Functional Characteristics of Fungal Communities in the Rhizosphere of the Endangered Plant Abies ziyuanensis
Abstract
1. Introduction
2. Materials and Methods
2.1. Overview of the Research Area and Collection of Soil Samples
2.2. Determination of Soil Physicochemical Properties
2.3. Rhizospheric Soil Fungal DNA Extraction, PCR Amplification, and High-Throughput Sequencing
2.4. Bioinformatics Analysis
2.5. Statistical Analysis of Data
3. Results
3.1. Soil Physicochemical Properties of the Rhizosphere of A. ziyuanensis
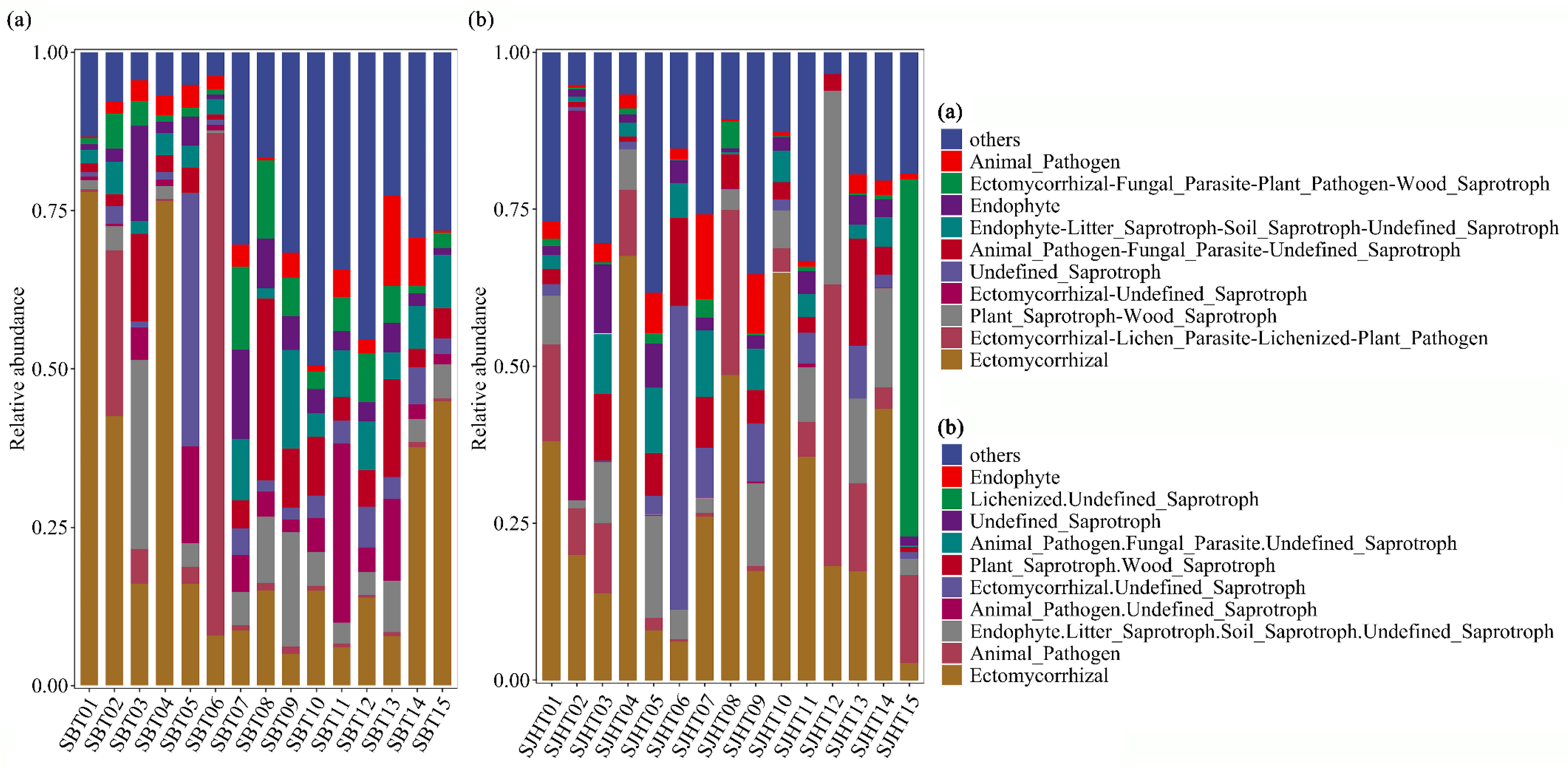
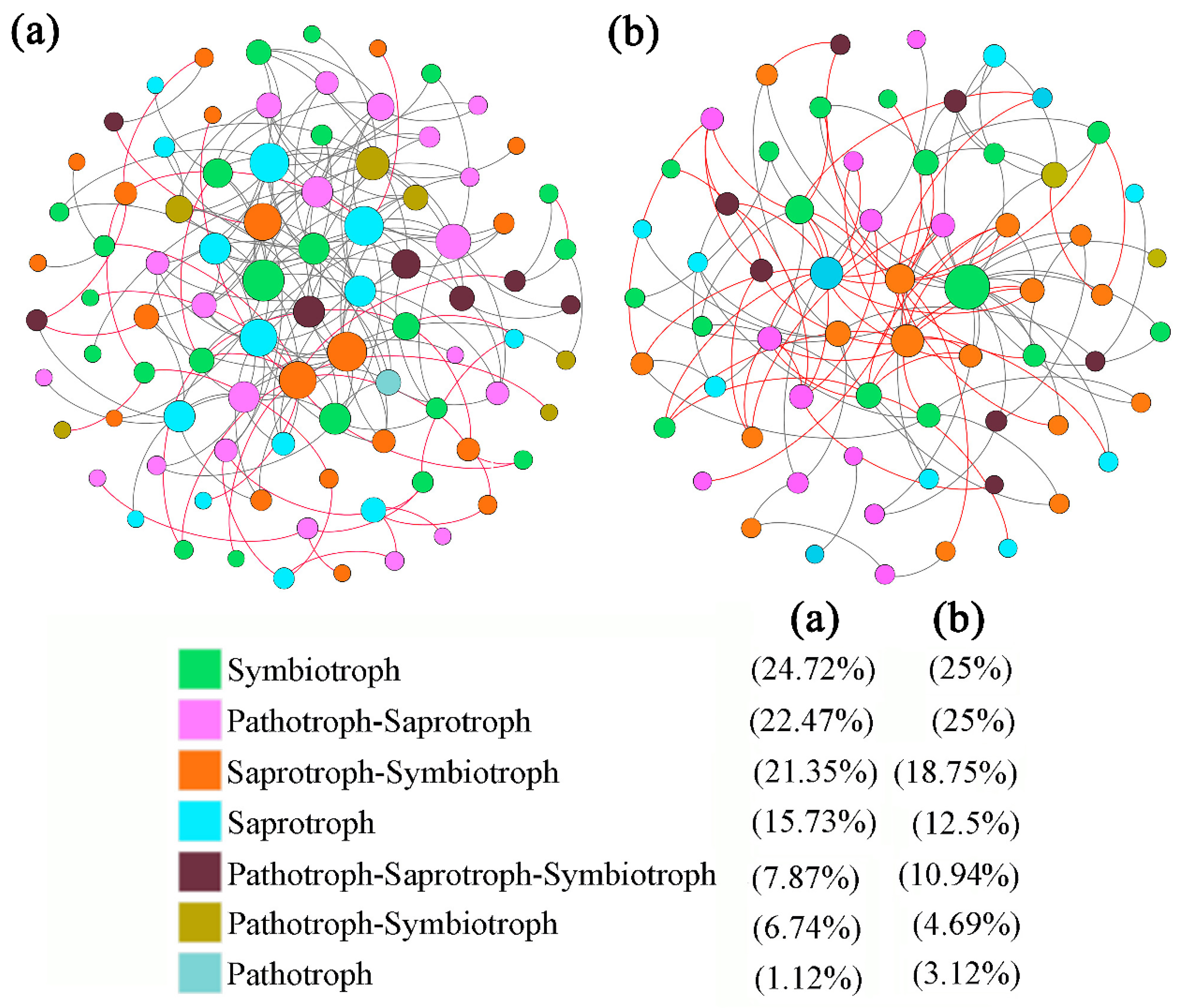
3.2. Trophic Modes and Functional Guilds of Rhizospheric Soil Fungal Communities of A. ziyuanensis
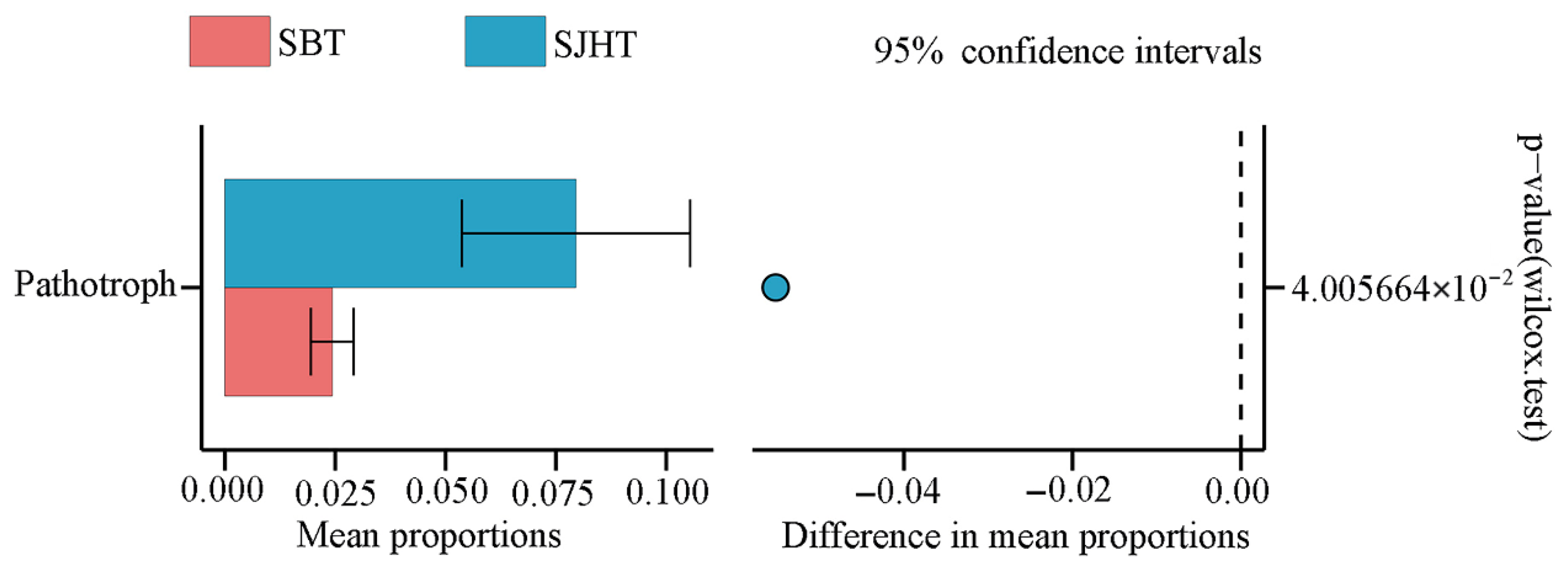
3.3. Differences in Trophic Modes and Functional Guilds of Rhizospheric Soil Fungal Communities of A. ziyuanensis
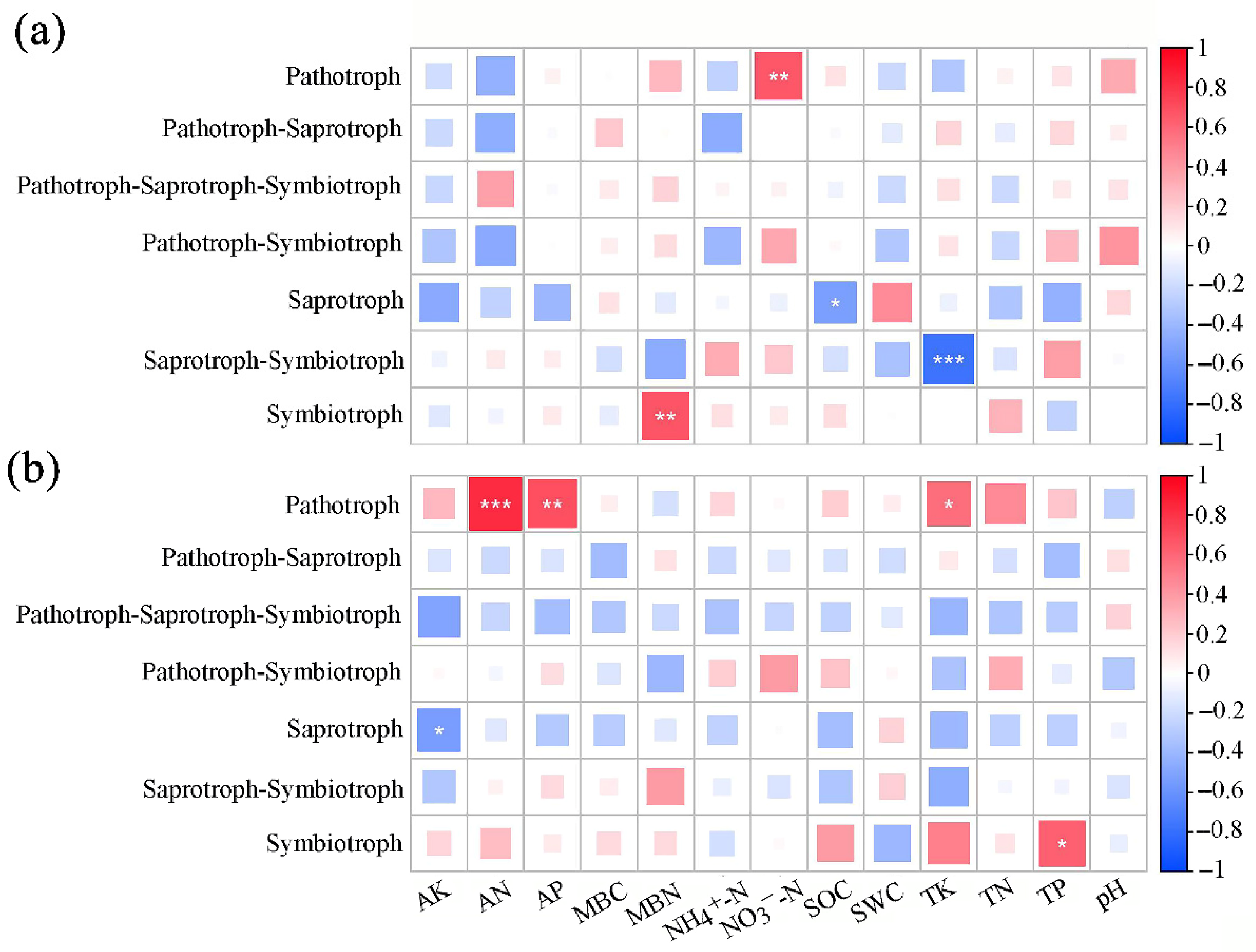
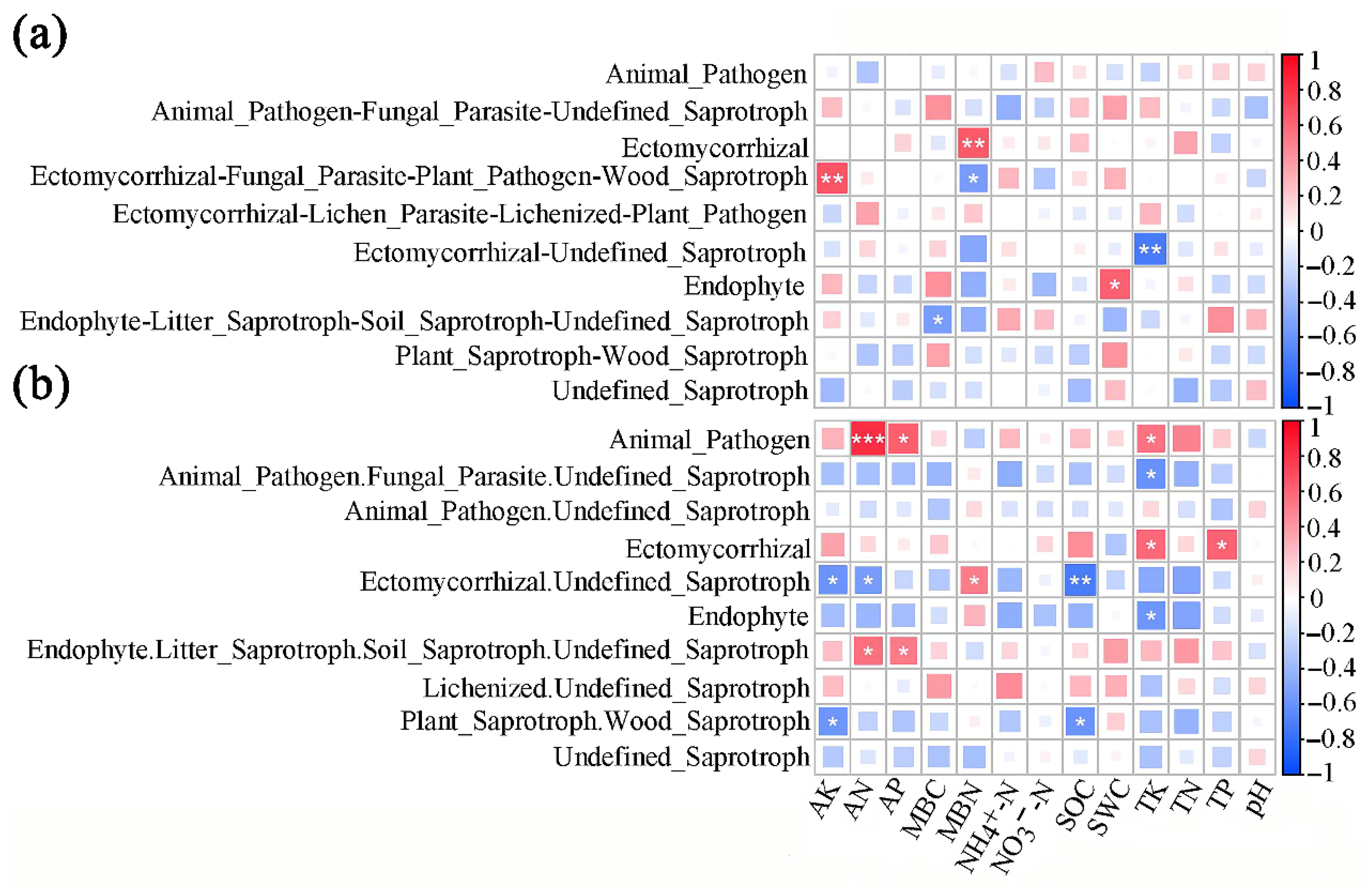
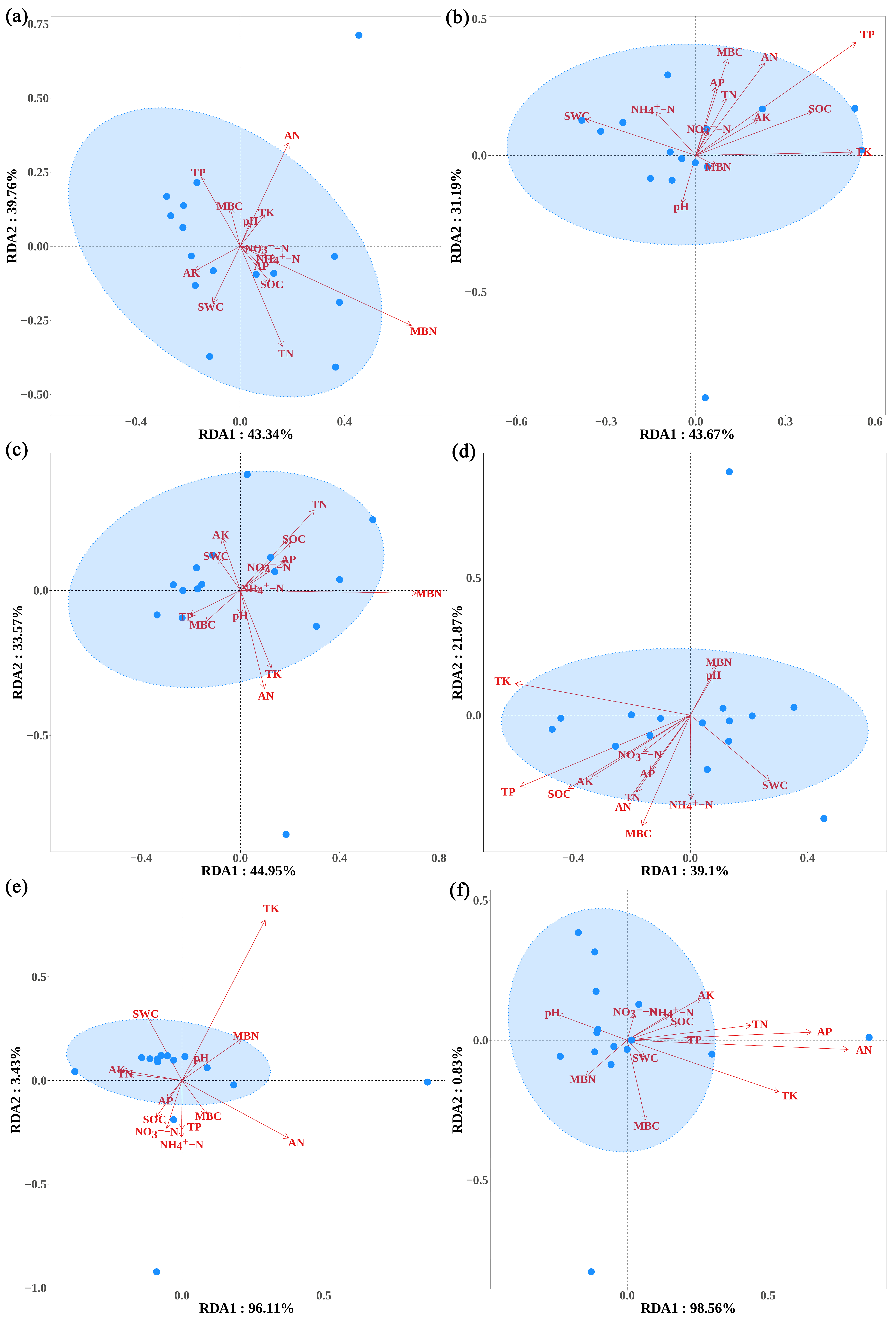
3.4. The Influence of Soil Physicochemical Properties on the Trophic Modes and Functional Groups of Fungal Communities in the Rhizosphere of A. ziyuanensis
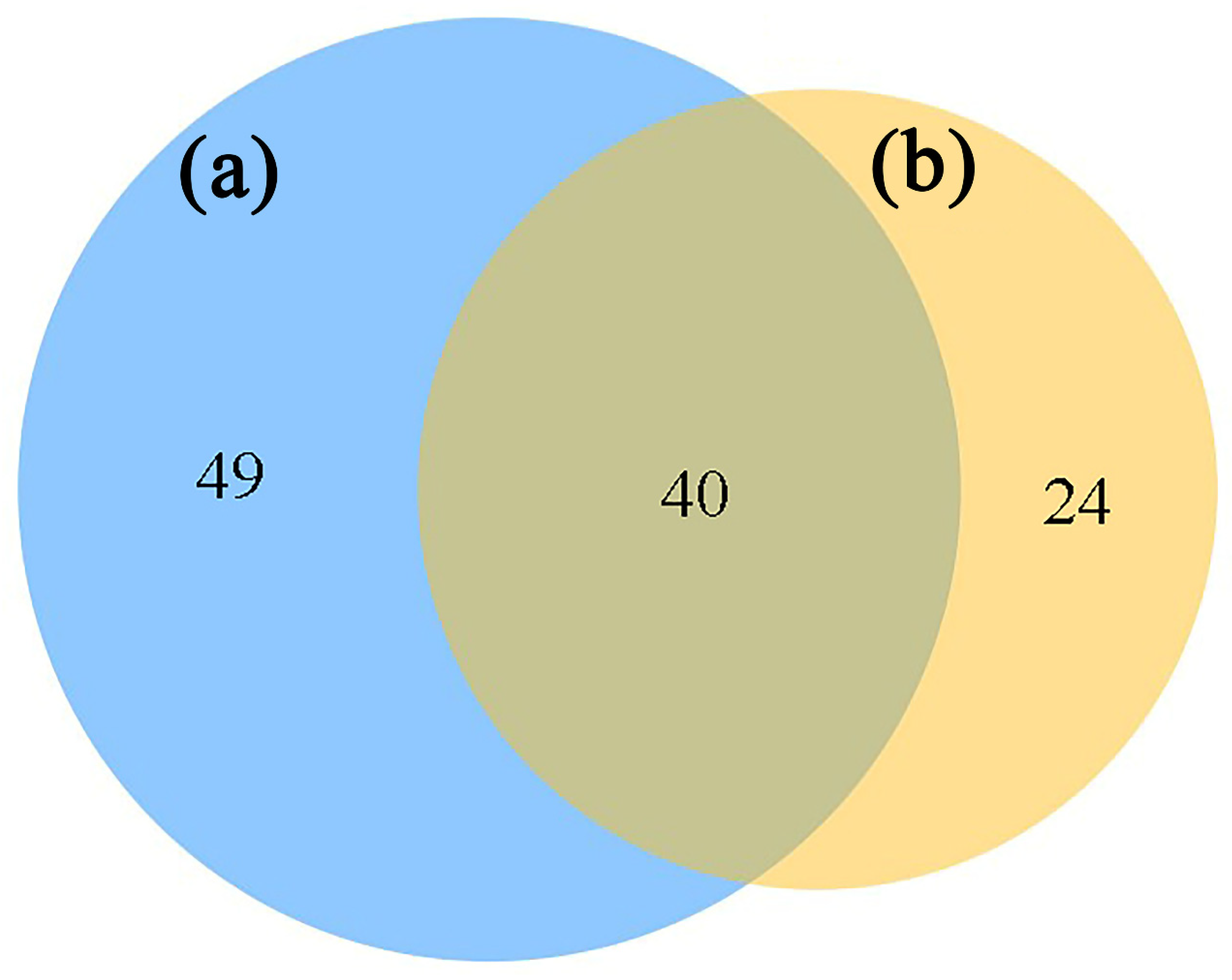
3.5. Characteristics of Fungal fMEN Structure in the Rhizosphere of A. ziyuanensis
3.6. Topological Roles of fMEN Nodes of Fungi in the Rhizosphere of A. ziyuanensis
3.7. The Influence of Soil Physicochemical Properties on the Ecological Network Structure of Fungal Functional Molecules
4. Discussion
4.1. Analysis of Functional Groups of Fungal Communities in the Rhizosphere of A. ziyuanensis
4.2. Functional Molecular Ecological Network Analysis of Fungal Communities in the Rhizosphere of A. ziyuanensis
4.3. Research Prospects of Functional Groups of Fungal Communities in the Rhizosphere of A. ziyuanensis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| FUNGuild | Fungi Functional Guild |
| fMEN | Functional Molecular Ecological Network |
