1. Introduction
During the early life stages of mammals, the colonization of gastrointestinal microbiota is a dynamic process that plays pivotal roles in nutrient digestion, immune system homeostasis and metabolic regulation, thereby contributing significantly to the maintenance of host physiological functions [
1,
2]. Furthermore, the symbiotic relationship between intestinal microbiota and their hosts closely interacts with health status, facilitating gastrointestinal development in the host [
3]. Carbohydrates serve as the primary energy source for animals; however, a critical limitation arises from the fact that most mammalian digestive enzymes in the intestinal tract cannot ferment or degrade plant lignocellulosic material. This host limitation underscores the critical role of symbiotic gut microbiota. For instance, in ruminants, their specialized digestive systems and symbiotic relationships with microorganisms enable gut microbiota to secrete abundant cellulases. These enzymes break down plant lignocellulosic substances into absorbable microbial proteins and volatile fatty acids (VFAs), thereby providing essential nutrients and energy for the ruminants [
4]. Beyond their involvement in nutrient digestion and absorption, microbial communities in the ruminant digestive tract also influence health and reproductive performance [
5]. Under normal physiological conditions, these microbiotas maintain a relatively stable dynamic equilibrium. Nevertheless, when animals are exposed to weakened immunity or other adverse environmental factors (such as a high-carbohydrate diet), the balance of the gastrointestinal microbiota will be disrupted, leading to the occurrence of diseases such as diarrhea, rumen acidosis and enteritis [
6]. Additionally, the distribution of gastrointestinal microbiota varies among individuals due to differences in growth stages, species, diet, living environments and other contributing factors [
7].
For ruminants, the digestive system predominantly operates in a monogastric hindgut fermentation pattern prior to weaning, transitioning to a forestomach fermentation model post-weaning concurrent with rumen development. The mature rumen harbors a highly diverse and dense microbial consortium comprising bacteria, fungi, archaea and other microorganisms, serving as the central organ of the digestive system. Occupying over 50% of the total stomach volume, the rumen plays critical roles in feed storage, rumination and microbial fermentation/digestion of complex plant-based diets such as cellulose [
8]. Given that ruminants primarily rely on rumen microbiota to degrade plant lignocellulosic substances, research on their gastrointestinal microbiota has predominantly focused on forestomach communities, often neglecting intestinal microbiota [
9]. For instance, Hao et al. investigated the rumen microbial community dynamics during weaning transitions in dairy calves, and found that no significant difference in α-diversity or β-diversity was observed between pre-weaning and post-weaning groups [
9]. However, linear discriminant analysis (LDA) demonstrated that the relative abundance of Fibrobacteres was significantly higher in the post-weaning group compared to the pre-weaning group. Additionally, the network node degree of rumen bacterial communities was markedly elevated after post-weaning (16.54 vs. 9.5) [
9]. Similarly, Zhang et al. demonstrated that dietary fiber content can modulate major rumen microbial populations (at phylum and genus levels) in sheep [
10]. Low-fiber energy feeds elevated the Actinobacteria abundance and reduce the Cyanobacteria and
Ruminococcaceae populations, whereas high-fiber diets increased VFAs concentrations and decreased Proteobacteria and
Ruminococcaceae abundances [
10]. These findings suggest that dietary fiber content influences metabolic activities of fiber-degrading bacteria, thereby mediating microbial interactions and competitive dynamics [
10]. Furthermore, longitudinal monitoring of rumen bacterial colonization in pre-weaned ruminants using 16S rDNA amplicon sequencing revealed dynamic, stage-specific and functionally structured microbial establishment during rumen development [
11,
12].
The suckling period represents the most rapid growth phase for lambs and serves as a critical window for the establishment of their intestinal microbiota. However, compared to the extensive research on rumen microbiota, studies focusing on the intestinal microbiome of suckling lambs remain relatively limited. Because the rumen of mammalian ruminants is not yet fully developed and functionally similar to that of monogastric animals, some indigestible nutrients enter the intestines for fermentation [
13]. Malmuthuge et al. identified that the intestinal lumen microbiota of one-week-old calves comprises bacteria, fungi, archaea, protozoa and viruses, with Firmicutes, Bacteroidetes, Proteobacteria and Actinobacteria detected across all intestinal segments [
14]. Concurrently, microbial communities with low biomass and diversity were detected in the intestines of prenatal lambs, mainly composed of Proteobacteria, Firmicutes and actinomycetes. This indicates that microorganisms are already present in the intestines of prenatal lambs, and the colonization of microorganisms in the fetal intestines begins in the uterus [
15,
16]. Contrarily, other investigations assert that the fetal gut of healthy sheep remains sterile during gestation, with microbial colonization commencing only after amniotic membrane rupture during parturition [
17]. This controversy regarding the timing of intestinal microbiota establishment (prenatal vs. postnatal) in lambs necessitates further systematic investigation to resolve existing discrepancies. The first month postpartum represents a critical window for the development of gastrointestinal microenvironments in lambs. During this period, the structural dynamics of intestinal microbiota exhibit high instability, with colonization processes co-regulated by feeding regimens, dietary transitions, host age, environmental exposures and other interacting factors. Beyond the strong constraints imposed by the above factors on microbial colonization, random colonization in the early stage of life may have a lasting impact on the long-term development of animal microbial communities [
18]. As the most abundant and phylogenetically diverse microbial group in pre-weaned ruminants, bacteria play pivotal roles in fiber degradation, immune modulation, VFAs production and gastrointestinal morphological development—processes fundamental to nutrient acquisition and energy metabolism in pre-weaned lambs [
18]. Despite high-throughput sequencing technology and data analysis methods have significantly deepened our understanding of the functions and diversity of gut microbiota, the temporal colonization patterns of bacterial communities in suckling lambs remain poorly characterized. During production, lambs usually start to consume solid feed after 7 days of age. By 28 days of age (lasting for three weeks), the microbiota in their intestines may enter a relatively stable stage after undergoing the screening pressure of dietary transition. Therefore, this study aims to analyze the bacterial colonization dynamics of the jejunum, ileum and cecum of lambs at three critical developmental time points (0, 7 and 28 days of age), to clarify the evolution pattern of the intestinal microbiota with age and intestinal segments, and to provide a theoretical basis for promoting the healthy growth and improving production efficiency of young ruminants through targeted regulation of the intestinal microbiota.
