | 5.10 (ddd, 9.1, 5.0, 2.5 Hz) | 80.0 | 5.44 (dd, 9.7, 4.6 Hz) | 76.1 | 5.09 (overlapped) | 80.2 |
| 14 | 1.67 (ddq, 14.2, 9.1, 7.4 Hz) 1.57 (dqd, 14.2, 7.4, 5.0 Hz) | 25.7 | 1.74 (ddq, 14.0, 9.6, 7.4 Hz) 1.51 (dqd, 14.0, 7.4, 4.6 Hz) | 26.9 | 1.66 (overlapped) 1.58 (overlapped) | 25.6 |
| 15 | 0.90 (t, 7.4 Hz) | 10.7 | 0.89 (t, 7.4 Hz) | 10.9 | 0.90 (t, 7.3 Hz) | 10.6 |
| OCH3 | 3.11 (s) | 50.0 | ||||
| 6 | 5.2 | |||
|---|---|---|---|---|
| No | δH (Multiplicity, J) | δC | δH (Multiplicity, J) | δC |
| 1 | - | 177.9 | - | 177.5 |
| 2 | 2.75 (dq, 10.4, 6.7 Hz) | 45.3 | 2.63 (dq, 11.0, 6.7 Hz) | 45.2 |
| 2-CH3 | 1.27 (d, 6.7 Hz) | 15.5 | 1.18 (d, 6.7 Hz) | 13.2 |
| 3 | 3.94 (dd, 10.4, 1.1 Hz) | 80.2 | 3.63 (d, 11.0 Hz) | 79.9 |
| 4 | 1.87 (qdd, 7.0, 2.9, 1.1) | 38.4 | 2.02 (overlapped) | 37.4 |
| 4-CH3 | 0.99 (d, 7.0 Hz) | 7.1 | 1.06 (d, 7.1 Hz) | 7.9 |
| 5 | 3.86 (d, 2.9 Hz) | 81.7 | 3.37 (d, 4.3 Hz) | 82.1 |
| 6 | - | 76.5 | - | 75.6 |
| 6-CH3 | 1.22 (s) | 27.1 | 1.24 (s) | 28.8 |
| 7 | 1.36 (dd, 14.8, 12.3 Hz) 1.71 (dd, 14.8, 2.6 Hz) | 44.3 | 1.63 (dd, 14.3, 5.9 Hz) 1.84 (dd, 14.3, 8.2 Hz) | 43.1 |
| 8 | 3.29 (overlapped) | 37.5 | 3.49 (dqd, 8.2, 6.7, 5.9 Hz) | 35.2 |
| 8-CH3 | 1.18 (d, 6.4 Hz) | 16.4 | 1.00 (d, 6.7 Hz) | 19.9 |
| 9 | - | 208.3 | - | 208.8 |
| 10 | - | 136.2 | - | 138.0 |
| 10-CH3 | 1.74 (d, 1.4 Hz) | 11.7 | 1.80 (d, 1.2 Hz) | 12.2 |
| 11 | 6.53 (dq, 8.9, 1.4 Hz) | 144.6 | 6.40 (dq, 8.3, 1.2 Hz) | 145.4 |
| 12 | 2.84 (dqd, 8.9, 7.0, 1.8 Hz) | 37.7 | 2.88 (dqd, 8.3, 6.7, 2.4 Hz) | 36.4 |
| 12-CH3 | 1.07 (d, 7.0 Hz) | 12.3 | 1.18 (d, 6.7 Hz) | 13.2 |
| 13 | 5.08 (ddd, 9.7, 4.3, 1.8 Hz) | 77.9 | 5.07 (overlapped) | 78.1 |
| 14 | 1.75 (dqd, 14.7, 9.7, 7.4 Hz) 1.61 (dqd, 14.7, 7.4, 4.3 Hz) | 26.1 | 1.68 (m) | 26.2 |
| 15 | 0.92 (t, 7.4 Hz) | 10.7 | 0.92 (t, 7.4 Hz) | 10.6 |
| 1 | - | 177.9 | - | 177.5 |
| 7 | Megalomicin C1 (3) | |||
|---|---|---|---|---|
| No | δH (Multiplicity, J) | δC | δH (Multiplicity, J) | δC |
| 1 | - | 180.3 | - | 178.1 |
| 2 | 2.53 (qd, 7.4, 6.0 Hz) | 50.5 | 2.94 (dq, 10.4, 7.2 Hz) | 46.3 |
| 2-CH3 | 1.01 (d, 7.4 Hz) | 16.0 | 1.20 (d, 7.2 Hz) | 16.8 |
| 3 | 4.03 (d, 6.0 Hz) | 80.3 | 4.55 (dd, 10.4, 1.5 Hz) | 83.1 |
| 4 | 2.23 (dq, 9.6, 7.2 Hz) | 46.9 | 2.04 (pd, 7.6, 6.2, 1.5 Hz) | 39.1 |
| 4-CH3 | 1.11 (d, 7.2 Hz) | 12.5 | 1.14 (d, 7.6 Hz) | 10.5 |
| 5 | 3.43 (d, 9.6 Hz) | 88.3 | 3.95 (d, 6.2 Hz) | 83.6 |