| SBT | Shenbaotang |
| SJHT | Sanjiaohutang |
References
- Lu, T.; Ke, M.; Lavoie, M.; Jin, Y.J.; Fan, X.J.; Zhang, Z.Y.; Fan, Z.W.; Sun, L.W.; Gillings, M.; Peñuelas, J.; et al. Rhizosphere microorganisms can influence the timing of plant flowering. Microbiome 2018, 6, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Mendes, R.; Kruijt, M.; Bruijn, I.D.; Dekkers, E.; Voort, M.V.D.; Schneider, J.H.M.; Piceno, Y.M.; DeSantis, T.Z.; Andersen, G.L.; Bakker, P.A.H.M.; et al. Deciphering the rhizosphere microbiome for disease-suppressive bacteria. Science 2011, 332, 1097–1100. [Google Scholar] [CrossRef] [PubMed]
- Geml, J.; Morgado, L.N.; Semenova-Nelsen, T.A.; Schilthuizen, M. Changes in richness and community composition of ectomycorrhizal fungi among altitudinal vegetation types on Mount Kinabalu in Borneo. New Phytol. 2017, 215, 454–468. [Google Scholar] [CrossRef]
- Clemmensen, K.E.; Finlay, R.D.; Dahlberg, A.; Stenlid, J.; Wardle, D.A.; Lindahl, B.D. Carbon sequestration is related to mycorrhizal fungal community shifts during long-term succession in boreal forests. New Phytol. 2015, 205, 1525–1536. [Google Scholar] [CrossRef]
- Averill, C.; Bhatnagar, J.M.; Dietze, M.C.; Pearse, W.D.; Kivlin, S.N. Global imprint of mycorrhizal fungi on whole-plant nutrient economics. Proc. Natl. Acad. Sci. USA 2019, 116, 23163–23168. [Google Scholar] [CrossRef]
- Root, R.B. The niche exploitation pattern of the blue-gray gnatcatcher. Ecol. Monogr. 1967, 37, 317–350. [Google Scholar] [CrossRef]
- Nguyen, N.H.; Song, Z.W.; Bates, S.T.; Branco, S.; Tedersoo, L.; Menke, J.; Schilling, J.S.; Kennedy, P.G. FUNGuild: An open annotation tool for parsing fungal community datasets by ecological guild. Fungal Ecol. 2016, 20, 241–248. [Google Scholar] [CrossRef]
- Pinzari, F.; Colaizzi, P.; Maggi, O.; Persiani, A.M.; Schütz, R.; Rabin, I. Fungal bioleaching of mineral components in a twentieth-century illuminated parchment. Anal. Bioanal. Chem. 2012, 402, 1541–1550. [Google Scholar] [CrossRef]