2. Materials and Methods
2.1. Animals and Sample Collection
The animal experimental procedures were approved by the Institutional Animal Care and Use Committee of Guangdong Ocean University (Zhanjiang, Guangdong Province, China; Approval Code: SYXK-2023-032) in compliance with the Regulations for the Administration of Laboratory Animals (issued by the State Science and Technology Commission of the People’s Republic of China, 2013).
A total of 24 male neonatal Hu sheep lambs with similar body weights (2.94 ± 0.22 kg) were selected as experimental subjects. At 0, 7 and 28 days of age, 5 lambs were randomly selected from each time point for slaughter and sampling, resulting in a total of 15 lambs used in the trial. Notably, lambs slaughtered on 0 days of age had not received colostrum, while the remaining lambs were bottle-fed colostrum in three equal portions (300 mL initially, followed by 150 mL each in subsequent feedings) within 18 h post-birth. The colostrum used in the experiment was collected via manual mammary gland massage of ewes, immediately sealed, and stored under frozen conditions. Prior to each feeding, colostrum was thawed in a 36 °C water bath and administered quantitatively via bottle. Beyond colostrum intake, all lambs were bottle-fed ewe milk three times daily at 15% of their body weight. Immediately after birth, all lambs were separated from their dams and, from 7 days of age onward, and began to freely feed on alfalfa hay and starter feed. The detailed composition and nutritional profiles are provided in
Supplementary Table S1. Experimental lambs were individually identified with ear tags and housed collectively in an 8 m × 8 m dedicated pens with straw bedding renewed every 3 days. Throughout the experimental period, lambs had free access to clean drinking water, with both ewe milk and water maintained at temperatures between 32 °C and 36 °C.
Lambs slaughtered on 7 and 28 days of age were subjected to a 12 h fasting period prior to euthanasia, which was performed via captive bolt stunning followed by humane exsanguination. All slaughter procedures adhered to the National Standard Operating Procedures (GB/T 43562-2023) [
19]. Postmortem, the abdominal cavity was opened, and the jejunum, ileum and cecum were carefully isolated using nylon ropes to prevent chyme backflow between adjacent intestinal regions. Chyme samples were collected from each intestinal segment, transferred into sterile centrifuge tubes, and immediately flash-frozen in liquid nitrogen. A total of 45 intestinal content samples were stored at −80 °C until total DNA extraction.
2.2. DNA Extraction
Total DNA was extracted from thawed (4 °C) intestinal chyme samples (200 mg) using the ZymoBIOMICS DNA Microprep Kit (D4301, Zymo Research, Irvine, CA, USA). This kit employs an optimized lysis buffer system for rapid (30 min) DNA isolation from high-inhibitor matrices while ensuring DNA purity and compatibility with downstream sequencing applications. Extraction procedures were performed in strict accordance with the manufacturer’s protocols. DNA integrity was verified via 0.8% agarose gel electrophoresis, followed by nucleic acid quantification using the Tecan F200 platform with PicoGreen dye-based fluorescence detection. Extracted DNA samples were normalized to 10 ng/μL using sterile ultrapure water and stored at −80 °C pending further molecular analyses.
2.3. PCR Amplification
The 16S rRNA V4 region was amplified from all DNA samples (
n = 45) using conventional PCR with universal primers 515F (5′-GTGYCAGCMGCCGCGGTAA-3′) and 806R (5′-GGACTACHVGGGTWTCTAAT-3′), each incorporating a 12 nt unique barcode sequence [
20]. PCR reactions were conducted in 50-μL mixtures containing 1 × PCR buffer, 5 μL of 2 mM dNTP mix, 3 μL of 25 mM MgSO
4, 1.5 μL of each primer, 1 U of KOD-Plus-Neo DNA polymerase, and 20 ng of template DNA. Amplification was performed on an Applied Biosystems GeneAmp PCR System 9700 (Thermo Scientific, Waltham, MA, USA) under the following conditions: initial denaturation at 94 °C for 1 min, followed by 25–30 cycles of denaturation at 94 °C for 20 s, annealing at 54 °C for 30 s, and extension at 72 °C for 30 s, with a final extension at 72 °C for 5 min and termination at 4 °C. Technical triplicates were performed for each sample, and PCR products from the exponential phase were pooled equimolarly for library construction. Amplicons were mixed with 6 × loading dye, resolved on 2% agarose gels for target fragment verification, and purified using the Zymoclean Gel Recovery Kit (D4008). Purified products were quantified via Qubit 2.0 Fluorometer (Thermo Scientific, Waltham, MA, USA) and pooled at equimolar concentrations for subsequent sequencing analyses.
2.4. High-Throughput Sequencing and Sequencing Data Analysis
Library preparation was performed using the NEBNext Ultra II DNA Library Prep Kit for Illumina (NEB #E7645L, New England BioLabs, Ipswich, MA, USA). Sequencing was conducted on an Illumina NovaSeq 6000 platform using the NovaSeq 6000 SP Reagent Kit v1.5 (Illumina, San Diego, CA, USA) with 2 × 250 bp paired-end (PE) sequencing configuration.
Paired-end reads were merged using FLASH (v1.2.11). Using saber, the sequences of each sample were separated from raw reads based on barcodes, and the barcode sequences were truncated. Sequences were quality filtered using QIIME2 (v2020.2) with the following criteria: exclusion of sequences with mean quality scores < 30, removal of sequences < 200 bp in length, and elimination of reads containing ambiguous bases (N > 0). Denoising and chimera removal were conducted via the Deblur algorithm in QIIME2 (v2024.10), generating amplicon sequence variant (ASV) feature tables and representative sequences. Taxonomic classification was performed using a Naive Bayes classifier trained on the SILVA database (v138), which was also used for ASV annotation. Representative sequences were aligned using QIIME2 (v2024.10)’s multiple sequence alignment tool, and phylogenetic trees were constructed using the FastTree plugin. Homogenize each sample and resampling based on the one with the least data volume among the samples.