| 6 | - | 84.4 | - | 82.3 |
| 6-CH3 | 1.52 (s) | 31.3 | 1.58 (s) | 19.8 |
| 7 | 2.31 (dd, 13.5, 12.8 Hz) 1.68 (dd, 12.8, 6.2 Hz) | 44.7 | 2.05 (d, 15.1 Hz) 1.70 (d, 15.1 Hz) | 39.8 |
| 8 | 2.44 (dqd, 13.5, 7.0, 6.2 Hz) | 41.5 | 3.23 (q, 6.9 Hz) | 38.9 |
| 8-CH3 | 0.95 (d, 7.0 Hz) | 12.3 | 1.15 (d, 7.0 Hz) | 12.5 |
| 9 | - | 121.2 | - | no |
| 10 | - | 139.9 | 2.55 (m) | 46.9 |
| 10-CH3 | 1.82 (d, 1.6 Hz) | 14.2 | 1.15 (d, 7.0 Hz) | 19.2 |
| 11 | 5.61 (q, 1.6 Hz) | 130.0 | 3.61 (d, 1.4 Hz) | 70.5 |
| 12 | - | 90.4 | - | 76.0 |
| 12-CH3 | 1.25 (s) | 23.4 | 1.19 (s) | 17.6 |
| 13 | 4.89 (overlapped) | 80.7 | 5.19 (dd, 11.3, 2.3 Hz) | 78.6 |
| 14 | 1.69 (overlapped) 1.40 (overlapped) | 25.1 | 1.90 (qd, 7.4, 2.3 Hz) 1.54 (dq, 11.3, 7.4 Hz) | 22.2 |
| 15 | 0.84 (t, 7.3 Hz) | 10.6 | 0.85 (t, 7.4 Hz) | 10.9 |
| D-desosamine | ||||
| 1′ | 4.15 (d, 7.3 Hz) | 106.9 | 4.55 (d, 7.1 Hz) | 103.6 |
| 2′ | 3.39 (overlapped) | no | 3.38 (d, 7.1 Hz) | 71.5 |
| 3′ | no | no | no | no |
| 4′ | 1.84 (overlapped) 1.33 (overlapped) | 32.0 | 1.91 (m) 1.41 (m) | 31.7 |
| 5′ | 3.54 (m) | 70.0 | 3.65 (p, 10.4, 6.3 Hz) | 69.1 |
| 6′ | 1.22 (d, 6.1 Hz) | 21.3 | 1.28 (d, 6.0 Hz) | 22.0 |
| 3′N(CH3)2 | 2.49 (brs) | no | 2.55/2.84 | 41.8 |
| L-megasamine | ||||
| 1″ | - | - | 4.98 (t, 6.8, 5.6 Hz) | 91.0 |
| 2″ | - | - | 3.34 (overlapped) 1.83 (m) | 30.6 |
| 3″ | - | - | no | 62.8 |
| 4″ | - | - | 3.82 (t, 1.8, 1.4 Hz) | 66.3 |
| 5″ | - | - | 4.30 (qd, 7.4, 1.4 Hz) | 75.1 |
| 6″ | - | - | 1.23 (d, 7.4 Hz) | 15.2 |
| 3′N(CH3)2 | - | - | 2.55/2.84 | 41.8 |
| L-mycarose | ||||
| 1″′ | 4.99 (d, 4.6 Hz) | 99.3 | 5.03 (d, 4.5 Hz) | 99.0 |
| 2″′ | 3.34 (overlapped) 1.80 (dd, 15.3, 4.7 Hz) | 36.5 | 3.34 (overlapped) 1.83 (m) | 37.3 |
| 3″′ | - | 79.7 | - | 79.7 |
| 3″′-CH3 | 1.42 (s) | 22.7 | 1.45 (s) | 22.9 |
| 4″′ | 4.61 (d, 9.8 Hz) | 79.1 | 4.60 (d, 9.6 Hz) | 78.9 |
| 5″′ | 4.38 (dq, 9.8, 6.8 Hz) | 64.2 | 4.25 (dq, 9.6, 6.1 Hz) | 64.4 |
| 6″′ | 1.15 (d, 6.3 Hz) | 17.6 | 1.20 (d, 6.1 Hz) | 19.2 |
| 3″′a | - | 172.4 | - | 172.7 |
| 3″′b | 2.01 (s) | 22.8 | 2.13 (s) | 20.7 |
| 4″′a | - | 172.2 | - | 172.0 |
| 4″′b | 2.15 (s) | 20.6 | 2.13 (s) | 23.6 |
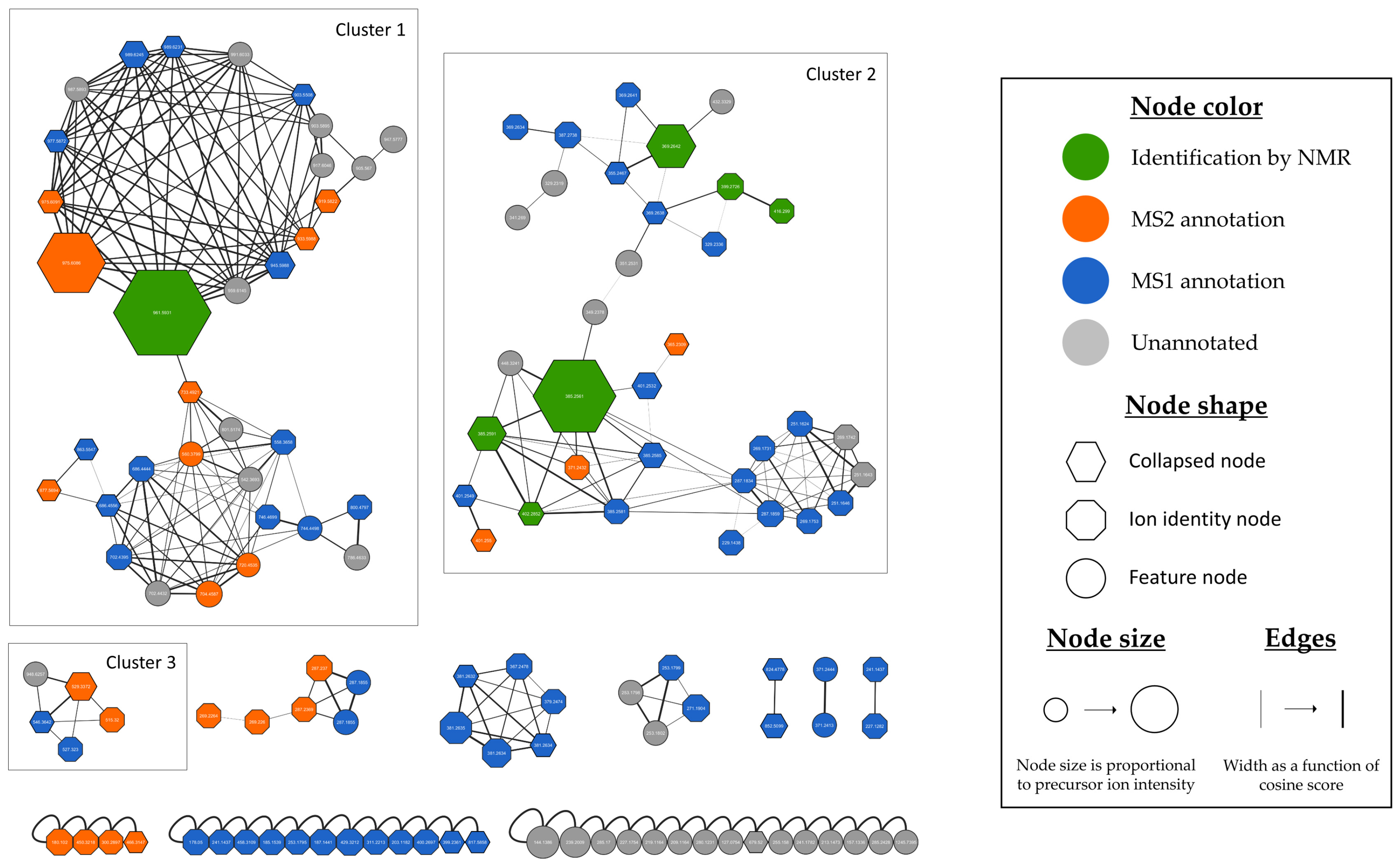
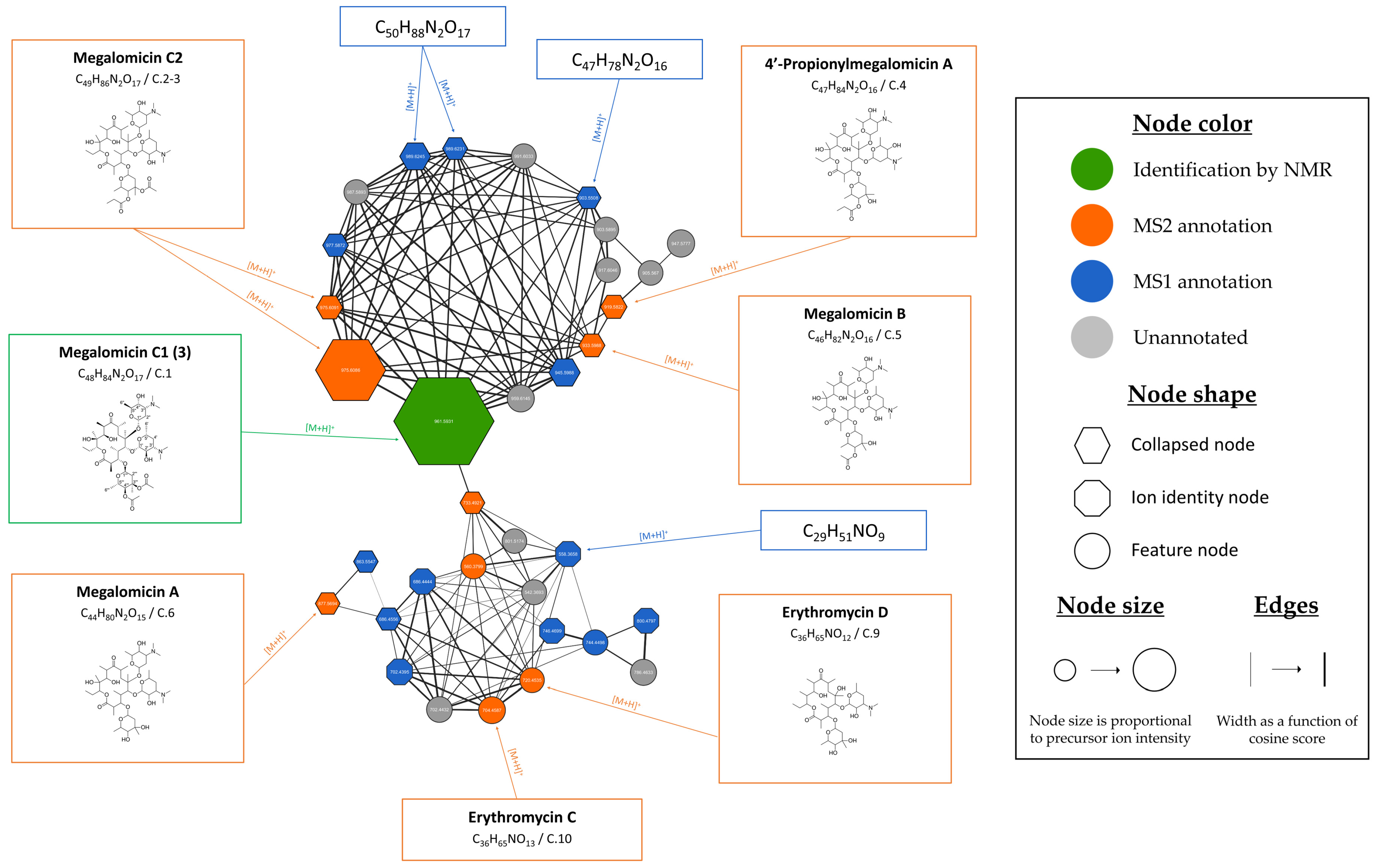
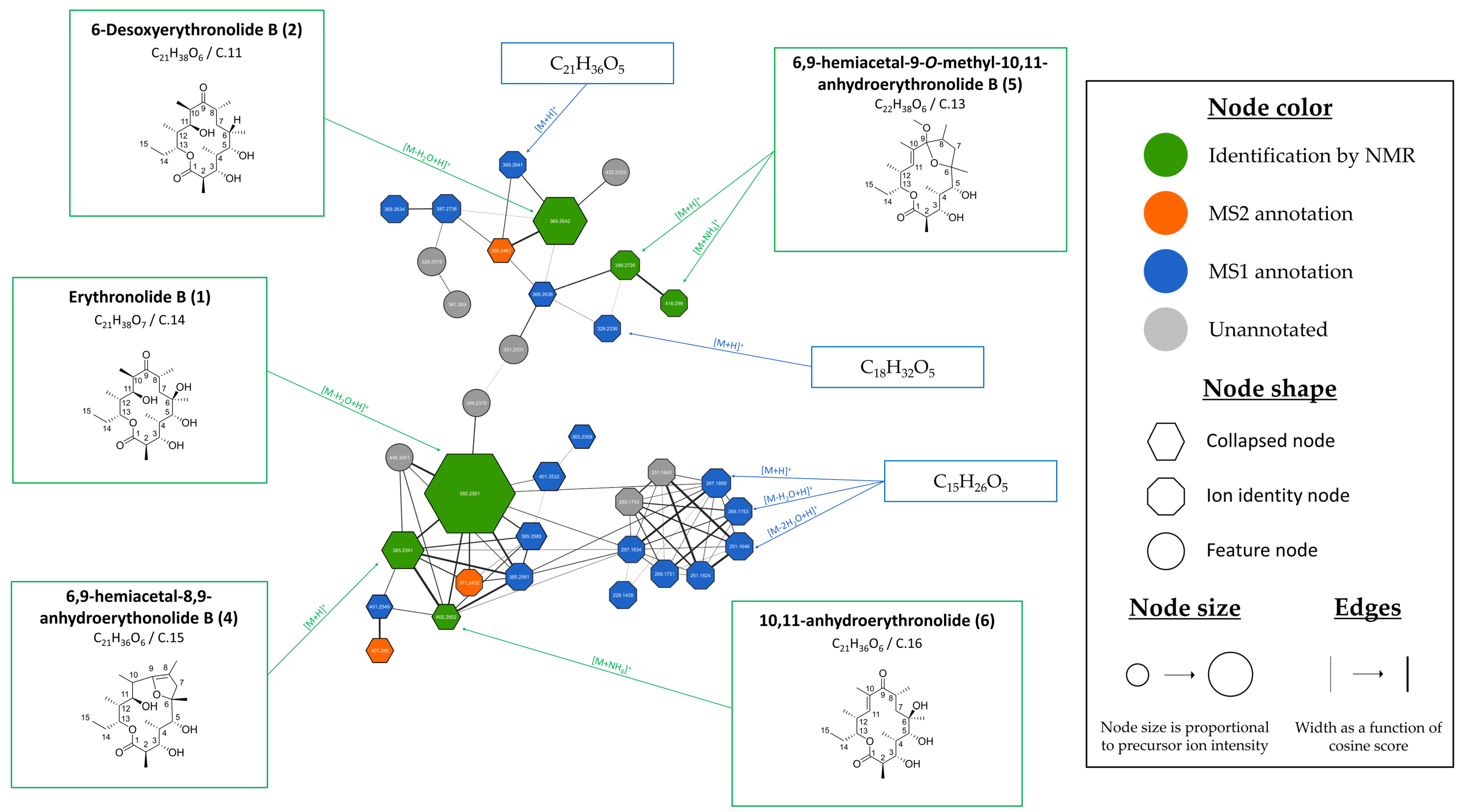
3.2.2. Evaluation of the Chemical Diversity and Biosynthetic Potential of Micromonospora sp. SH-82 Through Ion Identity Molecular Networking