- Kyaschenko, J.; Clemmensen, K.E.; Hagenbo, A.; Karltun, E.; Lindahl, B.D. Shift in fungal communities and associated enzyme activities along an age gradient of managed Pinus sylvestris stands. ISME J. 2017, 11, 863–874. [Google Scholar] [CrossRef]
- Frąc, M.; Hannula, S.E.; Bełka, M.; Jędryczka, M. Fungal biodiversity and their role in soil health. Front. Microbiol. 2018, 9, 707. [Google Scholar] [CrossRef]
- White, J.F.; Kingsley, K.L.; Verma, S.K.; Kowalski, K.P. Rhizophagy cycle: An oxidative process in plants for nutrient extraction from symbiotic microbes. Microorganisms 2018, 6, 95. [Google Scholar] [CrossRef]
- Clemmensen, K.E.; Bahr, A.; Ovaskainen, O.; Dahlberg, A.; Ekblad, A.; Wallander, H.; Stenlid, J.; Finlay, R.D.; Wardle, D.A.; Lindahl, B.D. Roots and associated fungi drive long-term carbon sequestration in boreal forest. Science 2013, 339, 1615–1618. [Google Scholar] [CrossRef]
- Moisan, K.; Cordovez, V.; Zande, E.M.V.D.; Raaijmakers, J.M.; Dicke, M.; Lucas-Barbosa, D. Volatiles of pathogenic and non-pathogenic soil-borne fungi affect plant development and resistance to insects. Oecologia 2019, 190, 589–604. [Google Scholar] [CrossRef] [PubMed]
- Fincham, W.N.W.; Dunn, A.M.; Brown, L.E.; Hesketh, H.; Roy, H.E. Invasion success of a widespread invasive predator may be explained by a high predatory efficacy but may be influenced by pathogen infection. Biol. Invasions 2019, 21, 3545–3560. [Google Scholar] [CrossRef]
- He, D.; Shen, W.; Eberwein, J.; Zhao, Q.; Ren, L.; Wu, Q.L. Diversity and co-occurrence network of soil fungi are more responsive than those of bacteria to shifts in precipitation seasonality in a subtropical forest. Soil Biol. Biochem. 2017, 115, 499–510. [Google Scholar] [CrossRef]