All statistical analyses were performed using R (v4.0.5). Phylogenetic diversity (PD) was calculated with the Picante package (v1.8.2), while other alpha and beta diversity metrics were computed using the Vegan package (v2.6-4). Bray–Curtis dissimilarity matrices were generated via Vegan’s vegdist function, and principal coordinate analysis (PCoA) was conducted using the ape package (v5.7-1). Analysis of similarities (ANOSIM) and permutational multivariate analysis of variance (PerMANOVA) were performed using Vegan’s anosim and adonis functions, respectively. Each row is normalized through Z-score standardization (z value = [Actual relative abundance of a certain genus in a specific intestinal region—average relative abundance of the genus in the intestine]/standard deviation), generating a heat map. This method is used to screen dominant genera and conduct cluster analysis at the regional level.
2.5. PICRUST2 Prediction
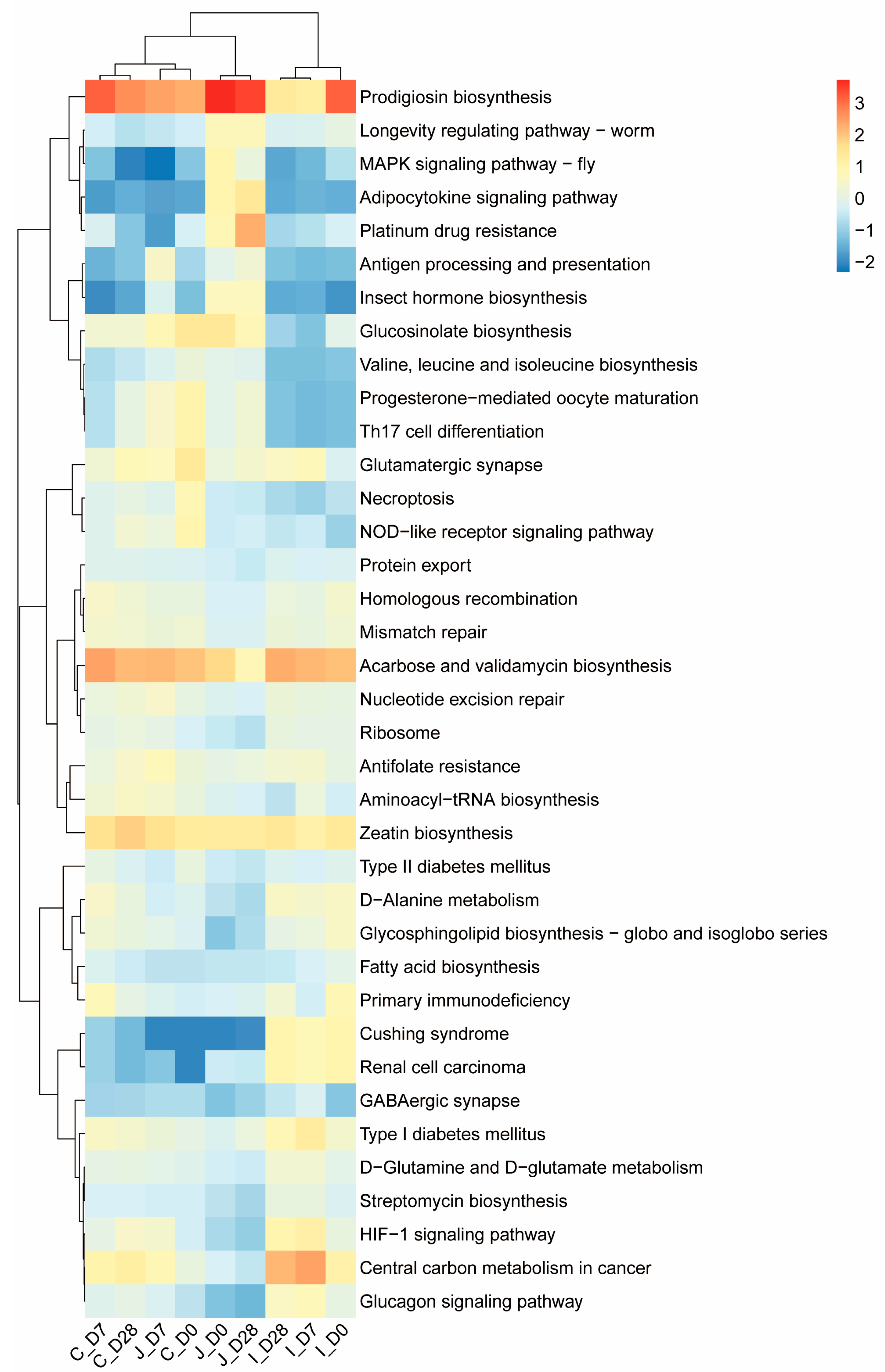
Phylogenetic Investigation of Communities by Reconstruction of Unobserved States 2 (PICRUSt2), an advanced evolution of the original PICRUSt framework, is currently the most frequently cited functional prediction tool based on amplicon sequencing. In this study, we leveraged PICRUSt2 to predict molecular functional potentials for each sample using 16S rRNA gene data. This method infers Kyoto Encyclopedia of Genes and Genomes (KEGG) Ortholog (KO) metabolic pathways by incorporating phylogenetic placement of OTUs along with 16S rRNA gene copy number-corrected abundances. Functional predictions were analyzed at three hierarchical levels of the KO pathway ontology. Following z-score standardization, heatmaps were generated to identify dominant pathways at level 3 and perform regional-level clustering analysis of microbial functional profiles.
2.6. Statistical Analysis
All data analyses were conducted using SPSS software (version 26 for Windows, SPSS, Chicago, IL, USA). Data were first tested for normality (Shapiro-Wilk test) and homogeneity of variances (Levene’s test). One-way analysis of variance was employed to compare α-diversity indices and bacterial relative abundances among lambs at 0, 7 and 28 days of age. For data failing normality or homogeneity assumptions, non-parametric alternatives (Kruskal–Wallis test) were applied. Results are presented as mean ± standard error (SE). Statistical significance was set at p < 0.05, with trends reported for 0.05 < p < 0.10.