3.3. Biological Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- World Health Organization. World Malaria Report 2022; World Health Organization: Geneva, Switzerland, 2022. [Google Scholar]
- Hassan, S.S.U.; Shaikh, A.L. Marine Actinobacteria as a Drug Treasure House. Biomed. Pharmacother. 2017, 87, 46–57. [Google Scholar] [CrossRef] [PubMed]
- Jose, P.A.; Maharshi, A.; Jha, B. Actinobacteria in Natural Products Research: Progress and Prospects. Microbiol. Res. 2021, 246, 126708. [Google Scholar] [CrossRef]
- Yan, S.; Zeng, M.; Wang, H.; Zhang, H. Micromonospora: A Prolific Source of Bioactive Secondary Metabolites with Therapeutic Potential. J. Med. Chem. 2022, 65, 8735–8771. [Google Scholar] [CrossRef]
- Barka, E.A.; Vatsa, P.; Sanchez, L.; Gaveau-Vaillant, N.; Jacquard, C.; Klenk, H.-P.; Clément, C.; Ouhdouch, Y.; van Wezel, G.P. Taxonomy, Physiology, and Natural Products of Actinobacteria. Microbiol. Mol. Biol. Rev. 2015, 80, 1–43. [Google Scholar] [CrossRef] [PubMed]
- Hifnawy, M.S.; Fouda, M.M.; Sayed, A.M.; Mohammed, R.; Hassan, H.M.; AbouZid, S.F.; Rateb, M.E.; Keller, A.; Adamek, M.; Ziemert, N.; et al. The Genus Micromonospora as a Model Microorganism for Bioactive Natural Product Discovery. RSC Adv. 2020, 10, 20939–20959. [Google Scholar] [CrossRef] [PubMed]
- Qi, S.; Gui, M.; Li, H.; Yu, C.; Li, H.; Zeng, Z.; Sun, P. Secondary Metabolites from Marine Micromonospora: Chemistry and Bioactivities. Chem. Biodivers. 2020, 17, e2000024. [Google Scholar] [CrossRef]
- Carro, L.; Nouioui, I.; Sangal, V.; Meier-Kolthoff, J.P.; Trujillo, M.E.; Montero-Calasanz, M.d.C.; Sahin, N.; Smith, D.L.; Kim, K.E.; Peluso, P.; et al. Genome-Based Classification of Micromonospora with a Focus on Their Biotechnological and Ecological Potential. Sci. Rep. 2018, 8, 525. [Google Scholar] [CrossRef]
- Weinstein, M.J.; Luedemann, G.M.; Oden, E.M.; Wagman, G.H.; Rosselet, J.P.; Marquez, J.A.; Coniglio, C.T.; Charney, W.; Herzog, H.L.; Black, J. Gentamicin, a New Antibiotic Complex from Micromonospora. J. Med. Chem. 1963, 6, 463–464. [Google Scholar] [CrossRef]
- Charan, R.D.; Schlingmann, G.; Janso, J.; Bernan, V.; Feng, X.; Carter, G.T. Diazepinomicin, a New Antimicrobial Alkaloid from a Marine Micromonospora sp. J. Nat. Prod. 2004, 67, 1431–1433. [Google Scholar] [CrossRef]