- Wagg, C.; Schlaeppi, K.; Banerjee, S.; Kuramae, E.E.; Heijden, M.G.A.V.D. Fungal-bacterial diversity and microbiome complexity predict ecosystem functioning. Nat. Commun. 2019, 10, 1–10. [Google Scholar] [CrossRef]
- Fuhrman, J.A.; Steele, J.A. Community structure of marine bacterioplankton: Patterns, networks, and relationships to function. Aquat. Microb. Ecol. 2008, 53, 69–81. [Google Scholar] [CrossRef]
- Deng, Y.; Jiang, Y.H.; Yang, Y.; He, Z.; Luo, F.; Zhou, J. Molecular ecological network analyses. BMC Bioinform. 2012, 13, 113. [Google Scholar] [CrossRef]
- Zhou, J.Z.; Deng, Y.; Luo, F.; He, Z.L.; Yang, Y.F. Phylogenetic molecular ecological network of soil microbial communities in response to elevated CO2. Mbio 2011, 2, e00122-11. [Google Scholar] [CrossRef]
- Fu, L.K.; Lu, Y.J.; Mo, S.L. The genus Abies discovered for first time in Guangxi and Hunan. Acta Phytotaxon. Sin. 1980, 18, 205–210. [Google Scholar]
- Xiang, Q.P. A preliminary survey on the distribution of rare and endangered plants of Abies in China. Guangxi Plants 2001, 113–117+126. [Google Scholar]
- Ning, S.J.; Tang, R.Q.; Cao, J.W. Current status and conservation countermeasures of germplasm resources of Abies ziyuanensis. Guangxi Plants 2005, 197–200+280. [Google Scholar]
- Tang, S.Q.; Dai, W.J.; Li, M.S.; Zhang, Y.; Geng, Y.P.; Wang, L.; Zhong, Y. Genetic diversity of relictual and endangered plant Abies ziyuanensis (Pinaceae) revealed by AFLP and SSR markers. Genetica 2008, 133, 21–30. [Google Scholar] [CrossRef]
- Li, S.; Mo, S.H.; Hu, X.H.; De, T. Prediction of potential suitable areas of endangered plant Abies ziyuanensis based on MaxEnt and ArcGIS. J. Ecol. 2024, 43, 533–541. [Google Scholar]
- Wang, Y.X.; Deng, T.; Ye, J.T.; Hu, X.H. Characterization of Microsatellites Sequence in Genome of Abies Ziyuanensis, an Endangered Species. Mol. Plant Breed. 1–14. Available online: http://kns.cnki.net/kcms/detail/46.1068.S.20240110.1531.002.html (accessed on 16 July 2024).
