Novel Thiazolidinedione Derivatives as Potential ZIKV Antiviral Inhibitors
Abstract
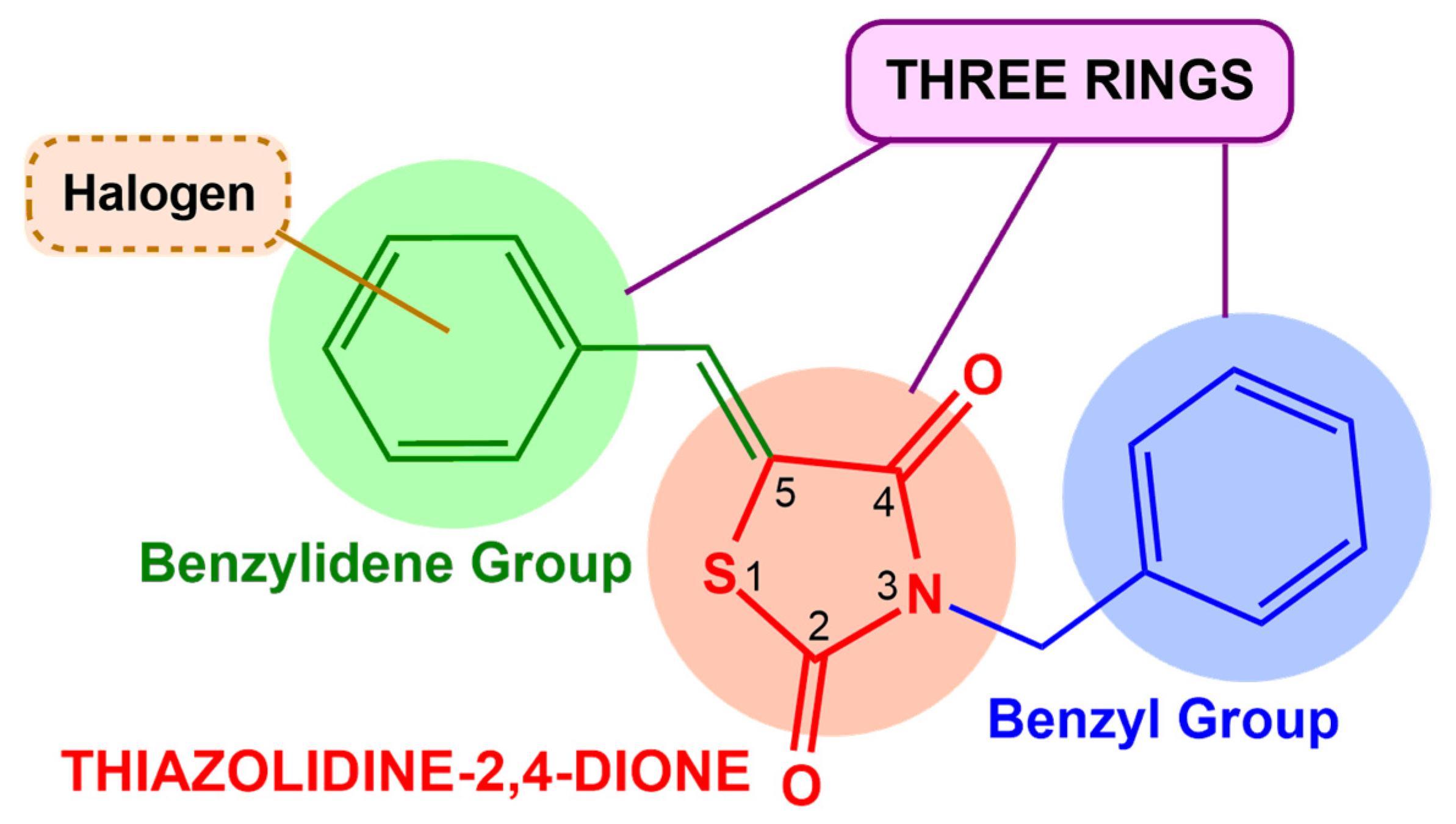
1. Introduction
2. Materials and Methods
2.1. Chemistry
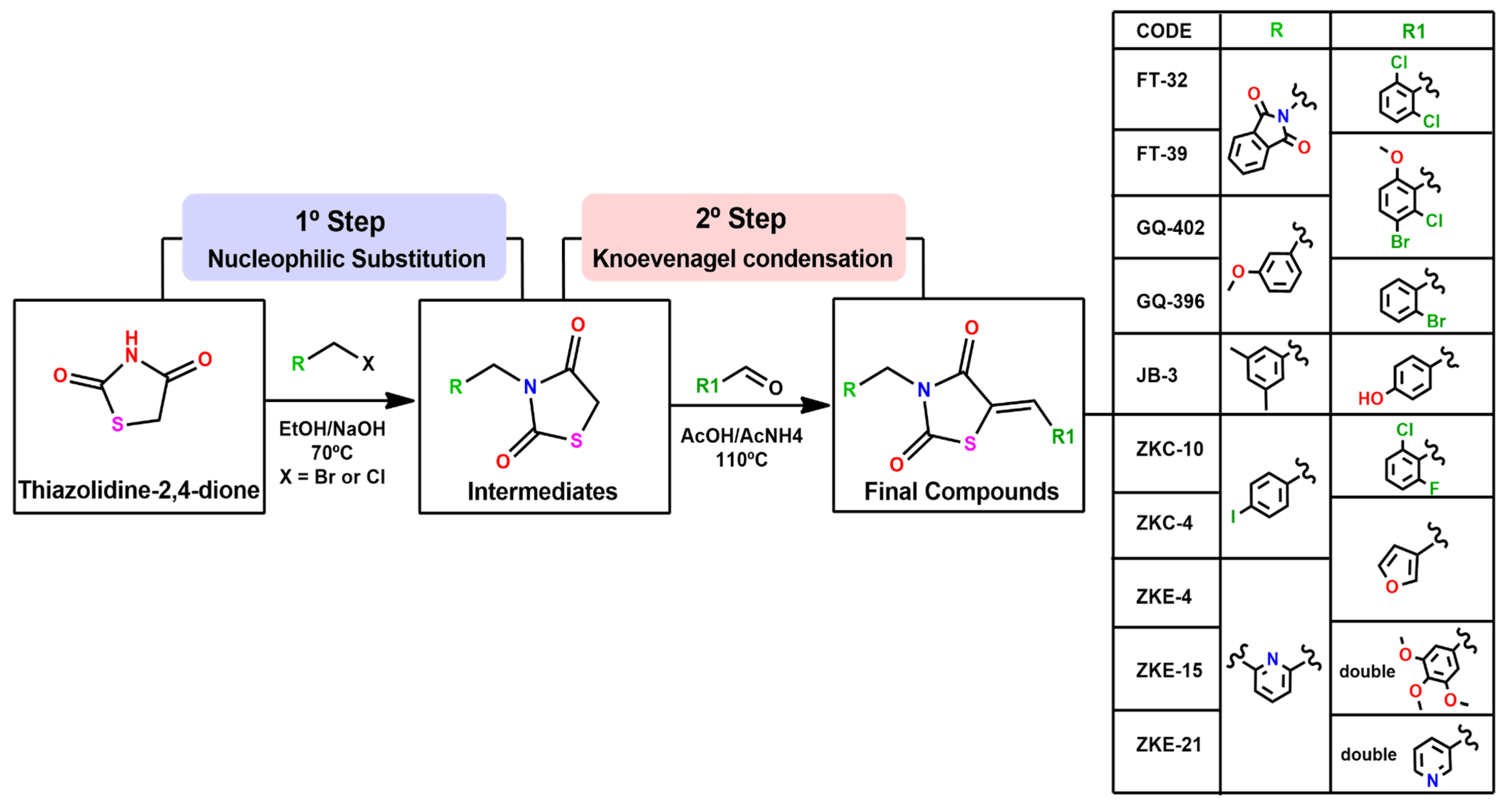
2.1.1. Obtaining Compounds for Screening
Procedure for Synthesis of 5-Benzylidene-3-(4-iodobenzyl)-thiazolidine-2,4-dione (ZKC-10)
General Procedure for Synthesis of 5-Benzylidene-3-(3-methoxybenzyl)-thiazolidine-2,4-dione (GQs)