4. Discussion
There are a large number of microbial communities in the gastrointestinal tract of ruminants, including bacteria, Archaea, and anaerobic fungi. These microbial communities coexist and restrict each other with the ruminant body, maintaining the stability of the intestinal microbial environment of ruminants in a long-term dynamic manner, which plays a crucial role in the health and production of ruminants. While numerous studies have investigated the dynamic distribution and colonization patterns of gastrointestinal microbiota in ruminants, research on microbial communities in the juvenile ruminant digestive system has largely been confined to the rumen and fecal microbiota [
21,
22]. This focus stems from the rumen’s status as the primary fermentative organ and the non-invasive nature of fecal sampling, which has garnered considerable attention from relevant research scholars [
23]. However, emerging evidence indicates that rumen and fecal microbiota inadequately represent microbial communities in other intestinal segments [
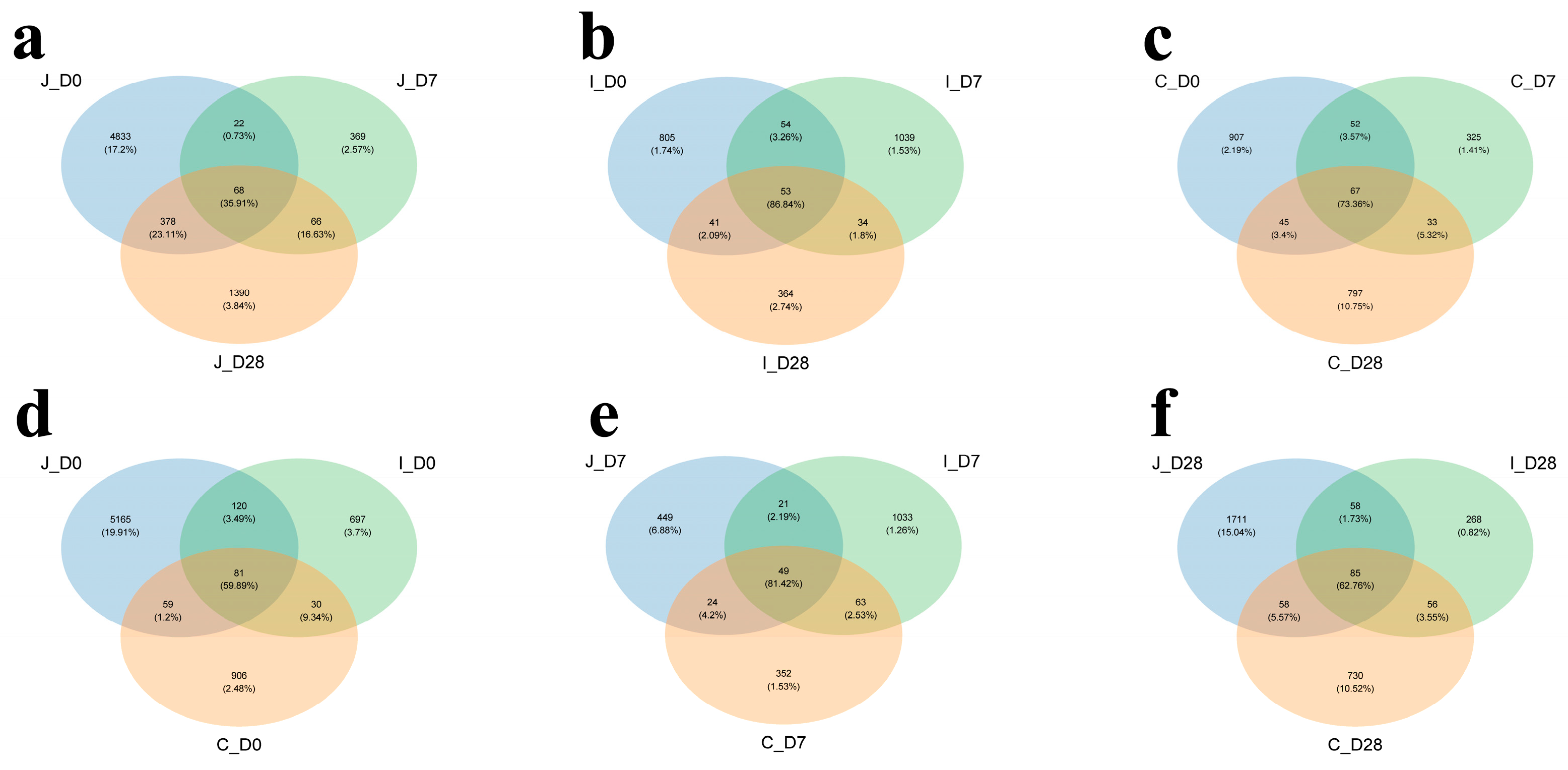
24]. Consequently, the bacterial communities in each segment of the intestinal tract still lack sufficient characteristic descriptions, and little is known about the colonization of the microbiota in the intestines of young ruminants. Therefore, this study aims to gain a deeper understanding of the bacterial composition and colonization in the jejunum, ileum and cecum regions of lambs on 0, 7 and 28 days of age.