- Reen, F.J.; Romano, S.; Dobson, A.D.W.; O’Gara, F. The Sound of Silence: Activating Silent Biosynthetic Gene Clusters in Marine Microorganisms. Mar. Drugs 2015, 13, 4754–4783. [Google Scholar] [CrossRef]
- Tay, D.W.P.; Tan, L.L.; Heng, E.; Zulkarnain, N.; Ching, K.C.; Wibowo, M.; Chin, E.J.; Tan, Z.Y.Q.; Leong, C.Y.; Ng, V.W.P.; et al. Exploring a General Multi-Pronged Activation Strategy for Natural Product Discovery in Actinomycetes. Commun. Biol. 2024, 7, 50. [Google Scholar] [CrossRef]
- Asamizu, S. Co-Cultivation Strategies for Natural Product Discovery from Actinomycetes: Unlocking Silent Secondary Metabolism with Mycolic Acid-Containing Bacteria. World J. Microbiol. Biotechnol. 2025, 41, 217. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhu, M.; Yang, C.; Zhu, S.; Tan, B.; Qi, S.-H.; Zhu, Y.; Zhang, C. Development of A Salt-Enhanced Promoter Strategy for Activating Silent Biosynthetic Gene Clusters from Streptomycetes. Metab. Eng. 2025, 92, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Du, X.; Yu, X.; Jiang, Q.; Zheng, K.; Xu, J.; Wang, P. Recent Advances in the Heterologous Expression of Biosynthetic Gene Clusters for Marine Natural Products. Mar. Drugs 2022, 20, 341. [Google Scholar] [CrossRef] [PubMed]
- Saïd Hassane, C.; Fouillaud, M.; Le Goff, G.; Sklirou, A.D.; Boyer, J.B.; Trougakos, I.P.; Jerabek, M.; Bignon, J.; de Voogd, N.J.; Ouazzani, J.; et al. Microorganisms Associated with the Marine Sponge Scopalina hapalia: A Reservoir of Bioactive Molecules to Slow down the Aging Process. Microorganisms 2020, 8, 1262. [Google Scholar] [CrossRef]
- Le Loarer, A.; Marcellin-Gros, R.; Dufossé, L.; Bignon, J.; Frédérich, M.; Ledoux, A.; Queiroz, E.F.; Wolfender, J.-L.; Gauvin-Bialecki, A.; Fouillaud, M. Prioritization of Microorganisms Isolated from the Indian Ocean Sponge Scopalina hapalia Based on Metabolomic Diversity and Biological Activity for the Discovery of Natural Products. Microorganisms 2023, 11, 697. [Google Scholar] [CrossRef]
- Le Loarer, A.; Dufossé, L.; Bignon, J.; Frédérich, M.; Ledoux, A.; Fouillaud, M.; Gauvin-Bialecki, A. OSMAC Method to Assess Impact of Culture Parameters on Metabolomic Diversity and Biological Activity of Marine-Derived Actinobacteria. Mar. Drugs 2024, 22, 23. [Google Scholar] [CrossRef]
- Andrews, S. FastQC: A Quality Control Tool for High Throughput Sequence Data. Available online: https://www.bioinformatics.babraham.ac.uk/projects/fastqc/ (accessed on 6 November 2023).