5-(2-Bromobenzylidene)-3-(3-methoxybenzyl)thiazolidine-2,4-dione (GQ-396)
5-(5-Bromo-2-methoxybenzylidene)-3-(3-methoxybenzyl)thiazolidine-2,4-dione (GQ-402)
2.2. Biology
2.2.1. Viruses and Cell Lines
2.2.2. Viral Production
2.2.3. Cell Viability Assays
2.2.4. Antiviral Measurement
2.2.5. Viral RNA Quantification
2.3. Molecular Docking of Ligands with Proteins
2.4. Statistical Analysis
3. Results
3.1. Synthesis of the Compounds
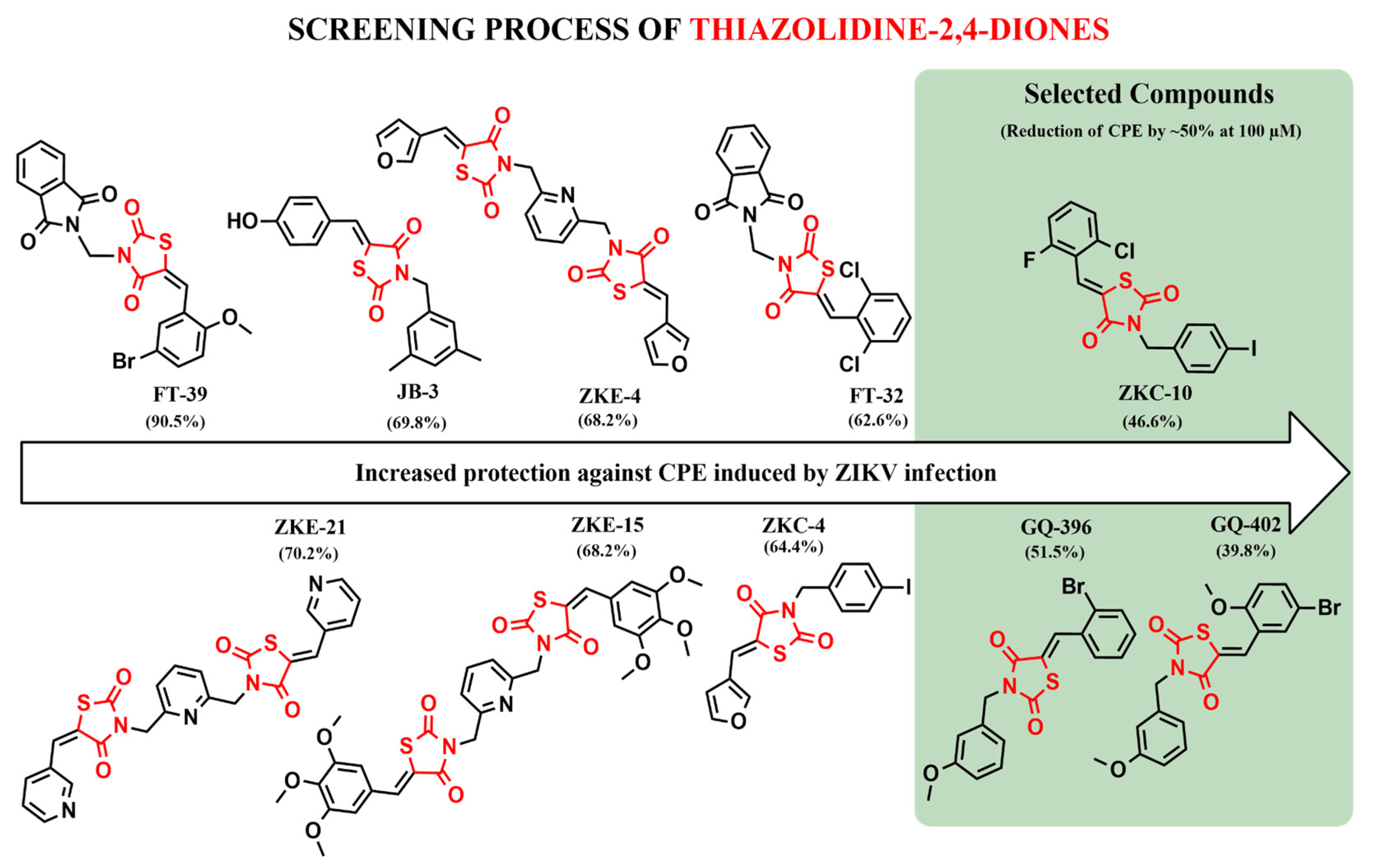