- Martínez-Romero, E.; Aguirre-Noyola, J.L.; Taco-Taype, N.; Martínez-Romero, J.; Zuñiga-Dávila, D. Plant microbiota modified by plant domestication. Syst. Appl. Microbiol. 2020, 43, 126106. [Google Scholar] [CrossRef] [PubMed]
- Olanrewaju, O.S.; Ayangbenro, A.S.; Glick, B.R.; Oluranti Babalola, O. Plant health: Feedback effect of root exudates-rhizobiome interactions. Appl. Microbiol. Biotechnol. 2019, 103, 1155–1166. [Google Scholar] [CrossRef]
- Contreras, M.J.; Leal, K.; Bruna, P.; Nuñez-Montero, K.; Goméz-Espinoza, O.; Santos, A.; Bravo, L.; Valenzuela, B.; Solis, F.; Gahona, G.; et al. Commonalities between the Atacama Desert and Antarctica rhizosphere microbial communities. Front. Microbiol. 2023, 14, 1197399. [Google Scholar] [CrossRef]
- Qu, Q.; Zhang, Z.Y.; Peijnenburg, W.J.G.M.; Liu, W.Y.; Lu, T.; Hu, B.L.; Chen, J.M.; Chen, J.; Lin, Z.F.; Qian, H.F. Rhizosphere Microbiome Assembly and Its Impact on Plant Growth. J. Agric. Food Chem. 2020, 68, 5024–5038. [Google Scholar] [CrossRef]
- Xie, F.; Ma, A.Z.; Zhou, H.C.; Liang, Y.; Yin, J.; Ma, K.; Zhuang, X.L.; Zhuang, G.Q. Revealing Fungal Communities in Alpine Wetlands Through Species Diversity, Functional Diversity and Ecological Network Diversity. Microorganisms 2020, 8, 632. [Google Scholar] [CrossRef]
- Bao, S.D. Soil Agricultural Chemistry Analysis; China Agricultural Press: Beijing, China, 2000. [Google Scholar]
- Daraz, U.; Erhunmwunse, A.S.; Dubeux, J.C.B., Jr.; Mackowiak, C.; Liao, H.L.; Wang, X.B. Soil fungal community structure and function response to rhizoma perennial peanut cultivars. BMC Plant Biol. 2024, 24, 582. [Google Scholar] [CrossRef]
- Geng, X.; Zuo, J.; Meng, Y.; Zhuge, Y.; Zhu, P.; Wu, N.; Bai, X.; Ni, G.; Hou, Y. Changes in nitrogen and phosphorus availability driven by secondary succession in temperate forests shape soil fungal communities and function. Ecol. Evol. 2023, 13, e10593. [Google Scholar] [CrossRef]