- Bushnell, B.; Rood, J.; Singer, E. BBMerge—Accurate Paired Shotgun Read Merging via Overlap. PLoS ONE 2017, 12, e0185056. [Google Scholar] [CrossRef]
- De Coster, W.; Rademakers, R. NanoPack2: Population-Scale Evaluation of Long-Read Sequencing Data. Bioinformatics 2023, 39, btad311. [Google Scholar] [CrossRef]
- Wick, R.R.; Judd, L.M.; Gorrie, C.L.; Holt, K.E. Completing Bacterial Genome Assemblies with Multiplex MinION Sequencing. Microb. Genom. 2017, 3, e000132. [Google Scholar] [CrossRef] [PubMed]
- Wick, R.R.; Judd, L.M.; Gorrie, C.L.; Holt, K.E. Unicycler: Resolving Bacterial Genome Assemblies from Short and Long Sequencing Reads. PLoS Comput. Biol. 2017, 13, e1005595. [Google Scholar] [CrossRef]
- Simão, F.A.; Waterhouse, R.M.; Ioannidis, P.; Kriventseva, E.V.; Zdobnov, E.M. BUSCO: Assessing Genome Assembly and Annotation Completeness with Single-Copy Orthologs. Bioinformatics 2015, 31, 3210–3212. [Google Scholar] [CrossRef]
- Parks, D.H.; Imelfort, M.; Skennerton, C.T.; Hugenholtz, P.; Tyson, G.W. CheckM: Assessing the Quality of Microbial Genomes Recovered from Isolates, Single Cells, and Metagenomes. Genome Res. 2015, 25, 1043–1055. [Google Scholar] [CrossRef]
- Seemann, T. Barrnap: Bacterial Ribosomal RNA Predictor. 2023. Available online: https://github.com/tseemann/barrnap (accessed on 6 August 2025).
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic Local Alignment Search Tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Katoh, K.; Misawa, K.; Kuma, K.; Miyata, T. MAFFT: A Novel Method for Rapid Multiple Sequence Alignment Based on Fast Fourier Transform. Nucleic Acids Res. 2002, 30, 3059–3066. [Google Scholar] [CrossRef]
- Stamatakis, A. RAxML Version 8: A Tool for Phylogenetic Analysis and Post-Analysis of Large Phylogenies. Bioinformatics 2014, 30, 1312–1313. [Google Scholar] [CrossRef]
- Jain, C.; Rodriguez-R, L.M.; Phillippy, A.M.; Konstantinidis, K.T.; Aluru, S. High Throughput ANI Analysis of 90K Prokaryotic Genomes Reveals Clear Species Boundaries. Nat. Commun. 2018, 9, 5114. [Google Scholar] [CrossRef] [PubMed]
- Shimoyama, Y. ANIclustermap: A Tool for Drawing ANI Clustermap Between All-vs-All Microbial Genomes. Available online: https://github.com/moshi4/ANIclustermap/blob/main/CITATION.cff (accessed on 6 November 2023).