3.2. Screening of Ten Thiazolidinedione Derivatives Against Zika Infection in Vero E6 Cells
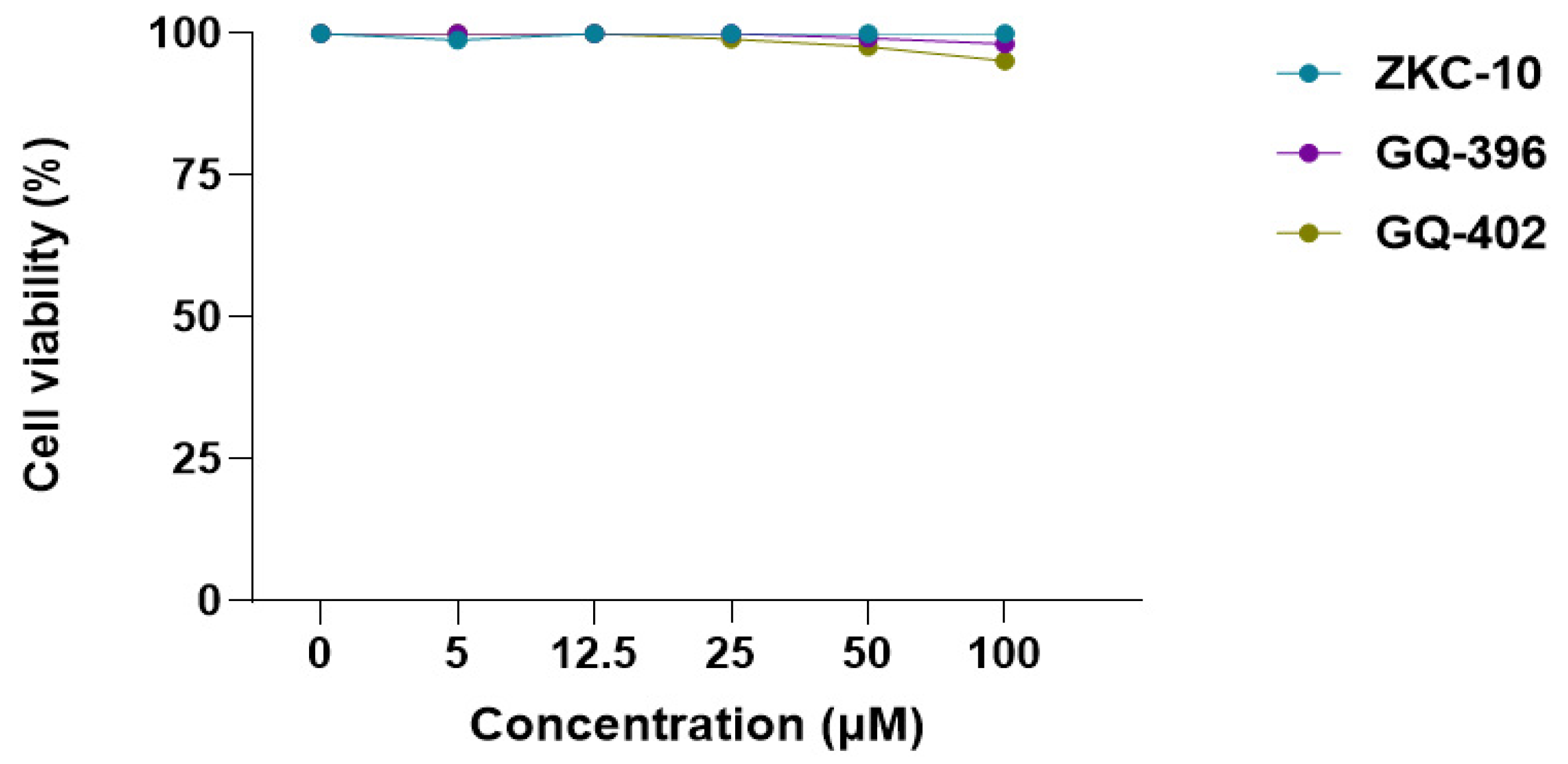
3.3. Cytotoxicity and Antiviral Activity of Derivatives of Thiazolidinedione
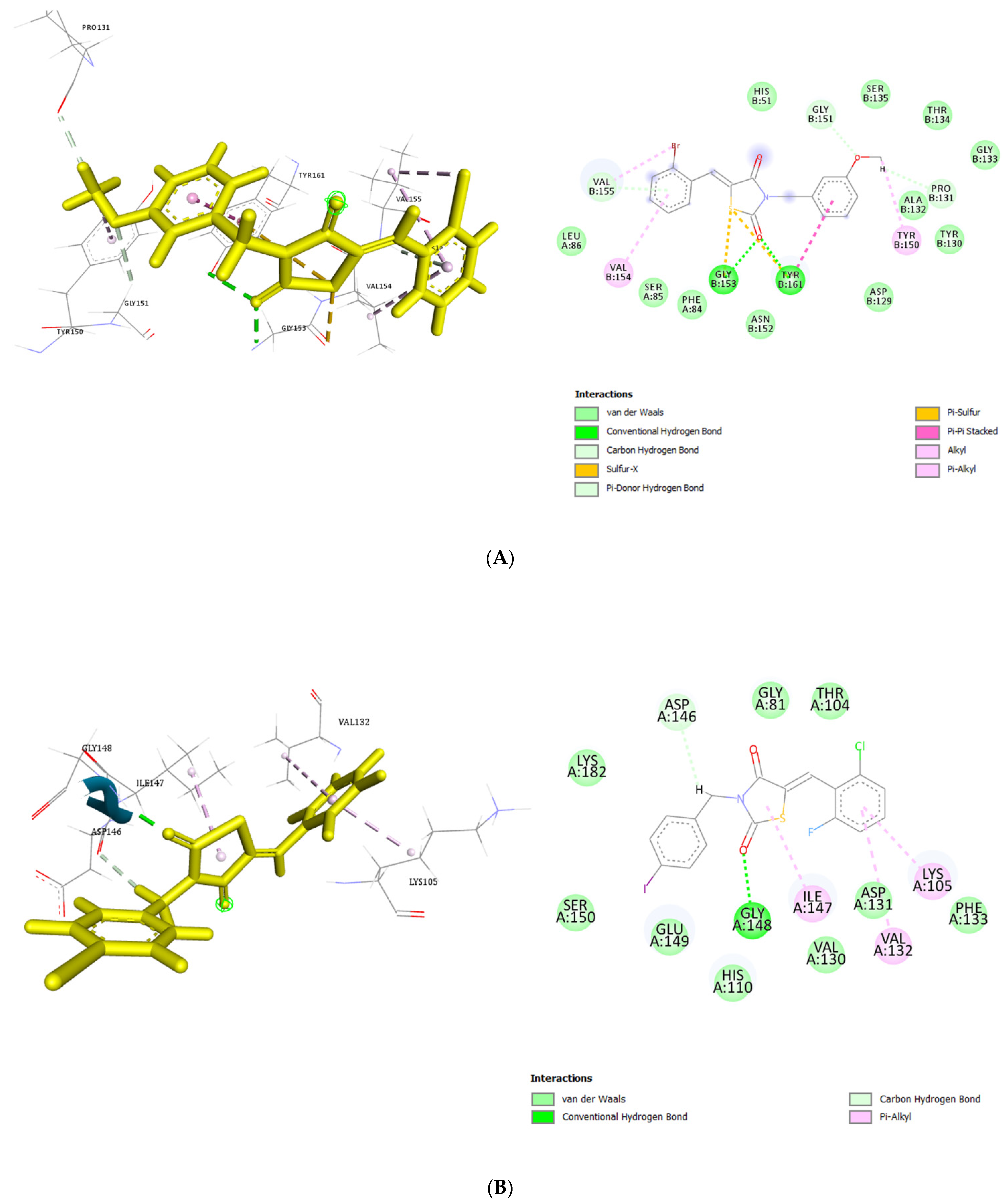
3.4. Molecular Docking of the Thiazolidinedione Derivatives
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Scott, C.; Weaver, W.K.R. Present and Future Arboviral Threats. Antivir. Res. 2010, 85, 328–345. [Google Scholar]
- Halstead, S.B. Chikungunya and Zika Disease; Elsevier Inc.: Amsterdam, The Netherlands, 2018; Chapter 3. [Google Scholar]
- Dick, G.W.A.; Kitchen, S.F.; Haddow, A.J. Zika Virus (I). Isolations and serological specificity. Trans. R. Soc. Trop. Med. Hyg. 1952, 46, 509–520. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control. Zika Virus Disease Epidemic: Potential Association with Microcephaly and Guillain-Barre Syndrome (First Update); ECDC: Stockholm, Sweden, 2016.
