Microbial Assembly and Stress-Tolerance Mechanisms in Salt-Adapted Plants Along the Shore of a Salt Lake: Implications for Saline–Alkaline Soil Remediation
Abstract
1. Introduction
2. Materials and Methods
2.1. Description of the Study Area
2.2. Site Selection and Soil Collection
2.3. Determination of Rhizosphere Soil Physicochemical Properties
2.4. DNA Extraction, PCR Amplification, and Illumina MiSeq Sequencing
2.5. Isolation and Characterization of Rhizosphere Soil Bacteria
2.6. Screening of Plant Growth Promoting Bacteria
2.7. Data Processing
3. Results
3.1. Rhizosphere Soil Properties
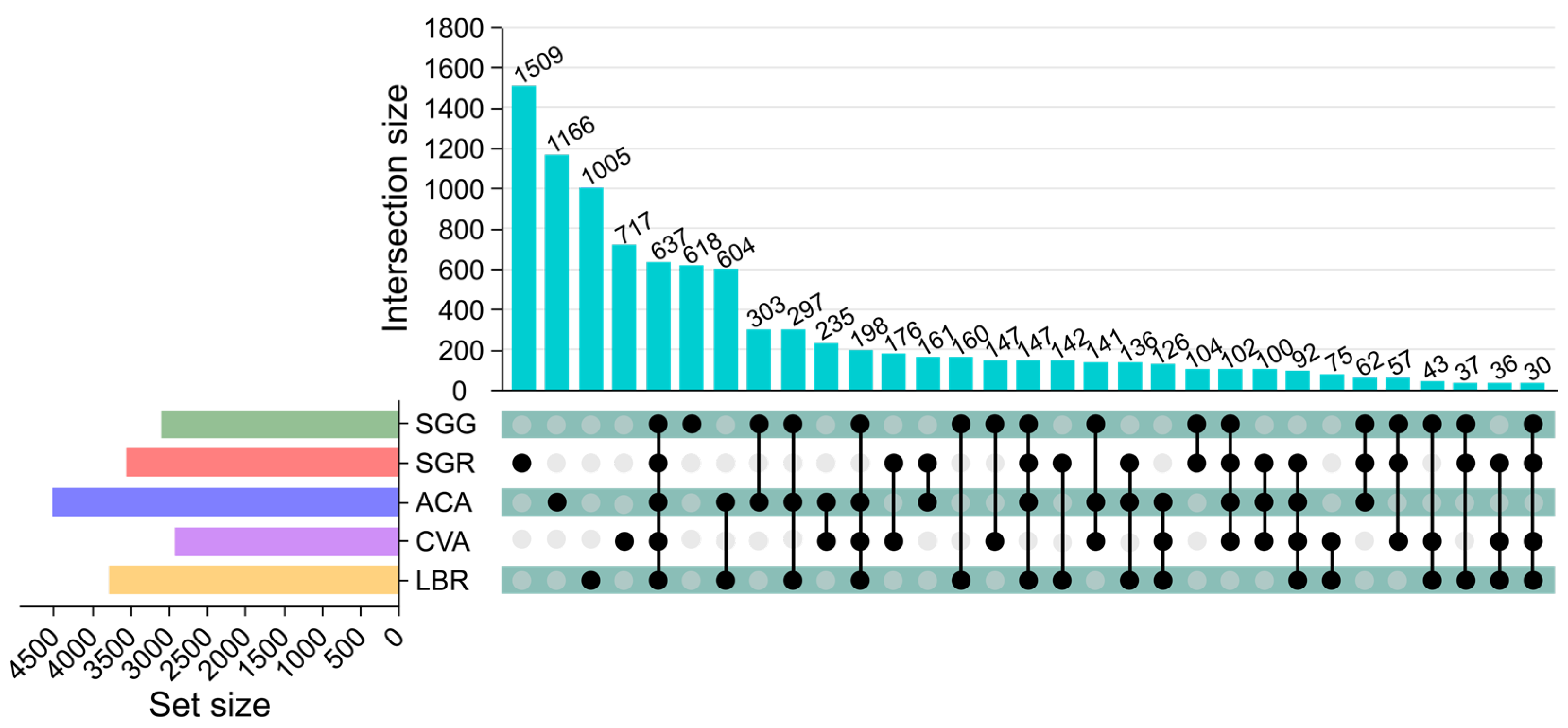
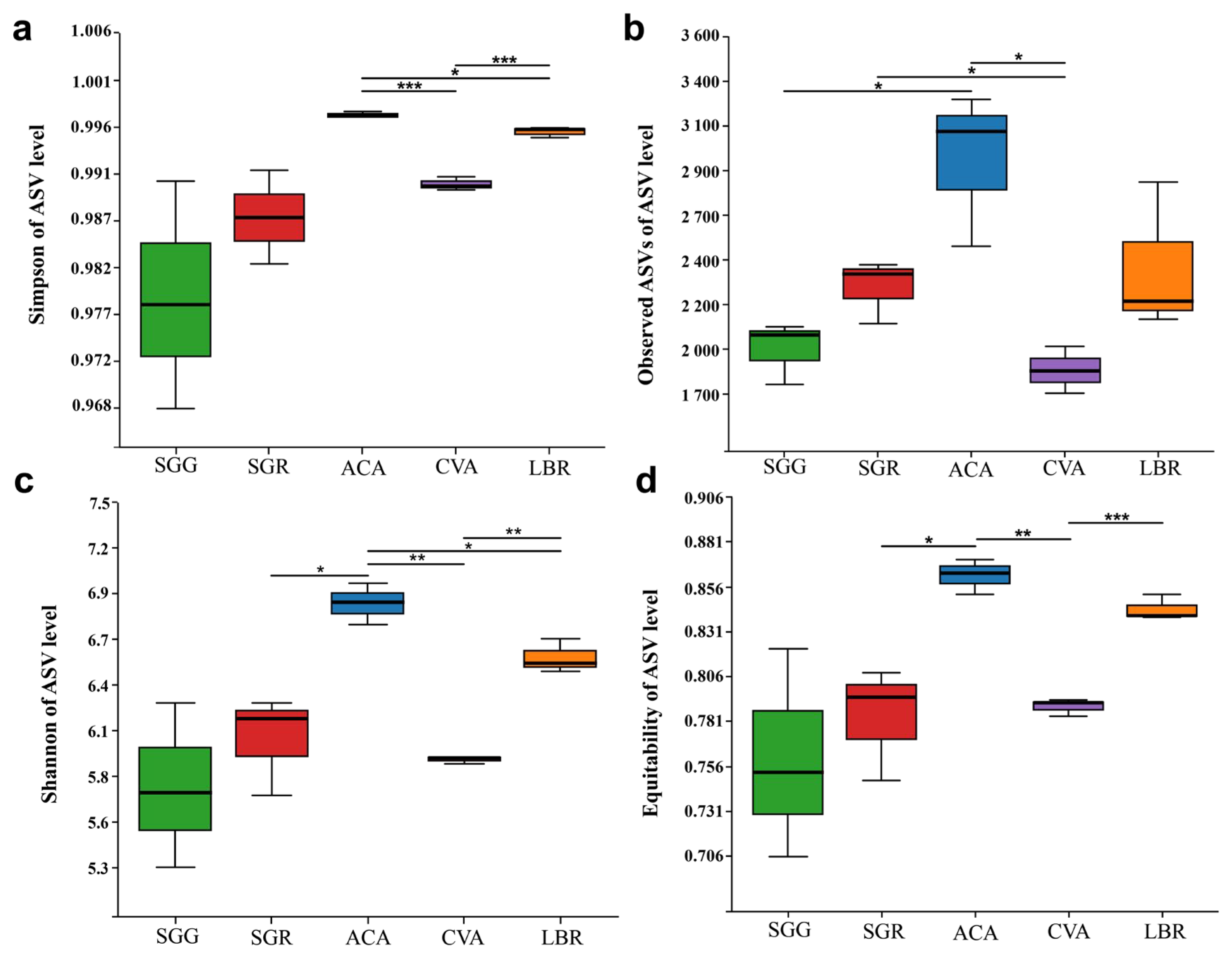
3.2. Diversity of Rhizosphere Soil Microbial Communities
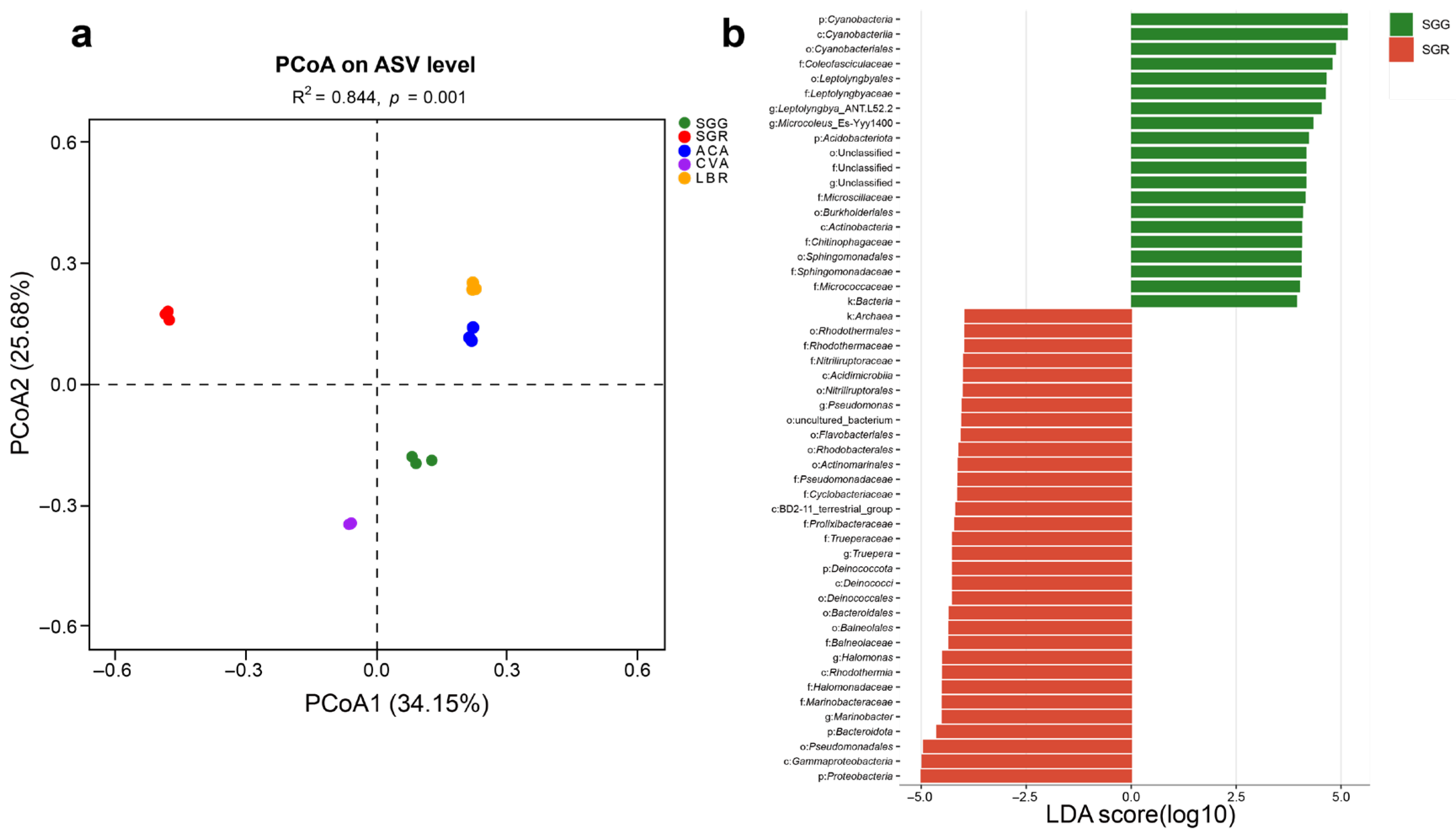
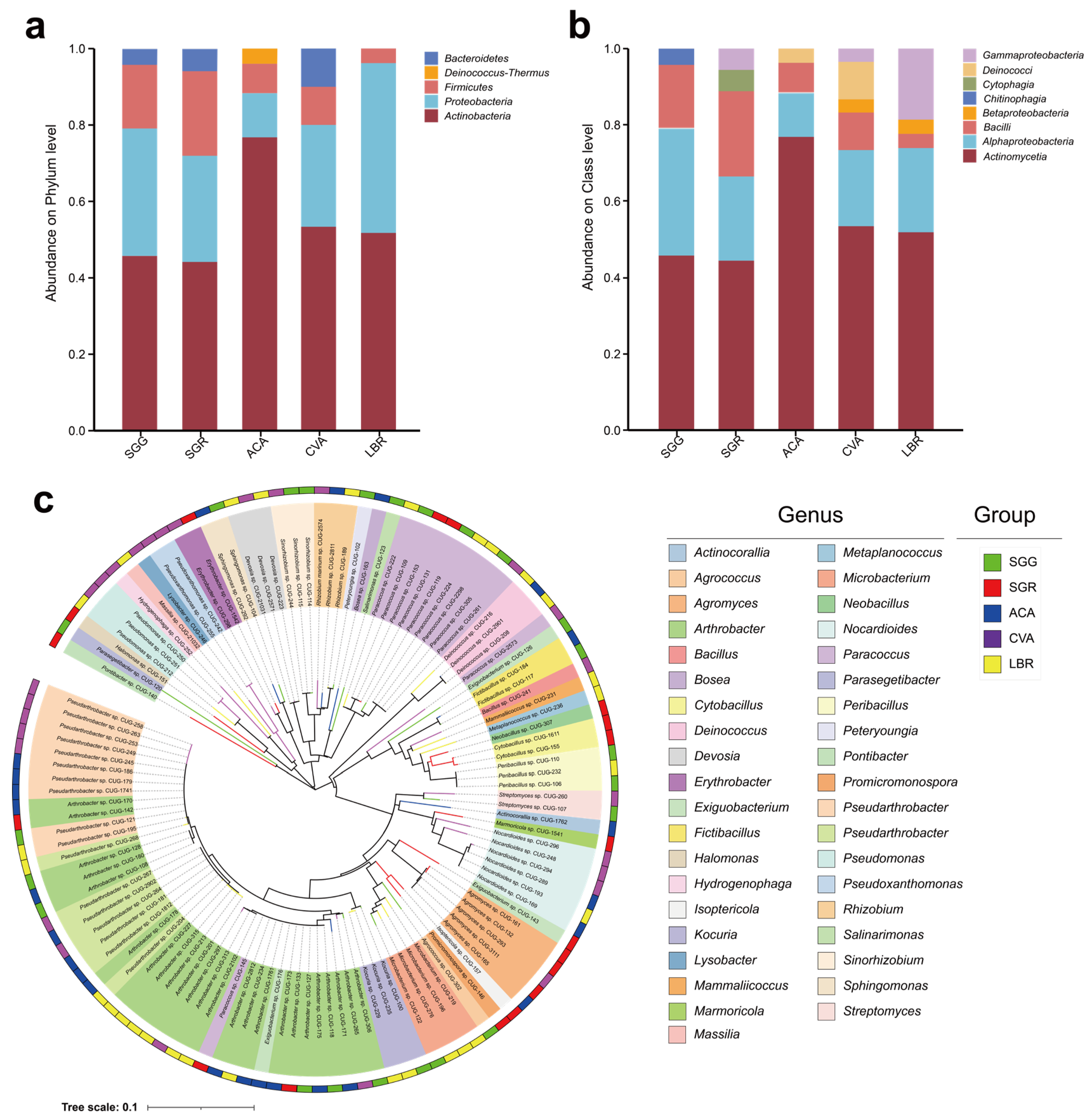
3.3. Composition of Rhizosphere Soil Microbial Communities
3.4. Structure of Rhizosphere Soil Microbial Communities