- Aziz, R.K.; Bartels, D.; Best, A.A.; DeJongh, M.; Disz, T.; Edwards, R.A.; Formsma, K.; Gerdes, S.; Glass, E.M.; Kubal, M.; et al. The RAST Server: Rapid Annotations Using Subsystems Technology. BMC Genom. 2008, 9, 75. [Google Scholar] [CrossRef] [PubMed]
- Seemann, T. Prokka: Rapid Prokaryotic Genome Annotation. Bioinformatics 2014, 30, 2068–2069. [Google Scholar] [CrossRef]
- Blin, K.; Shaw, S.; Augustijn, H.E.; Reitz, Z.L.; Biermann, F.; Alanjary, M.; Fetter, A.; Terlouw, B.R.; Metcalf, W.W.; Helfrich, E.J.N.; et al. antiSMASH 7.0: New and Improved Predictions for Detection, Regulation, Chemical Structures and Visualisation. Nucleic Acids Res. 2023, 51, W46–W50. [Google Scholar] [CrossRef] [PubMed]
- Guillarme, D.; Nguyen, D.T.T.; Rudaz, S.; Veuthey, J.-L. Method Transfer for Fast Liquid Chromatography in Pharmaceutical Analysis: Application to Short Columns Packed with Small Particle. Part II: Gradient Experiments. Eur. J. Pharm. Biopharm. 2008, 68, 430–440. [Google Scholar] [CrossRef]
- Queiroz, E.F.; Alfattani, A.; Afzan, A.; Marcourt, L.; Guillarme, D.; Wolfender, J.-L. Utility of Dry Load Injection for an Efficient Natural Products Isolation at the Semi-Preparative Chromatographic Scale. J. Chromatogr. A 2019, 1598, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Schmid, R.; Heuckeroth, S.; Korf, A.; Smirnov, A.; Myers, O.; Dyrlund, T.S.; Bushuiev, R.; Murray, K.J.; Hoffmann, N.; Lu, M.; et al. Integrative Analysis of Multimodal Mass Spectrometry Data in MZmine 3. Nat. Biotechnol. 2023, 41, 447–449. [Google Scholar] [CrossRef] [PubMed]
- Myers, O.D.; Sumner, S.J.; Li, S.; Barnes, S.; Du, X. One Step Forward for Reducing False Positive and False Negative Compound Identifications from Mass Spectrometry Metabolomics Data: New Algorithms for Constructing Extracted Ion Chromatograms and Detecting Chromatographic Peaks. Anal. Chem. 2017, 89, 8696–8703. [Google Scholar] [CrossRef]
- Schmid, R.; Petras, D.; Nothias, L.-F.; Wang, M.; Aron, A.T.; Jagels, A.; Tsugawa, H.; Rainer, J.; Garcia-Aloy, M.; Dührkop, K.; et al. Ion Identity Molecular Networking for Mass Spectrometry-Based Metabolomics in the GNPS Environment. Nat. Commun. 2021, 12, 3832. [Google Scholar] [CrossRef]
- Wang, M.; Carver, J.J.; Phelan, V.V.; Sanchez, L.M.; Garg, N.; Peng, Y.; Nguyen, D.D.; Watrous, J.; Kapono, C.A.; Luzzatto-Knaan, T.; et al. Sharing and Community Curation of Mass Spectrometry Data with Global Natural Products Social Molecular Networking. Nat. Biotechnol. 2016, 34, 828–837. [Google Scholar] [CrossRef]
- Nothias, L.F.; Petras, D.; Schmid, R.; Dührkop, K.; Rainer, J.; Sarvepalli, A.; Protsyuk, I.; Ernst, M.; Tsugawa, H.; Fleischauer, M.; et al. Feature-Based Molecular Networking in the GNPS Analysis Environment. Nat. Methods 2020, 17, 905–908. [Google Scholar] [CrossRef]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A Software Environment for Integrated Models of Biomolecular Interaction Networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]
- Dührkop, K.; Fleischauer, M.; Ludwig, M.; Aksenov, A.A.; Melnik, A.V.; Meusel, M.; Dorrestein, P.C.; Rousu, J.; Böcker, S. SIRIUS 4: A Rapid Tool for Turning Tandem Mass Spectra into Metabolite Structure Information. Nat. Methods 2019, 16, 299–302. [Google Scholar] [CrossRef]
- Allard, P.-M.; Péresse, T.; Bisson, J.; Gindro, K.; Marcourt, L.; Pham, V.C.; Roussi, F.; Litaudon, M.; Wolfender, J.-L. Integration of Molecular Networking and In-Silico MS/MS Fragmentation for Natural Products Dereplication. Anal. Chem. 2016, 88, 3317–3323. [Google Scholar] [CrossRef]
- Rutz, A.; Dounoue-Kubo, M.; Ollivier, S.; Bisson, J.; Bagheri, M.; Saesong, T.; Ebrahimi, S.N.; Ingkaninan, K.; Wolfender, J.-L.; Allard, P.-M. Taxonomically Informed Scoring Enhances Confidence in Natural Products Annotation. Front. Plant Sci. 2019, 10, 1329. [Google Scholar] [CrossRef] [PubMed]
- Rutz, A.; Sorokina, M.; Galgonek, J.; Mietchen, D.; Willighagen, E.; Gaudry, A.; Graham, J.G.; Stephan, R.; Page, R.; Vondrášek, J.; et al. The LOTUS Initiative for Open Knowledge Management in Natural Products Research. eLife 2022, 11, e70780. [Google Scholar] [CrossRef]
- Trager, W.; Jensen, J.B. Human Malaria Parasites in Continuous Culture. Science 1976, 193, 673–675. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, C.; Anwesh, M.; Alessia, T.; Giuffrida, D.; La Tella, R.; Chiaia, V.; Mondello, L.; Anil, K.; Le Loarer, A.; Gauvin-Bialecki, A.; et al. Genome and Compound Analysis of Sioxanthin-Producing Marine Actinobacterium Micromonospora sp. Nov. Strain SH-82 Isolated from Sponge Scopalina hapalia. Curr. Microbiol. 2024, 81, 298. [Google Scholar] [CrossRef]
- Ciufo, S.; Kannan, S.; Sharma, S.; Badretdin, A.; Clark, K.; Turner, S.; Brover, S.; Schoch, C.L.; Kimchi, A.; DiCuccio, M. Using Average Nucleotide Identity to Improve Taxonomic Assignments in Prokaryotic Genomes at the NCBI. Int. J. Syst. Evol. Microbiol. 2018, 68, 2386–2392. [Google Scholar] [CrossRef]