- Pan American Health Organization (PAHO). ZIKA Cumulative Cases. Available online: https://www.paho.org/en/documents/zika-cumulative-cases-4-january-2018 (accessed on 4 January 2018).
- Roos, R.P. Zika Virus—A Public Health Emergency of International Concern. JAMA Intern. Med. 2016, 73, 1395–1396. [Google Scholar] [CrossRef][Green Version]
- Ming, G.; Tang, H.; Song, H. Advances in Zika Virus Research: Stem Cell Models, Challenges and Opportunities. Cell Stem Cell 2016, 19, 690–702. [Google Scholar] [CrossRef]
- García, L.L.; Padilla, L.; Castaño, J.C. Inhibitors compounds of the flavivirus replication process. Virol. J. 2017, 14, 95. [Google Scholar] [CrossRef] [PubMed]
- Hossain, S.; Shovon, T.I.; Hasan, R.; Hakim, F.T.; Hasan, M.M.; Esha, S.A.; Tasnim, S.; Nazir, S.; Akhter, F.; Ali, A.; et al. Therapeutic Potential of Antiviral Peptides against the NS2B/NS3 Protease of Zika Virus. ACS Omega 2023, 8, 35207–35218. [Google Scholar] [CrossRef] [PubMed]
- Fiorucci, D.; Meaccini, M.; Poli, G.; Stincarelli, M.A.; Vagaggini, C.; Giannecchini, S.; Sutto-Ortiz, P.; Canard, B.; Decroly, E.; Dreassi, E.; et al. Identification of Novel Non-Nucleoside Inhibitors of Zika Virus NS5 Protein Targeting MTase Activity. Int. J. Mol. Sci. 2024, 25, 2437. [Google Scholar] [CrossRef]
- Sokolova, A.S.; Yarovaya, O.I.; Bormotov, N.I.; Shishkinac, L.N.; Salakhutdinovab, N.F. Synthesis and antiviral activity of camphor-based 1,3-thiazolidin-4-one and thiazole derivatives as Orthopoxvirus-reproduction inhibitors. MedChemComm 2018, 9, 1746–1753. [Google Scholar] [CrossRef]
- Sahiba, N.; Sethiya, A.; Soni, J.; Agarwal, D.K.; Agarwal, S. Saturated Five-Membered Thiazolidines and Their Derivatives: From Synthesis to Biological Applications. Top. Curr. Chem. 2020, 378, 34. [Google Scholar] [CrossRef] [PubMed]
- Hwu, J.R.; Gupta, N.K.; Tsay, S.C.; Huang, W.C.; Albulescu, I.C.; Kovacikova, K.; van Hemert, M.J. Bis(benzofuran-thiazolidinone)s and bis(benzofuran-thiazinanone)s as inhibiting agents for chikungunya virus. Antivir. Res. 2017, 146, 96–101. [Google Scholar] [CrossRef]
- Tanaka, T.; Okuyama-Dobashi, K.; Motohashi, R.; Yokoe, H.; Takahashi, K.; Wiriyasermkul, P.; Kasai, H.; Yamashita, A.; Maekawa, S.; Enomoto, N.; et al. Inhibitory effect of a novel thiazolidinedione derivative on hepatitis B virus entry. Antivir. Res. 2021, 194, 105165. [Google Scholar] [CrossRef]
- Hassan, G.S.; Georgey, H.H.; Mohammed, E.Z.; Omar, F.A. Anti-hepatitis-C virus activity and QSAR study of certain thiazolidinone and thiazolotriazine derivatives as potential NS5B polymerase inhibitors. Eur. J. Med. Chem. 2019, 184, 111747. [Google Scholar] [CrossRef]
- Zheng, Y.; Murugesan, S. One-Pot Synthesis of Novel Hydrazono-1,3-Thıazolıdın-4-One Derivatives as Anti-HIV and Anti-Tubercular Agents: Synthesıs, Bıologıcal Evalu- atıon, Molecular Modelling and Admet Studıes. Curr. HIV Res. 2022, 20, 255–271. [Google Scholar]