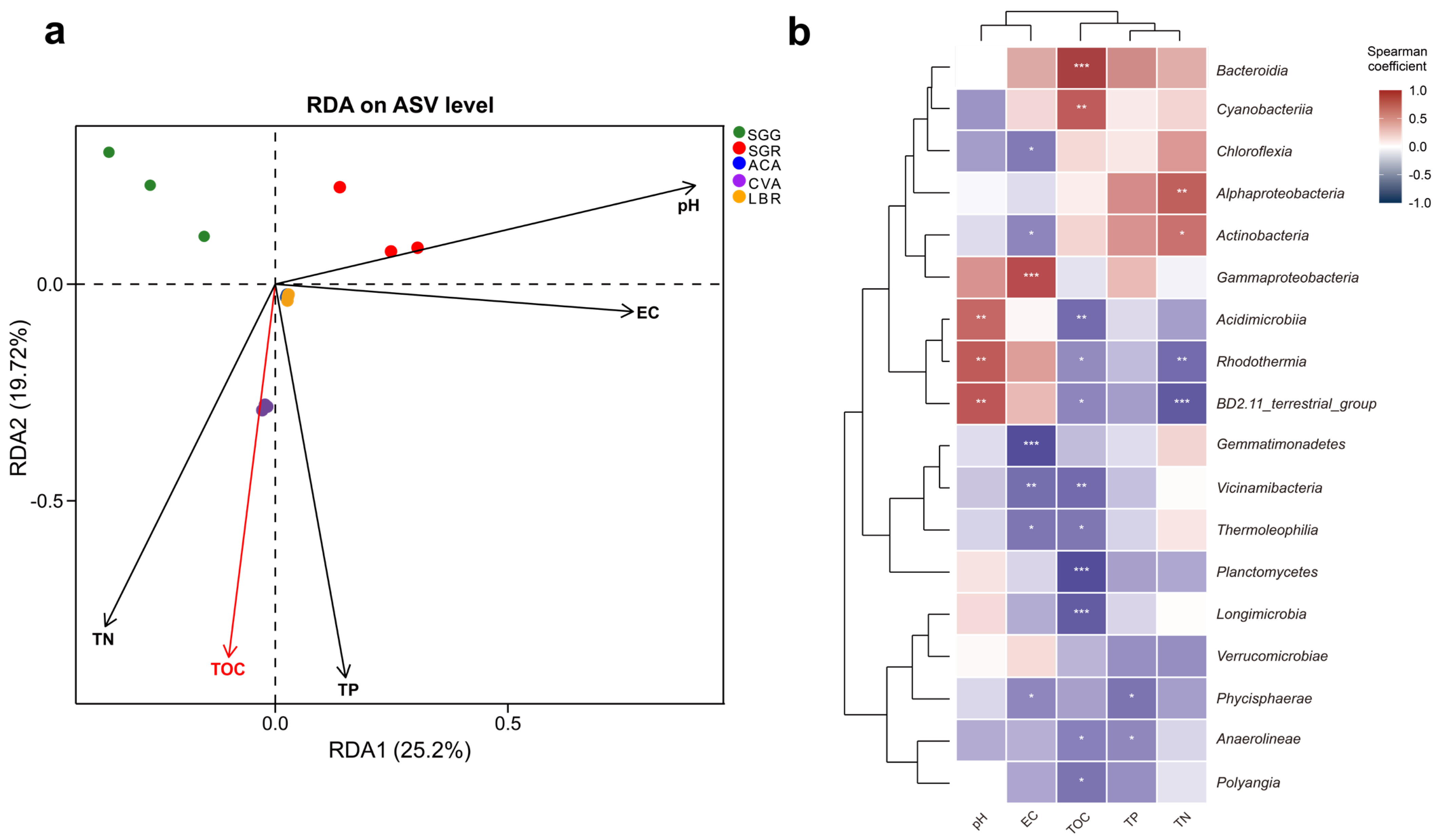
3.5. Correlations Between Rhizosphere Soil Physicochemical Properties and Microbial Communities
3.6. Diversity of Culturable Rhizosphere Soil Microorganisms
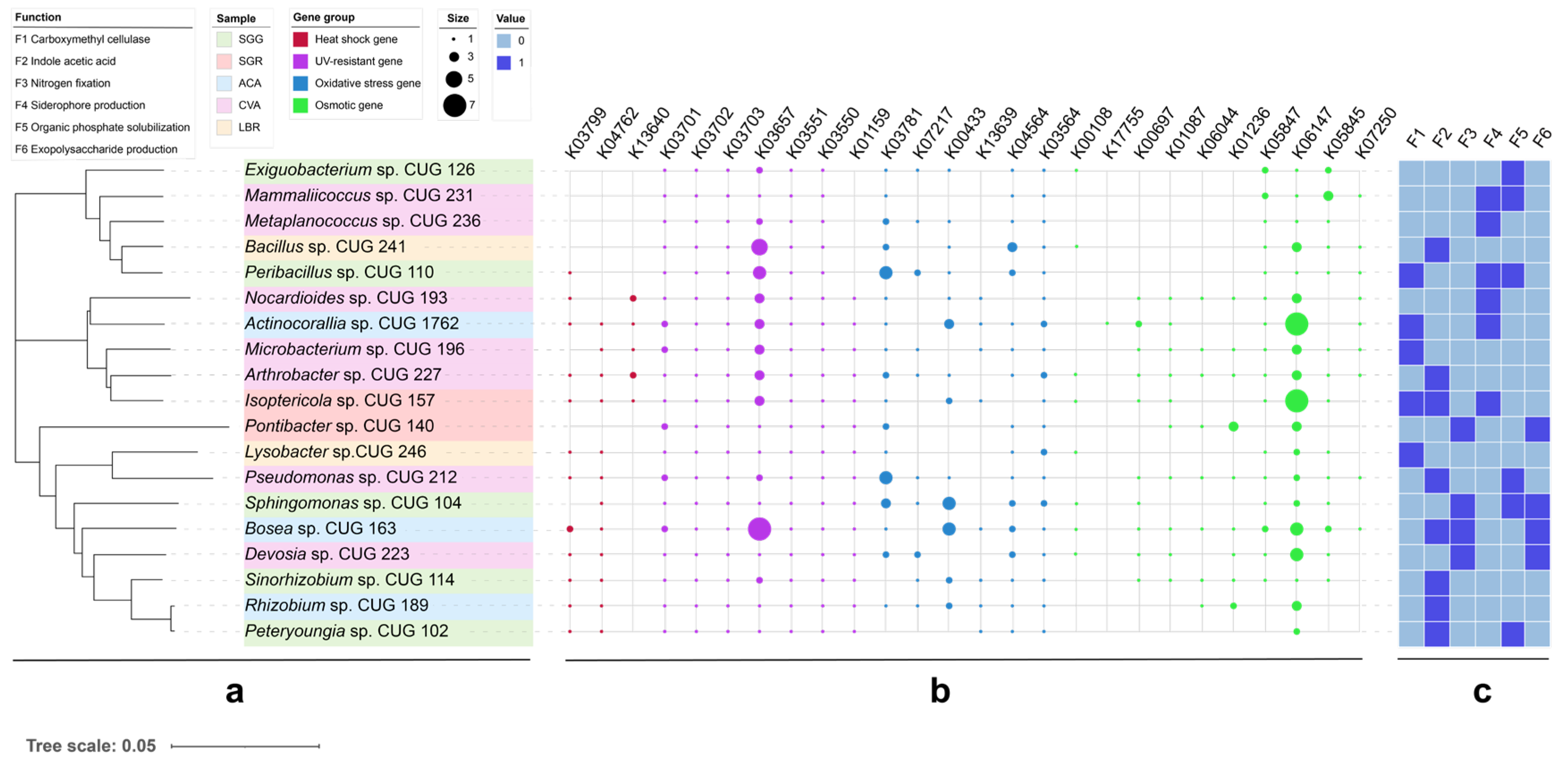
3.7. Results of Stress Resistance Screening
4. Discussion
4.1. Habitat-Specific Selection Drives Rhizosphere Microbial Assembly in Salt-Adapted Plants
4.2. Relationships Between Soil Factors and Rhizosphere Soil Microbial Communities
4.3. Functional Traits of Culturable PGPR and Stress Resistance Mechanisms
4.4. Implications of Rhizosphere Microorganisms in Salt Lake Ecosystems and Agricultural Applications
4.5. Limitations and Future Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gu, D.; Andreev, K.; Dupre, M.E. Major trends in population growth around the world. China CDC Wkly. 2021, 3, 604–613. [Google Scholar] [CrossRef] [PubMed]