- Richards, T.A.; Jones, M.D.M.; Leonard, G.; Bass, D. Marine fungi: Their ecology and molecular diversity. Ann. Rev. Mar. Sci. 2012, 4, 495–522. [Google Scholar] [CrossRef]
- Yang, H.Q.; Xiang, Y.Q.; Lü, Q.; Yin, B.R.; Tang, Z.R.; Zhang, Y.; Chen, G.; Lai, J.M.; Fan, C.; Li, X.W. A comparative study the soil fungal community structure across three mixed forests at the initial stage of afforestation. Acta Ecol. Sin. 2024, 44, 3360–3371. [Google Scholar]
- Xi, S.H.; Ming, A.G.; Tan, L.; He, J.; Qin, L. Comparison of soil fungal community diversity and functional groups between native tree species and Eucalyptus plantations in south subtropical China. Guangxi Plants 2024, 44, 1232–1244. [Google Scholar]
- Wen, X.; Dang, P. Structural and functional characteristics of soil fungal communities under 4 subtropical forest stands. J. Fujian Agric. For. Univ. (Nat. Sci. Ed.) 2024, 53, 267–275. [Google Scholar]
- Shan, L.; Schmid, B.; Deyn, G.B.D.; Yu, S. Soil microbes promote complementarity effects among co-existing trees through soil nitrogen partitioning. Funct. Ecol. 2018, 32, 1879–1889. [Google Scholar]
- Bennett, J.A.; Maherali, H.; Reinhart, K.O.; Lekberg, Y.; Hart, M.M.; Klironomos, J. Plant-soil feedbacks and mycorrhizal type influence temperate forest population dynamics. Science 2017, 355, 181–184. [Google Scholar] [CrossRef]
- Frew, A.P.; Jeff, R.G.; Glauser, G.; Bennett, A.E.; Johnson, S.N. Mycorrhizal fungi enhance nutrient uptake but disarm defences in plant roots, promoting plant-parasitic nematode populations. Soil Biol. Biochem. 2018, 126, 123–132. [Google Scholar] [CrossRef]
- Li, M. Ectomycorrhizae Fungi Diversity in Main Community of Pinus Massoniana in Guizhou. Master’s Thesis, Guizhou University, Guiyang, China, 2017. [Google Scholar]
- Li, H.F.; Huang, X.B.; Shen, J.Y. Effects of plant diversity and soil properties on soil fungal community structure with secondary succession in the Pinus yunnanensis forest. Geoderma 2020, 379, 114646. [Google Scholar] [CrossRef]