- Manvar, D.; Küçükgüzel, İ.; Erensoy, G.; Tatar, E.; Deryabaşoğulları, G.; Reddy, H.; Talele, T.T.; Cevik, O.; Kaushik-Basu, N. Biochemical and Biophysical Research Communications Discovery of conjugated thiazolidinone-thiadiazole scaffold as anti-dengue virus polymerase inhibitors. Biochem. Biophys. Res. Commun. 2016, 469, 743–747. [Google Scholar] [CrossRef]
- Petrou, A.; Zagaliotis, P.; Theodoroula, N.F.; Mystridis, G.A.; Vizirianakis, I.S.; Walsh, T.J.; Geronikaki, A. Thiazole/Thiadiazole/Benzothiazole Based Thiazolidin-4-One Derivatives as Potential Inhibitors of Main Protease of SARS-CoV-2. Molecules 2022, 27, 2180. [Google Scholar] [CrossRef]
- Skoreński, M.; Sieńczyk, M. The Fellowship of Privileged Scaffolds—One Structure to Inhibit Them All. Pharmaceuticals 2021, 14, 1164. [Google Scholar] [CrossRef]
- Mushtaq, M.; Naz, S.; Parang, K.; Ul-Haq, Z. Exploiting Dengue Virus Protease as a Therapeutic Target: Current Status, Challenges and Future Avenues. Curr Med. Chem. 2021, 28, 7767–7802. [Google Scholar] [CrossRef] [PubMed]
- Murtuja, S.; Shilkar, D.; Sarkar, B.; Sinha, B.N.; Jayaprakash, V. A critical observation on the design and development of reported peptide inhibitors of DENV NS2B-NS3 protease in the last two decades. Mini Rev. Med. Chem. 2022, 22, 1108–1130. [Google Scholar] [CrossRef]
- Jitonnom, J.; Meelua, W.; Tue-Nguen, P.; Saparpakorn, P.; Hannongbua, S.; Chotpatiwetchkul, W. 3D-QSAR and molecular docking studies of peptide-hybrids as dengue virus NS2B/NS3 protease inhibitors. Chem. Biol. Interact. 2024, 396, 111040. [Google Scholar] [CrossRef] [PubMed]
- Starvaggi, J.; Previti, S.; Zappalà, M.; Ettari, R. The Inhibition of NS2B/NS3 Protease: A New Therapeutic Opportunity to Treat Dengue and Zika Virus Infection. Int. J. Mol. Sci. 2024, 25, 4376. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, S.M.C.; Galdino, L.V.; Lima, M.C.; Moura, J.A.d.S.; Viana, D.C.F.; da Rosa, M.M.; Ferreira, L.F.G.R.; Hernandes, M.Z.; Pereira, M.C.; Rêgo, M.J.B.d.M.; et al. Evaluation of thiazolidine derivatives with potential anti-ZIKV activity. Curr. Top. Med. Chem. 2024, 24, 2224–2237. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, M.C.V.A.; Viana, D.C.F.; Silva, A.A.; Pereira, M.C.; Duarte, F.S.; Pitta, M.G.R.; Pitta, I.R.; Pitta, M.G.R. Bioorganic Chemistry Synthesis of novel thiazolidinic-phthalimide derivatives evaluated as new multi-target antiepileptic agents. Bioorganic Chem. 2022, 119, 105548. [Google Scholar] [CrossRef]
- Moura, J.A.d.S. (Federal University of Pernambuco, Recife, Pernambuco, Brazil). Unpublished work, 2023.
- Branco Junior. (Federal University of Pernambuco, Recife, Pernambuco, Brazil). Unpublished work, 2017.