- Flowers, T.J.; Colmer, T.D. Salinity tolerance in halophytes. New Phytol. 2008, 179, 945–963. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yasen, M.; Gong, M.; Zhou, Q.; Li, M. Structural variability in the rhizosphere bacterial communities of three halophytes under different levels of salinity-alkalinity. Plant Soil. 2024, 502, 709–723. [Google Scholar] [CrossRef]
- Xavier, J.F.; da Costa, D.P.; da Silva Gonçalves, J.V.; Pinto, M.C.F.; Goulart, R.S.; Zonta, E.; da Silva Coelho, I. Different halophytes orchestrate microbial diversity in the rhizosphere of salinity-impacted soils. Appl. Soil. Ecol. 2024, 202, 105588. [Google Scholar] [CrossRef]
- Garbeva, P.; van Elsas, J.D.; van Veen, J.A. Rhizosphere microbial community and its response to plant species and soil history. Plant Soil. 2008, 302, 19–32. [Google Scholar] [CrossRef]
- Han, Y.; Xu, L.; Liu, L.; Yi, M.; Guo, E.; Zhang, A.; Yi, H. Illumina sequencing reveals a rhizosphere bacterial community associated with foxtail millet smut disease suppression. Plant Soil. 2017, 410, 411–421. [Google Scholar] [CrossRef]
- Li, M.; Wang, J.; Yao, T.; Zhang, T.; Zhou, Q.; Mutallip, M. Bacterial diversity and community structure in the rhizosphere of four halophytes. Curr. Microbiol. 2021, 78, 2720–2732. [Google Scholar] [CrossRef]
- Li, Y.; Kong, Y.; Teng, D.; Zhang, X.; He, X.; Zhang, Y.; Lv, G. Rhizobacterial communities of five co-occurring desert halophytes. PeerJ 2018, 6, e5508. [Google Scholar] [CrossRef]
- Hollister, E.B.; Engledow, A.S.; Hammett, A.J.M.; Provin, T.L.; Wilkinson, H.H.; Gentry, T.J. Shifts in microbial community structure along an ecological gradient of hypersaline soils and sediments. ISME J. 2010, 4, 829–838. [Google Scholar] [CrossRef]