- Chen, Z.J.; Gao, S.K.; Chen, Y.; He, P.H.; He, Q.; Qiu, Q.; Li, J.Y. Effects of short-term fertilization on soil fungal community structure and functional group in Eucalyptus artificial forest. Acta Ecol. Sin. 2020, 40, 3813–3821. [Google Scholar]
- Nasholm, T.; Ekblad, A.; Nordin, A.; Giesler, R.; Hoegberg, M.; Hoegberg, P. Boreal forest plants take up organic nitrogen. Nature 1998, 392, 914–916. [Google Scholar] [CrossRef]
- Zhou, J.; Deng, Y.; Luo, F.; He, Z.; Tu, Q.; Zhi, X. Functional molecular ecological networks. Mbio 2010, 1, e00169-10. [Google Scholar] [CrossRef]
- Qin, L.; Wang, Y.F.; Ming, A.G.; Xi, S.H.; Xiao, Z.R.; Teng, J.Q.; Tan, L. Molecular Ecological Network Structure and Potential Function of the Bacterial Community in the Soil Profile under Indigenous Tree Plantations in Subtropical China. Forests 2023, 14, 803. [Google Scholar] [CrossRef]
- Eo, J.K.; Eom, A.H. Community of Endophytic Fungi from Alpine Conifers on Mt. Seorak. Mycobiology 2022, 50, 317–325. [Google Scholar] [CrossRef]
- Li, Q. Responses of Alpine Meadow Vegetation, Soil and Microorganism to Altitudes and Aspect in Eastern Qilian Mountains and its Microbial Adaptation Mechanisms. Ph.D. Thesis, Gansu Agricultural University, Lanzhou, China, 2021. [Google Scholar]
- Huang, W.; Chen, Y.Z.; Zhuang, Y.H. Seasonal variations in nutrient content and microbial populations in rhizosphere and non-rhizosphere soils of Camellia oleifera. Jiangsu Agric. Sci. 2017, 45, 265–270. [Google Scholar]
- Yu, Q.; Weng, L.L.; Zhang, N.; Wang, B.; Xiao, C.P. Study on the Dynamics of Fungal Community Diversity in Rhizosphere Soil Cultivated Ginseng with Different Cultivation Years. Mol. Plant Breed. 1–15. Available online: http://kns.cnki.net/kcms/detail/46.1068.S.20240319.1801.017.html (accessed on 11 May 2025).