- Verdonk, M.L.; Chessari, G.; Cole, J.C.; Hartshorn, M.J.; Murray, C.W.; Nissink, J.W.; Taylor, R.D.; Taylor, R. Modeling Water Molecules in Protein—Ligand Docking Using GOLD. J. Med. Chem. 2005, 48, 6504–6515. [Google Scholar] [CrossRef]
- Bruno, G.; Costantino, L.; Curinga, C.; Maccari, R.; Monforte, F.; Nicoló, F.; Ottanà, R.; Vigorita, M.G. Synthesis and aldose reductase inhibitory activity of 5-arylidene-2,4-thiazolidinediones. Bioorg. Med. Chem. 2002, 10, 1077–1084. [Google Scholar] [CrossRef]
- Xia, Z.; Knaak, C.; Ma, J.; Beharry, Z.M.; McInnes, C.; Wang, W.; Kraft, A.S.; Smith, C.D. Synthesis and Evaluation of Novel Inhibitors of Pim-1 and Pim-2 Protein Kinases. J. Med. Chem. 2009, 52, 74–86. [Google Scholar] [CrossRef]
- Dong, H.; Zhang, B.; Shi, P.Y. Flavivirus methyltransferase: A novel antiviral target. Antivir. Res. 2008, 80, 1–10. [Google Scholar] [CrossRef]
- Bollati, M.; Alvarez, K.; Assenberg, R.; Baronti, C.; Canard, B.; Cook, S.; Coutard, B.; Decroly, E.; de Lamballerie, X.; Gould, E.A.; et al. Structure and functionality in flavivirus NS-proteins: Perspectives for drug design. Antivir. Res. 2010, 87, 125–148. [Google Scholar] [CrossRef]
- Wei, Y.; Li, J.; Qing, J.; Huang, M.; Wu, M.; Gao, F.; Li, D.; Hong, Z.; Kong, L.; Huang, W.; et al. Discovery of Novel Hepatitis C Virus NS5B Polymerase Inhibitors by Combining Random Forest, Multiple e-Pharmacophore Modeling and Docking. PLoS ONE 2016, 11, e0148181. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Feng, Y.; Wang, H.; Yang, J.; Chen, X.; Wang, Y.; Lai, C.C.; Zhang, Y.; Li, C.; Xia, X.; et al. Synthesis and biological evaluation of novel anti-hepatitis C virus (HCV) agents: 2-hydroxylphenethyl sulfanyl-oxopyrimidines. Med. Chem. Res. 2017, 26, 1388–1396. [Google Scholar] [CrossRef]
- Kaptein, F.; Neyts, J. The Viral Polymerase Inhibitor 7-Deaza-2’—C-Methyladenosine Is a Potent Inhibitor of In Vitro Zika Virus Replication and Delays Disease Progression in a Robust Mouse Infection Model. PLoS Neglected Trop. Dis. 2016, 10, e0004695. [Google Scholar]
- Gonzalez, S.; Brzuska, G.; Ouarti, A.; Gallier, F.; Solarte, C.; Ferry, A.; Uziel, J.; Krol, E.; Lubin-Germain, N. Bioorganic & Medicinal Chemistry Anti-HCV and Zika activities of ribavirin C -nucleosides analogues. Bioorganic Med. Chem. 2022, 68, 116858. [Google Scholar]
- Kamiyama, N.; Soma, R.; Hidano, S.; Watanabe, K.; Umekita, H.; Fukuda, C.; Noguchi, K.; Gendo, Y.; Ozaki, T.; Sonoda, A.; et al. Ribavirin inhibits Zika virus (ZIKV) replication in vitro and suppresses viremia in ZIKV-infected STAT1-deficient mice. Antivir. Res. 2017, 146, 1–11. [Google Scholar] [CrossRef]
- Kamzeeva, P.N.; Aralov, A.V.; Alferova, V.A.; Korshun, V.A. Recent Advances in Molecular Mechanisms of Nucleoside Antivirals. Curr. Issues Mol. Biol. 2023, 45, 6851–6879. [Google Scholar] [CrossRef]
- Qian, X.; Qi, Z. Mosquito-Borne Flaviviruses and Current Therapeutic Advances. Viruses 2022, 14, 1226. [Google Scholar] [CrossRef]
- Bassetto, M.; Cima, C.M.; Basso, M.; Salerno, M.; Schwarze, F.; Friese, D.; Bugert, J.J.; Brancale, A. Novel Nucleoside Analogues as Effective Antiviral Agents for Zika Virus Infections. Molecules 2020, 25, 4813. [Google Scholar] [CrossRef]
- He, X.; Xing, R.; Liu, S.; Qin, Y.; Li, K.; Yu, H.; Li, P. The improved antiviral activities of amino- modified chitosan derivatives on Newcastle virus The improved antiviral activities of amino-modified chitosan derivatives on. Drug Chem. Toxicol. 2019, 44, 335–340. [Google Scholar] [CrossRef]
- Curreli, F.; Choudhury, S.; Pyatkin, I.; Zagorodnikov, V.P.; Bulay, A.K.; Altieri, A.; Kwon, Y.D.; Kwong, P.D.; Debnath, A.K. Design, Synthesis, and Antiviral Activity of Entry Inhibitors That Target the CD4-Binding Site of HIV-1. J. Med. Chem. 2012, 55, 4764–4775. [Google Scholar] [CrossRef]
- Rifaioglu, A.S.; Atas, H.; Martin, M.J.; Cetin-Atalay, R.; Atalay, V.; Doğan, T. Recent applications of deep learning and machine intelligence on in silico drug discovery: Methods, tools and databases. Brief. Bioinform. 2019, 20, 1878–1912. [Google Scholar] [CrossRef]