- Chun, J.; Oren, A.; Ventosa, A.; Christensen, H.; Arahal, D.R.; da Costa, M.S.; Rooney, A.P.; Yi, H.; Xu, X.-W.; De Meyer, S.; et al. Proposed Minimal Standards for the Use of Genome Data for the Taxonomy of Prokaryotes. Int. J. Syst. Evol. Microbiol. 2018, 68, 461–466. [Google Scholar] [CrossRef]
- Zhao, B.; Moody, S.C.; Hider, R.C.; Lei, L.; Kelly, S.L.; Waterman, M.R.; Lamb, D.C. Structural Analysis of Cytochrome P450 105N1 Involved in the Biosynthesis of the Zincophore, Coelibactin. Int. J. Mol. Sci. 2012, 13, 8500–8513. [Google Scholar] [CrossRef]
- Lasch, C.; Gummerlich, N.; Myronovskyi, M.; Palusczak, A.; Zapp, J.; Luzhetskyy, A. Loseolamycins: A Group of New Bioactive Alkylresorcinols Produced after Heterologous Expression of a Type III PKS from Micromonospora endolithica. Molecules 2020, 25, 4594. [Google Scholar] [CrossRef]
- Alarcón, B.; González, M.E.; Carrasco, L. Megalomycin C, a Macrolide Antibiotic That Blocks Protein Glycosylation and Shows Antiviral Activity. FEBS Lett. 1988, 231, 207–211. [Google Scholar] [CrossRef] [PubMed]
- Amsden, G.W. Erythromycin, Clarithromycin, and Azithromycin: Are the Differences Real? Clin. Ther. 1996, 18, 56–72, discussion 55. [Google Scholar] [CrossRef] [PubMed]
- Bonay, P.; Durán-Chica, I.; Fresno, M.; Alarcón, B.; Alcina, A. Antiparasitic Effects of the Intra-Golgi Transport Inhibitor Megalomicin. Antimicrob. Agents Chemother. 1998, 42, 2668–2673. [Google Scholar] [CrossRef]
- Nakornchai, S.; Konthiang, P. Activity of Azithromycin or Erythromycin in Combination with Antimalarial Drugs against Multidrug-Resistant Plasmodium falciparum in Vitro. Acta Trop. 2006, 100, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Waitz, J.A.; Moss, E.L.; Oden, E.M.; Weinstein, M.J. Biological Activity of Megalomicin, a New Micromonospora-Produced Macrolide Antibiotic Complex. J. Antibiot. 1969, 22, 265–272. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Useglio, M.; Peirú, S.; Rodríguez, E.; Labadie, G.R.; Carney, J.R.; Gramajo, H. TDP-L-Megosamine Biosynthesis Pathway Elucidation and Megalomicin A Production in Escherichia coli. Appl. Env. Microbiol. 2010, 76, 3869. [Google Scholar] [CrossRef]
- Volchegursky, Y.; Hu, Z.; Katz, L.; McDaniel, R. Biosynthesis of the Anti-Parasitic Agent Megalomicin: Transformation of Erythromycin to Megalomicin in Saccharopolyspora erythraea. Mol. Microbiol. 2000, 37, 752–762. [Google Scholar] [CrossRef]
- Mulzer, J.; Kirstein, H.M.; Buschmann, J.; Lehmann, C.; Luger, P. Total Synthesis of 9-Dihydroerythronolide B Derivatives and of Erythronolide B. J. Am. Chem. Soc. 1991, 113, 910–923. [Google Scholar] [CrossRef]
- Egan, R.S.; Perun, T.J.; Martin, J.R.; Mitscher, L.A. The Conformation of Erythronolide, the 14-Membered Aglycone Ring of the Erythromycin Antibiotics. Tetrahedron 1973, 29, 2525–2538. [Google Scholar] [CrossRef]
- Nourse, J.G.; Roberts, J.D. Nuclear Magnetic Resonance Spectroscopy. Carbon-13 Spectra of Some Macrolide Antibiotics and Derivatives. Substituent and Conformational Effects. J. Am. Chem. Soc. 1975, 97, 4584–4594. [Google Scholar] [CrossRef]
- Tay, J.-H.; Argüelles, A.J.; DeMars, M.D.; Zimmerman, P.M.; Sherman, D.H.; Nagorny, P. Regiodivergent Glycosylations of 6-Deoxy-Erythronolide B and Oleandomycin-Derived Macrolactones Enabled by Chiral Acid Catalysis. J. Am. Chem. Soc. 2017, 139, 8570–8578. [Google Scholar] [CrossRef]
- Bartner, P.; Boxler, D.L.; Brambilla, R.; Mallams, A.K.; Morton, J.B.; Sancilio, F.D.; Surprenant, H.; Tomalesky, G.; Lukacs, G.; Olesker, A.; et al. The Megalomicins. Part 7. A Structural Revision by Carbon-13 Nuclear Magnetic Resonance and X-Ray Crystallography. Synthesis and Conformational Analysis of 3-Dimethylamino- and 3-Azido-D- and -L-Hexopyranosides, and the Crystal structure of 4″-O-(4-Iodobenzoyl)Megalomicin A. J. Chem. Soc. Perkin Trans. 1 1979, 1600–1624. [Google Scholar]
- Martin, J.R.; Rosenbrook, W. Studies on the Biosynthesis of the Erythromycins. II. Isolation and Structure of a Biosynthetic Intermediate, 6-Deoxyerythronolide B. Biochemistry 1967, 6, 435–440. [Google Scholar] [CrossRef]
- Marquez, J.; Murawski, A.; Wagman, G.H.; Jaret, R.S.; Reimann, H. Isolation, Purification and Preliminary Characterization of Megalomicin. J. Antibiot. 1969, 22, 259–264. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sumner, L.W.; Amberg, A.; Barrett, D.; Beale, M.H.; Beger, R.; Daykin, C.A.; Fan, T.W.-M.; Fiehn, O.; Goodacre, R.; Griffin, J.L.; et al. Proposed Minimum Reporting Standards for Chemical Analysis. Metabolomics 2007, 3, 211–221. [Google Scholar] [CrossRef]
- McDaniel, R.; Thamchaipenet, A.; Gustafsson, C.; Fu, H.; Betlach, M.; Betlach, M.; Ashley, G. Multiple Genetic Modifications of the Erythromycin Polyketide Synthase to Produce a Library of Novel “Unnatural” Natural Products. Proc. Natl. Acad. Sci. USA 1999, 96, 1846–1851. [Google Scholar] [CrossRef]