The α-diversity refers to the biodiversity within a specific sample or habitat, with common evaluation metrics including the Chao1, PD, Simpson and Shannon diversity indexes, etc. [
25] The Chao1 index primarily estimates the total number of species in a community, with particular emphasis on rare species. The PD (Phylogenetic Diversity) index evaluates the evolutionary history or lineage diversity embedded within a community based on phylogenetic relationships among species. The Simpson diversity index focuses on community dominance, measuring the probability that two randomly selected individuals belong to the same species. The Shannon diversity index integrates both species richness and evenness information. A previous study has demonstrated that among gastrointestinal bacteria in goats, the highest alpha diversity is found in the four chambers of the stomach (rumen, reticulum, omasum and abomasum), followed by the duodenum and posterior intestine (cecum, colon and rectum), while the lowest alpha diversity is observed in the jejunum and ileum [
26]. Result mentioned earlier indicates significant variations in bacterial richness and diversity as milk and chyme transition between intestinal regions, which is consistent with the present study. This phenomenon may be attributed to differences in intestinal motility, redox potential, physiological functions and pH levels across distinct intestinal regions. Furthermore, bacterial richness and diversity may differ across age groups even within the same intestinal segment. While lamb intestinal microbiota typically exhibit increasing richness and diversity with age, this ecological succession remains fragile and dynamic, influenced by maternal microbial transmission, environmental temperatures, stressors, host developmental stages and dietary transitions. Our results demonstrate distinct α-diversity patterns across intestinal regions and age groups: in the jejunum, the 0-day-old group exhibited the highest bacterial richness and diversity, followed by the 28-day-old group, with the 7-day-old group showing the lowest values; in the ileum, while α-diversity indices showed no significant age-related differences, the 28-day-old group displayed the highest diversity; in the cecum, the 28-day-old group presented the greatest diversity, followed by the 0-day-old group, with the 7-day-old group again showing the lowest values. However, the α diversity indices among different intestinal regions within the same age group showed significant differences. Lv et al. conducted a study on the effects of early supplementation of starter on rumen development and microbial communities [
27]. The study found that the rumen microbial diversity of the group fed with milk replacer and starter (Group S) was lower than that of the group fed with milk replacer (Group C). However, supplementing the animals with appetizers before they were weaned was beneficial for promoting the development of rumen microorganisms [
27]. In the present study, lambs in the 28-day-old group received solid feed supplementation in addition to milk, resulting in the highest bacterial diversity and richness observed in both the ileum and cecum of 28-day-old lambs. This phenomenon may be attributed to solid feed intake which can stimulate gastrointestinal tract development, alter the physicochemical environment of the digestive tract and provide more diverse and complex fermentation substrates for gut microbiota. Additionally, the highest bacterial richness and diversity observed in the jejunum of day-0 lambs could be explained by the fact that the jejunum serves as the primary site for receiving nutrients and microorganisms from chyme in the middle digestive tract. Newborn lambs are initially exposed to microorganisms derived from the maternal vagina, feces and skin, and subsequently ingest high concentrations of maternal microbiota through colostrum suckling. These microbial populations rapidly colonize the jejunum, forming a transient state of high diversity. Supporting this observation, a related study found that the intestinal microbiota of bottle-fed lambs within 3 days of age is primarily composed of bacteria from the maternal vagina (46%), ambient air (30%), and pen floor (12%) [