- Kuntz, I.D.; Blaney, J.M.; Oatley, S.J.; Langridge, R.; Ferrin, T.E. A Geometric Approach to Macromolecule-Ligand Interactions. J. Mol. Biol. 1982, 161, 269–288. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Li, Y.; Loh, Y.R.; Phoo, W.W.; Hung, A.W.; Kang, C.; Luo, D. Crystal structure of unlinked NS2B-NS3 protease from Zika virus. Sci. Rep. 2016, 354, 1597–1600. [Google Scholar] [CrossRef] [PubMed]
- Nitsche, C.; Holloway, S.; Schirmeister, T.; Klein, C.D. Biochemistry and Medicinal Chemistry of the Dengue Virus Protease. Chem. Rev. 2014, 114, 11348–11381. [Google Scholar] [CrossRef]
- Rawlings, N.D.; Barrett, A.J. Families of cysteine peptidases. Methods Enzymol. 1994, 244, 1743–1773. [Google Scholar]
- Luo, D.; Vasudevan, S.; Lescar, J. The flavivirus NS2B—NS3 protease—Helicase as a target for antiviral drug development. Antivir. Res. 2015, 118, 148–158. [Google Scholar] [CrossRef] [PubMed]
- Erbel, P.; Schiering, N.; D’ARcy, A.; Renatus, M.; Kroemer, M.; Lim, S.P.; Yin, Z.; Keller, T.H.; Vasudevan, S.G.; Hommel, U. Structural basis for the activation of flaviviral NS3 proteases from dengue and West Nile virus. Nat. Struct. Mol. Biol. 2006, 13, 372–373. [Google Scholar] [CrossRef]
- Mohamed, N.; Asari, A. The data on molecular docking of cinnamic acid amide on dengue viral target NS2B/NS3. Data Brief 2022, 42, 108036. [Google Scholar] [CrossRef]
- Dos Santos Nascimento, I.J.; da Silva Rodrigues, É.E.; da Silva, M.F.; de Araújo-Júnior, J.X.; de Moura, R.O. Advances in Computational Methods to Discover New NS2B-NS3 Inhibitors Useful Against Dengue and Zika Viruses. Curr. Top. Med. Chem. 2022, 22, 2435–2462. [Google Scholar] [CrossRef]
- Kumar, S.; El-Kafrawy, S.A.; Bharadwaj, S.; Maitra, S.S.; Alandijany, T.A.; Faizo, A.A.; Khateb, A.M.; Dwivedi, V.D.; Azhar, E.I. Discovery of Bispecific Lead Compounds from Azadirachta indica against ZIKA NS2B-NS3 Protease and NS5 RNA Dependent RNA Polymerase Using Molecular Simulations. Molecules 2022, 27, 2562. [Google Scholar] [CrossRef]
- Lei, J.; Santos, N.P.; Santos, L.H.; de Magalhães, M.T.Q.; Hilgenfeld, R.; Ferreira, R.S.; Bleicher, L. Characterization of an Allosteric Pocket in Zika Virus NS2B-NS3 Protease. J. Chem. Inf. Model. 2022, 62, 945–957. [Google Scholar]
- Saleem, H.N.; Batool, F.; Mansoor, H.J.; Shahzad-ul-Hussan, S.; Saeed, M. Inhibition of Dengue Virus Protease by Eugeniin, Isobiflorin, and Biflorin Isolated from the Flower Buds of Syzygium aromaticum (Cloves). Am. Chem. Soc. 2019, 4, 1525–1533. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ralph de Oliveira, I.L.; da Silva Moura, J.A.; Recordon-Pinson, P.; Lagadec, F.; da Rosa, M.M.; Gonçalves, S.M.C.; Viana, D.C.F.; de Moraes Gomes, P.A.T.; da Rocha Pitta, M.G.; de Melo Rêgo, M.J.B.; et al. Novel Thiazolidinedione Derivatives as Potential ZIKV Antiviral Inhibitors. Microorganisms 2025, 13, 1967. https://doi.org/10.3390/microorganisms13091967
Ralph de Oliveira IL, da Silva Moura JA, Recordon-Pinson P, Lagadec F, da Rosa MM, Gonçalves SMC, Viana DCF, de Moraes Gomes PAT, da Rocha Pitta MG, de Melo Rêgo MJB, et al. Novel Thiazolidinedione Derivatives as Potential ZIKV Antiviral Inhibitors. Microorganisms. 2025; 13(9):1967. https://doi.org/10.3390/microorganisms13091967
Chicago/Turabian StyleRalph de Oliveira, Isabella Luiza, José Arion da Silva Moura, Patricia Recordon-Pinson, Floriane Lagadec, Michelle Melgarejo da Rosa, Sayonara Maria Calado Gonçalves, Douglas Carvalho Francisco Viana, Paulo André Teixeira de Moraes Gomes, Marina Galdino da Rocha Pitta, Moacyr Jesus Barreto de Melo Rêgo, and et al. 2025. "Novel Thiazolidinedione Derivatives as Potential ZIKV Antiviral Inhibitors" Microorganisms 13, no. 9: 1967. https://doi.org/10.3390/microorganisms13091967
APA StyleRalph de Oliveira, I. L., da Silva Moura, J. A., Recordon-Pinson, P., Lagadec, F., da Rosa, M. M., Gonçalves, S. M. C., Viana, D. C. F., de Moraes Gomes, P. A. T., da Rocha Pitta, M. G., de Melo Rêgo, M. J. B., Pereira, M. C., Métifiot, M., Andreola, M.-L., & da Rocha Pitta, M. G. (2025). Novel Thiazolidinedione Derivatives as Potential ZIKV Antiviral Inhibitors. Microorganisms, 13(9), 1967. https://doi.org/10.3390/microorganisms13091967