- Zhang, X.S.; Lu, J. Advances in the study of root biomass and its response to rhizosphere ecosystem. Jiangsu Agric. Sci. 2021, 49, 7. [Google Scholar]
- Ye, J.T.; Deng, T.; Cen, H.F.; Duang, Y.B.; Zhu, X.Z.; Hu, X.H. Niche and interspecific association of dominant trees in Abies ziyuanensis community of Yinzhu Laoshan, Guangxi. Acta Ecol. Sin. 2025, 45, 3921–3932. [Google Scholar]


| Physicochemical Properties | SBT | SJHT | p Value | Significance |
|---|---|---|---|---|
| SWC (%) | 40.54 ± 2.54 | 48.83± 4.25 | 0.11 | - |
| pH | 4.30 ± 0.06 | 4.38 ± 0.05 | 0.31 | - |
| TN (g/kg) | 5.57 ± 0.29 | 5.38 ± 0.51 | 0.75 | - |
| AN (mg/kg) | 285.60 ± 17.81 | 279.88 ± 30.69 | 0.87 | - |
| NO3−-N (mg/kg) | 5.01 ± 1.66 | 8.74 ± 3.02 | 0.29 | - |
| NH4+-N (mg/kg) | 31.07 ± 3.51 | 30.90 ± 3.37 | 0.97 | - |
| SOC (g/kg) | 72.71 ± 4.79 | 64.15 ± 4.10 | 0.19 | - |
| TP (g/kg) | 0.44 ± 0.08 | 0.71 ± 0.15 | 0.13 | - |
| AP (mg/kg) | 3.44 ± 0.53 | 4.66 ± 1.64 | 0.49 | - |
| TK (g/kg) | 22.27 ± 1.11 | 17.55 ± 1.05 | 0.00 | ** |
| AK (mg/kg) | 95.73 ± 4.25 | 80.80 ± 5.32 | 0.04 | * |
| MBC (mg/kg) | 1062.40 ± 98.64 | 1092.67 ± 129.60 | 0.85 | - |
| MBN (mg/kg) | 456.00 ± 46.43 | 365.47 ± 30.31 | 0.12 | - |
| Fungal Trophic Mode | Relative Abundance (%) | |
|---|---|---|
| SBT | SJHT | |
| Symbiotroph | 20.97 | 22.00 |
| Saprotroph-Symbiotroph | 10.61 | 14.16 |
| Saprotroph | 7.76 | 6.26 |
| Pathotroph-Symbiotroph | 2.51 | 2.20 |
| Pathotroph-Saprotroph-Symbiotroph | 11.17 | 3.96 |
| Pathotroph-Saprotroph | 5.88 | 8.35 |
| Pathotroph | 2.43 | 7.95 |
| Total | 61.33 | 64.88 |
| Fungal Functional Guilds | SBT | SJHT | p Value | Significance |
|---|---|---|---|---|
| Animal Pathogen | 0.0199 ± 0.0048 | 0.0758 ± 0.0262 | 0.0327 | * |
| Arbuscular Mycorrhizal | 0.0011 ± 0.0004 | 0.0036 ± 0.0013 | 0.0136 | * |
| Bryophyte Parasite-Dung Saprotroph-Ectomycorrhizal-Fungal Parasite-Leaf Saprotroph-Plant Parasite-Undefined Saprotroph-Wood Saprotroph | 0.0146 ± 0.0087 | 0.0030 ± 0.0025 | 0.0063 | ** |
| Dung Saprotroph | 0.0002 ± 0.0001 | 0.0003 ± 0.0003 | 0.0442 | * |
| Dung Saprotroph-Plant Saprotroph-Wood Saprotroph | 0.0004 ± 0.0002 | 0.0001 ± 0.0001 | 0.0231 | * |
| Ectomycorrhizal-Lichen Parasite-Lichenized-Plant Pathogen | 0.0693 ± 0.0492 | 0.0010 ± 0.0003 | 0.0000 | *** |
| Ectomycorrhizal-Undefined Saprotroph-Wood Saprotroph | 0.0003 ± 0.0001 | 0.0027 ± 0.0019 | 0.0337 | * |
| Endomycorrhizal-Plant Pathogen-Undefined Saprotroph | 0.0028 ± 0.0009 | 0.0009 ± 0.0004 | 0.0284 | * |
| NULL | 0.0005 ± 0.0002 | 0.0002 ± 0.0001 | 0.0434 | * |
| Plant Parasite-Wood Saprotroph | 0.0009 ± 0.0005 | 0.0001 ± 0.0001 | 0.0044 | ** |
| Plant Saprotroph | 0.0004 ± 0.0001 | 0.0006 ± 0.0006 | 0.0020 | ** |
| Plot | Molecular Ecological Networks | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cutoff | Total Nodes | Total Links | R2 R2 of Power-Law | Number of Positive Connections | Number of Negative Connections | Average Degree (avg K) | Average Clustering Coefficient (avg CC) | Average Path Distance (GD) | Modularity Index (Module Number) | |
| SBT | 0.690 | 89 | 184 | 0.69 | 40 | 144 | 4.135 | 0.057 | 3.708 | 0.448 (6) |
| SJHT | 0.690 | 64 | 115 | 0.74 | 60 | 55 | 3.594 | 0.169 | 3.391 | 0.524 (6) |
| Fungal Trophic Mode | Module Hub | Connector | ||
|---|---|---|---|---|
| SBT | SJHT | SBT | SJHT | |
| Symbiotroph | OTU85 | OTU143, OTU103 | ||
| Pathotroph–Saprotroph–Symbiotroph | OTU298 | |||
| Saprotroph–Symbiotroph | OTU27 | OTU199 | OTU44, OTU117 | |
| Saprotroph | OTU92, OTU411 | |||
| Pathotroph–Saprotroph | OTU321 | |||
| Pathotroph–Symbiotroph | ||||
| Pathotroph | OTU182 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Wu, J.; Deng, T.; Ye, J.; Hu, X. Functional Characteristics of Fungal Communities in the Rhizosphere of the Endangered Plant Abies ziyuanensis. Microorganisms 2025, 13, 1989. https://doi.org/10.3390/microorganisms13091989
Wang Y, Wu J, Deng T, Ye J, Hu X. Functional Characteristics of Fungal Communities in the Rhizosphere of the Endangered Plant Abies ziyuanensis. Microorganisms. 2025; 13(9):1989. https://doi.org/10.3390/microorganisms13091989
Chicago/Turabian StyleWang, Yufeng, Jiahao Wu, Tao Deng, Jiatong Ye, and Xinghua Hu. 2025. "Functional Characteristics of Fungal Communities in the Rhizosphere of the Endangered Plant Abies ziyuanensis" Microorganisms 13, no. 9: 1989. https://doi.org/10.3390/microorganisms13091989
APA StyleWang, Y., Wu, J., Deng, T., Ye, J., & Hu, X. (2025). Functional Characteristics of Fungal Communities in the Rhizosphere of the Endangered Plant Abies ziyuanensis. Microorganisms, 13(9), 1989. https://doi.org/10.3390/microorganisms13091989