15]. These bacterial communities rapidly colonized in the jejunum, resulting in instantaneous high diversity. The 7-day-old group, lacking solid feed intake and remaining in an unstable transitional phase, displayed the lowest α-diversity. In addition, PCoA based on the Bray-Curtis difference matrix and PERMANOVA separated different intestinal regions of the same age group and different age groups of the same intestinal region. The results showed that distinct clustering patterns in jejunal microbiota among the three age groups, as well as clear separation between three intestinal regions in 0-day-old and 7-day-old lambs. The results of this study indicate that the intestinal bacterial community structure of lambs shows dynamic changes with the increase in age and different intestinal regions. This change reflects that during the growth and development of lambs, the population structure of the gastrointestinal flora undergoes dynamic changes according to the host’s gastrointestinal location, diet, environment and age [
28,
29].
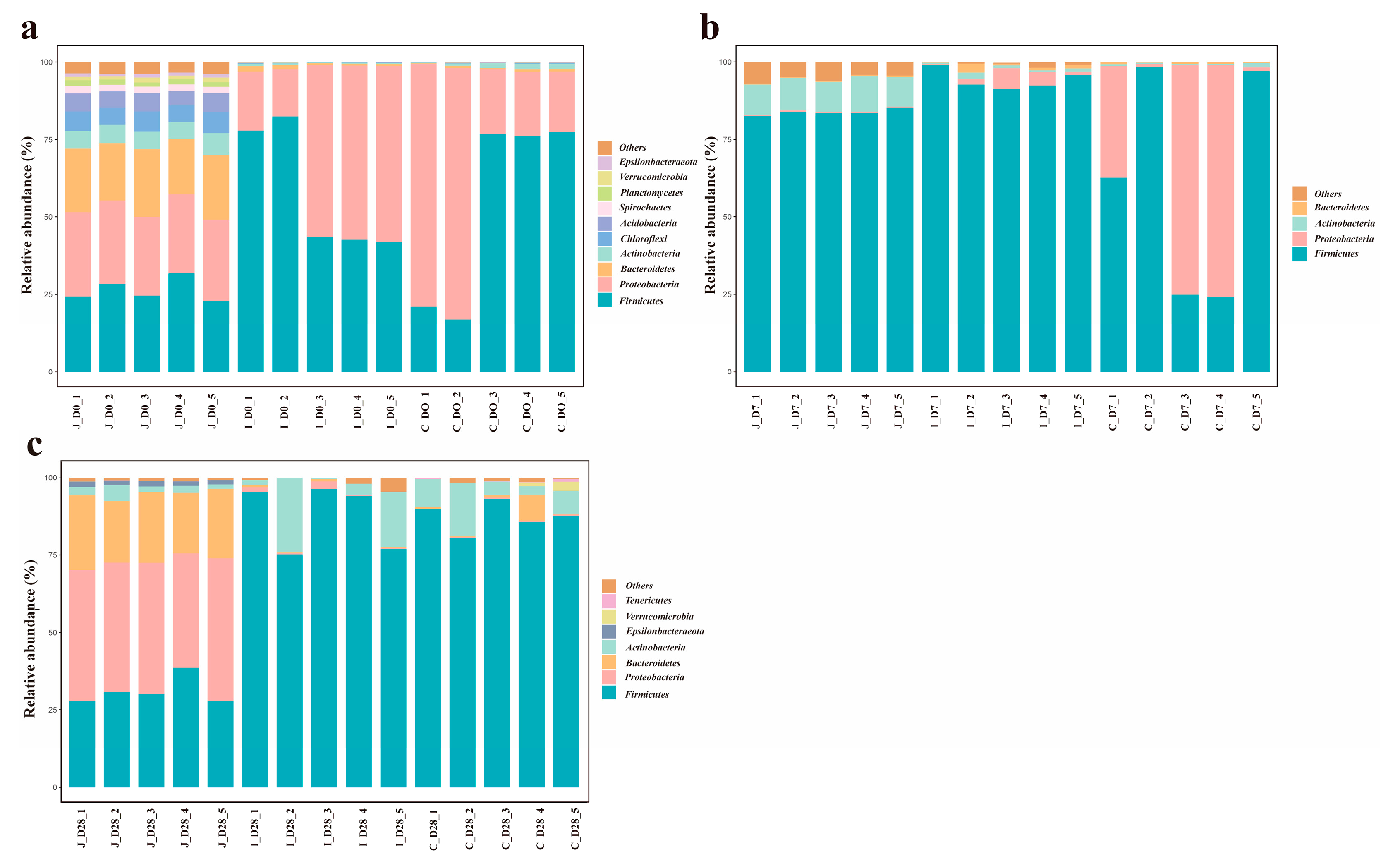
The distribution of microorganisms in the animal’s intestinal tract shows an uneven feature, which will lead to differences in the quantity and types of microorganisms in different intestinal regions. Across all chyme samples, 1062 bacterial genera spanning 42 phyla were identified. Firmicutes and Proteobacteria dominated all intestinal segments, which were in accordance with findings in cattle [
30], yaks [
31], sheep [
32] and goats [
33], but different from microbiome profiles of camels [
34]. In the intestine of camels, the Firmicutes is the most abundant phylum, followed by Verrucomicrobia. Although ruminants vary in species, the dominant bacterial phyla in some intestinal regions are similar [
35]. The ubiquity of these phyla in ruminants indicates that they play a key role in the intestinal microbiota ecology of ruminants. In addition, these two bacterial phyla account for over 85% of all bacteria, while Bacteroidetes and Actinomycetes also have a certain proportion of distribution. Moreover, the relative abundance of these major phyla varies in the intestinal region of lambs at different ages, and there may be a dynamic balance among them. Consistent with previous reports [
36,
37], Bacteroidetes and Firmicutes were followed by Actinobacteria, Proteobacteria and Spirochaetes as key contributors to polysaccharide and aromatic compound degradation. In addition, it was found in this study that the relative abundance of Firmicutes in the ileum of lambs at 7 days and 28 days of age was significantly higher than that of lambs at 0 days of age, while the Proteobacteria showed the opposite trend. This indicates that in 0-day-old lambs, due to the higher oxygen content in the intestinal tract, it is conducive to the colonization of aerobic/facultative anaerobic bacteria such as Proteobacteria. However, after 7 days of age, a strict anaerobic environment is formed in the intestinal tract, and the relative abundance of Firmicutes increases, which is conducive to the degradation of complex polysaccharides by fiber-degrading bacteria. Zhang et al. employed high-throughput sequencing technology to study the structural composition and spatial distribution characteristics of the intestinal microbial community in small-tailed Han sheep [
38]. They found that the dominant bacterial phyla in the cecum and rectum of small-tailed Han sheep were Firmicutes and Bacteroides, while the relative abundance of Firmicutes and Cyanobacteria was higher in the jejunum [
37]. Meanwhile, the intestinal microbiota of calves before weaning is mainly composed of Firmicutes, Bacteroides and Proteobacteria. This characteristic of the microbiota in the gastrointestinal tract is similar to that of adult cattle, indicating that the core bacterial community in the intestines of these mature animals was formed in the early stage of life. Our research results are consistent with this. The results of this study indicate that Firmicutes and Bacteroidetes dominate the gastrointestinal microbiota and play a significant role in the digestion and absorption of proteins and carbohydrates in the gastrointestinal tract of ruminants. In addition, the Firmicutes phylum occupies a relatively high proportion in multiple age groups and sites. It not only plays a significant role in the process of energy absorption but also participates in the degradation of oligosaccharides, cellulose and starch, which helps lambs maintain intestinal energy supply, internal environment stability and digest and absorb nutrients. The Proteobacteria phylum may be related to the early immune response in the lamb’s intestinal tract and its resistance to the invasion of external pathogens. This study is conducive to a deeper understanding of the succession patterns of the intestinal flora in lambs during their growth and development, providing a theoretical basis for improving the health and production performance of lambs by regulating the intestinal flora.