- Perun, T.J. Chemistry of Erythronolide B. Acid-Catalyzed Transformations of the Aglycon of Erythromycin B. J. Org. Chem. 1967, 32, 2324–2330. [Google Scholar] [CrossRef]
- Cheng, Z.; Zhang, Q.; Peng, J.; Zhao, X.; Ma, L.; Zhang, C.; Zhu, Y. Genomics-Driven Discovery of Benzoxazole Alkaloids from the Marine-Derived Micromonospora sp. SCSIO 07395. Molecules 2023, 28, 821. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, S.; Gong, J.; Zhen, Y. Erythromycin inhibits the proliferation of HERG K+ channel highly expressing cancer cells and shows synergy with anticancer drugs. Zhonghua Yi Xue Za Zhi 2006, 86, 3353–3357. [Google Scholar] [PubMed]
- Hamoya, T.; Miyamoto, S.; Tomono, S.; Fujii, G.; Nakanishi, R.; Komiya, M.; Tamura, S.; Fujimoto, K.; Toshima, J.; Wakabayashi, K.; et al. Chemopreventive Effects of a Low-Side-Effect Antibiotic Drug, Erythromycin, on Mouse Intestinal Tumors. J. Clin. Biochem. Nutr. 2017, 60, 199–207. [Google Scholar] [CrossRef]
- Zhanel, G.G.; Dueck, M.; Hoban, D.J.; Vercaigne, L.M.; Embil, J.M.; Gin, A.S.; Karlowsky, J.A. Review of Macrolides and Ketolides: Focus on Respiratory Tract Infections. Drugs 2001, 61, 443–498. [Google Scholar] [CrossRef] [PubMed]
- Laxmi Pradhan, B.; Lodhi, L.; Kishor Dey, K.; Ghosh, M. Analyzing Atomic Scale Structural Details and Nuclear Spin Dynamics of Four Macrolide Antibiotics: Erythromycin, Clarithromycin, Azithromycin, and Roxithromycin. RSC Adv. 2024, 14, 17733–17770. [Google Scholar] [CrossRef]
- Qadri, H.; Haseeb Shah, A.; Mudasir Ahmad, S.; Alshehri, B.; Almilaibary, A.; Ahmad Mir, M. Natural Products and Their Semi-Synthetic Derivatives against Antimicrobial-Resistant Human Pathogenic Bacteria and Fungi. Saudi J. Biol. Sci. 2022, 29, 103376. [Google Scholar] [CrossRef] [PubMed]
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; International Natural Product Sciences Taskforce; Supuran, C.T. Natural Products in Drug Discovery: Advances and Opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef] [PubMed]
- Subramani, R.; Sipkema, D. Marine Rare Actinomycetes: A Promising Source of Structurally Diverse and Unique Novel Natural Products. Mar. Drugs 2019, 17, 249. [Google Scholar] [CrossRef]
- Jagannathan, S.V.; Manemann, E.M.; Rowe, S.E.; Callender, M.C.; Soto, W. Marine Actinomycetes, New Sources of Biotechnological Products. Mar. Drugs 2021, 19, 365. [Google Scholar] [CrossRef] [PubMed]


| Percentage of Cell Viability a | ||||||
|---|---|---|---|---|---|---|
| MDA-MB-231 Cell Line | HCT-116 Cell Line | RPE1 Cell Line | ||||
| Concentration Tested b | 10 µg/mL or 10 µM | 1 µg/mL or 1 µM | 10 µg/mL or 10 µM | 1 µg/mL or 1 µM | 10 µg/mL or 10 µM | 1 µg/mL or 1 µM |
| Micromonospora sp. SH-82 extract | 101 ± 1.22 | 101 ± 0.67 | 93.1 ± 1.82 | 98.3 ± 1.73 | 94.55 ± 1.89 | 98.34 ± 1.48 |
| Erythronolide B (1) | 100 ± 0.76 | 107 ± 0.86 | 104 ± 1.16 | 101 ± 0.5 | 97.28 ± 1.03 | 101.53 ± 1.58 |
| 6-deoxyerythronolide B (2) | 101 ± 1.13 | 105 ± 1.56 | 103 ± 3.56 | 101 ± 1.62 | 95.28 ± 0.96 | 99.08 ± 1.40 |
| Megalomicin C1 (3) | 98.6 ± 1.38 | 102 ± 1.74 | 97.3 ± 2.22 | 100 ± 1.66 | 103.84 ± 1.94 | 104.19 ± 1.32 |
| Erythronolide B derivative (5) | 99 ± 3.28 | 100 ± 1.74 | 99.7 ± 0.59 | 99.1 ± 0.32 | 103.38 ± 1.77 | 103.19 ± 1.52 |
| IC50 (µg/mL or µM) a,b | |
|---|---|
| Micromonospora sp. SH-82 extract | 16.29 ± 1.22 µg/mL |
| Megalomicin C1 (3) | 6.12 ± 2.87 µg/mL 6.37 ± 2.99 µM |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Le Loarer, A.; Marcourt, L.; Marcellin-Gros, R.; Dufossé, L.; Ramesh, C.; Anwesh, M.; Bignon, J.; Frédérich, M.; Ledoux, A.; Ferreira Queiroz, E.; et al. New Marine Actinobacteria Strain, Micromonospora sp. SH-82: Characterization, Specialized Metabolites and Biological Activities. Microorganisms 2025, 13, 2045. https://doi.org/10.3390/microorganisms13092045
Le Loarer A, Marcourt L, Marcellin-Gros R, Dufossé L, Ramesh C, Anwesh M, Bignon J, Frédérich M, Ledoux A, Ferreira Queiroz E, et al. New Marine Actinobacteria Strain, Micromonospora sp. SH-82: Characterization, Specialized Metabolites and Biological Activities. Microorganisms. 2025; 13(9):2045. https://doi.org/10.3390/microorganisms13092045
Chicago/Turabian StyleLe Loarer, Alexandre, Laurence Marcourt, Rémy Marcellin-Gros, Laurent Dufossé, Chatragadda Ramesh, Maile Anwesh, Jérome Bignon, Michel Frédérich, Allison Ledoux, Emerson Ferreira Queiroz, and et al. 2025. "New Marine Actinobacteria Strain, Micromonospora sp. SH-82: Characterization, Specialized Metabolites and Biological Activities" Microorganisms 13, no. 9: 2045. https://doi.org/10.3390/microorganisms13092045
APA StyleLe Loarer, A., Marcourt, L., Marcellin-Gros, R., Dufossé, L., Ramesh, C., Anwesh, M., Bignon, J., Frédérich, M., Ledoux, A., Ferreira Queiroz, E., Wolfender, J.-L., Fouillaud, M., & Gauvin-Bialecki, A. (2025). New Marine Actinobacteria Strain, Micromonospora sp. SH-82: Characterization, Specialized Metabolites and Biological Activities. Microorganisms, 13(9), 2045. https://doi.org/10.3390/microorganisms13092045