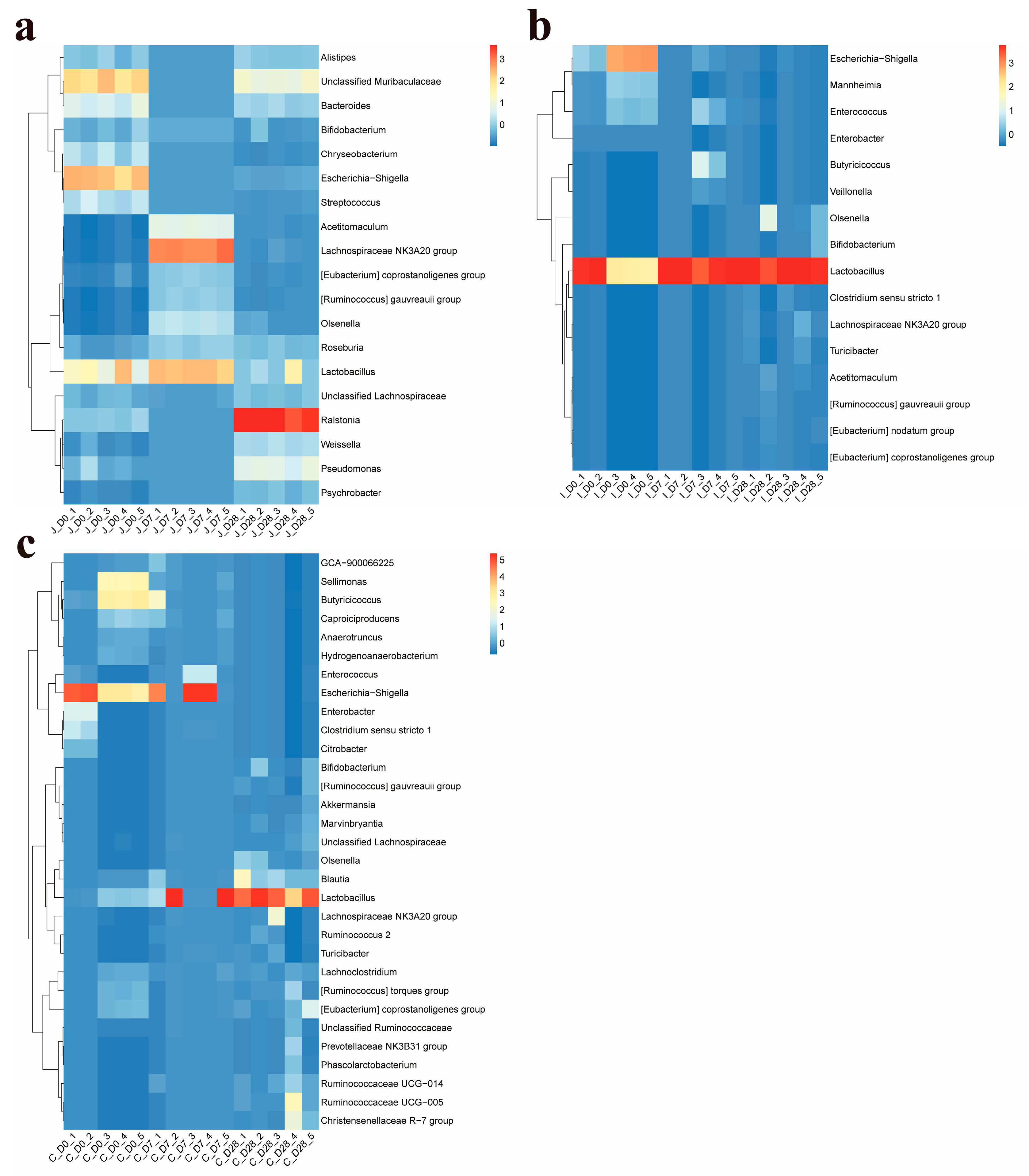
In this study, the core bacterial genera, in addition to the above four major phyla, also include the
Verrucomycota phylum. Relevant studies have shown that some bacterial species, including
Prevotella,
Bacteroides and
Ruminococcus, already exist in the gastrointestinal tract of newborn calves [
39,
40]. These bacteria mainly participate in the degradation of fiber and starch, and this phenomenon is usually found in adult ruminants. Notably, these microbial communities establish in 1-week-old calves receiving only liquid diets, indicating early-life colonization of beneficial digestive bacteria across gastrointestinal segments even in the absence of solid feed substrates [
40]. Our lamb study parallels these findings, demonstrating similar early microbial establishment. Furthermore, Bi et al. identified
Lactobacillus as a dominant maternal-derived genus in lamb jejunum [
15], a pattern corroborated by our detection of consistently high
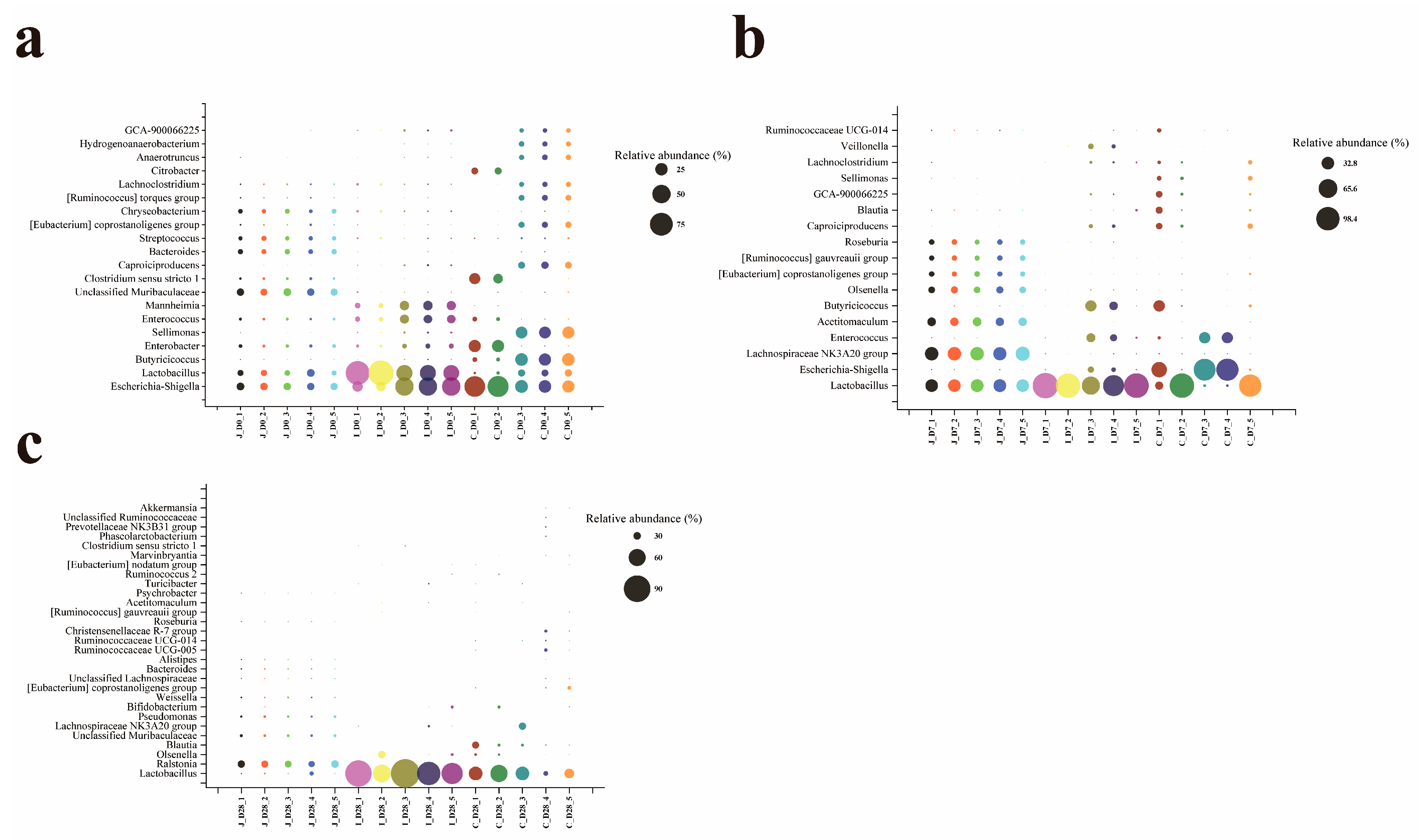
Lactobacillus abundance across all examined ages (0, 7 and 28-day-old lambs). As key drivers of small intestinal microbiome succession, Lactobacillus produce lactic acid, creating an acidic milieu that suppresses pathogens while promoting commensal proliferation, thereby maintaining intestinal homeostasis. In addition, the research found that different feeding methods of lambs also have a certain impact on the microorganisms in their intestines. Compared with breastfeeding, bottle feeding significantly increased the abundance of
Escherichia/Shigella,
Butyricicoccus and
Clostridium XIVa. The increase in the content of
Escherichia/Shigella indicates that artificial feeding may increase the number of potential pathogens [
15]. This phenomenon is consistent with the results of this study. With the increase in age, the number of
Escherichia/Shigella in the jejunum, ileum and cecum of lambs decreases, indicating that the intestinal environment of lambs is gradually maturing and their immune system is in the process of continuous development and improvement. The reduction in
Escherichia/
Shigella may reflect the effective control of these pathogens by the host immune system. Different types of bacterial genera exist in different parts of the gastrointestinal tract of ruminants, and the relative abundance of these bacterial genera varies significantly in different parts of the gastrointestinal tract. In 3-day-old lambs, the jejunum is the main site for food digestion and absorption. Research has found that the most abundant genus of bacteria in the jejunum during this period is
Bacteroides [
15], which is different from the results of this study. Another study has found that compared with Bacteroides strains, the main bacterial genera of the Firmiculata phylum are more concentrated in the intestine rather than the rumen [
41]. The results of this study indicate that
Lactobacillus and
Bifidobacterium, among others, have already begun to colonize initially from the environment or the mother’s body at birth in lambs. They may serve as the foundation for the establishment of beneficial bacteria in the intestines of newborn lambs, facilitating their initial adaptation to the external environment and digestion of breast milk. With the increase in age, the abundance of related genera such as
Ruminococcus and
Helicobacter increases. These genera play an important role in the generation of broken-chain fatty acids and the degradation of carbohydrates.
Based on PICRUSt2, the molecular functions of the intestinal bacterial community were predicted. At the KEGG level 2, the most significant functional categories were gene families related to cell motility, aging, translation, replication and repair, drug resistance, antineoplastic and nucleotide metabolism, and folding, sorting, and degradation. This is different from most current studies on weaned lambs over 60 days old or adult ruminants, where the majority of functional genes belong to carbohydrate metabolism, amino acid metabolism, membrane transport and energy metabolism, etc. In this study, although genes related to carbohydrate metabolism and amino acid metabolism also accounted for a certain proportion, they were not the main functional categories. The essence of the results stems from the particularity of the developmental stage of young lambs and the priority of microbial survival strategies. Because lactose in milk can be directly degraded by lactase, microorganisms do not need to be involved in large quantities. It is not until 28-day-old lambs (who have already consumed solid feed) that the need for the degradation of fiber and starch becomes prominent. In the 0-days-of-age lambs, the intestinal microbiota, in order to seize ecological niches and multiply rapidly, thus functional genes such as cell motility, replication and repair and translation are dominant. In the 7 days of age lambs, the intestinal microorganisms adapt to the milk environment. The main functions of the microorganisms are to resist acidic stress and maintain protein stability. Therefore, functional genes such as folding, sorting and degradation and translation are dominant. Among the 28 days of age lambs, due to the intake of solid feed after 7 days of age, the relative abundance of various function-related gene families showed significant differences among different intestinal regions, indicating that the functional development of the microbial community was relatively mature at this time, and the functional differentiation in different regions was obvious. The microorganisms in the intestines of newborn lambs are confronted with three survival pressures: host immune attack (requiring resistance genes), antibacterial substances in breast milk (requiring erroneous protein repair) and niche competition (movement, repair and translation). Therefore, the results of this study reveal the core biological laws of the development of intestinal microorganisms in young ruminants. That is, the transformation from the early stage of planting and survival to the later stage where nutrient metabolism is the main focus. Meanwhile, these findings reveal regional differences in the functional communities of gut microbiota, which are closely related to the characteristics of different gastrointestinal segments and their microbiota interactions [
42].
Taken together, this phenomenon is attributed to the complex system of the ruminant digestive tract, where different segments perform varied functions. In 0- to 28-day-old lambs, the diversity and composition of intestinal microbial communities demonstrate significant disparities, likely resulting from physiological and biochemical variations across intestinal regions, including factors such as oxygen content, redox potential, and pH values. The colonization of intestinal microbes progresses from an unstable initial stage to a transitional phase, gradually stabilizing under the influence of multiple factors. In this study, although the lambs were fed a fixed diet after 7 days of age, this period still represents a transitional phase in microbial community development rather than a relatively stable stage. Consequently, the gastrointestinal microbial communities remain dynamic across both temporal and spatial dimensions during this phase. Investigating and elucidating the composition and colonization patterns of gut microbiota in ruminants at different ages and intestinal regions, as well as their interactions, can enhance our understanding of the microbiome’s role in nutrient digestion.