- An, X.; Wang, Z.; Teng, X.; Zhou, R.; Wang, X.; Xu, M.; Lian, B. Rhizosphere bacterial diversity and environmental function prediction of wild salt-tolerant plants in coastal silt soil. Ecol. Indic. 2022, 134, 108503. [Google Scholar] [CrossRef]
- Gong, Y.; Bai, J.; Yang, H.; Zhang, W.; Xiong, Y.; Ding, P.; Qin, S. Phylogenetic diversity and investigation of plant growth-promoting traits of actinobacteria in coastal salt marsh plant rhizospheres from Jiangsu, China. Syst. Appl. Microbiol. 2018, 41, 516–527. [Google Scholar] [CrossRef]
- Song, Y.; Ma, L.; Zhang, H.; Fu, R.; Liang, X.; Li, J.; Li, J.; Li, M.; Shan, Y.; Cheng, J. The diversity and structure of diazotrophic communities in the rhizosphere of coastal saline plants is mainly affected by soil physicochemical factors but not host plant species. Front. Mar. Sci. 2022, 9, 1100289. [Google Scholar] [CrossRef]
- Han, M.; Yu, H.; Huang, J.; Wang, C.; Li, X.; Wang, X.; Xu, L.; Zhao, J.; Jiang, H. Limited microbial contribution in salt lake sediment and water to each other’s microbial communities. Microorganisms 2024, 12, 2534. [Google Scholar] [CrossRef]
- Berg, G.; Grube, M.; Schloter, M.; Smalla, K. Unraveling the plant microbiome: Looking back and future perspectives. Front. Microbiol. 2014, 5, 148. [Google Scholar] [CrossRef] [PubMed]
- Babalola, O.O. Beneficial bacteria of agricultural importance. Biotechnol. Lett. 2010, 32, 1559–1570. [Google Scholar] [CrossRef] [PubMed]
- Vejan, P.; Abdullah, R.; Khadiran, T.; Ismail, S.; Boyce, A.N. Role of plant growth promoting rhizobacteria in agricultural sustainability—A Review. Molecules 2016, 21, 573. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.Z. Characterization of Adsorbed Organic Matter on Mineral Surfaces. Ph.D. Thesis, University of Massachusetts, Amherst, MA, USA, 2008. [Google Scholar]
- Morcillo, R.J.L.; Manzanera, M. The effects of plant-associated bacterial exopolysaccharides on plant abiotic stress tolerance. Metabolites 2021, 11, 337. [Google Scholar] [CrossRef]
- Semenov, A.V.; Pereira e Silva, M.C.; Szturc-Koestsier, A.E.; Schmitt, H.; Salles, J.F.; van Elsas, J.D. Impact of incorporated fresh 13C potato tissues on the bacterial and fungal community composition of soil. Soil. Biol. Biochem. 2012, 49, 88–95. [Google Scholar] [CrossRef]
- Upadhyay, S.; Singh, D. Effect of salt-tolerant plant growth-promoting rhizobacteria on wheat plants and soil health in a saline environment. Plant Biol. 2015, 17, 288–293. [Google Scholar] [CrossRef]
- Mayak, S.; Tirosh, T.; Glick, B.R. Plant growth-promoting bacteria confer resistance in tomato plants to salt stress. Plant Physiol. Biochem. 2004, 42, 565–572. [Google Scholar] [CrossRef]
- Zeng, F.; Zhu, Y.; Zhang, D.; Zhao, Z.; Li, Q.; Ma, P.; Zhang, G.; Wang, Y.; Wu, S.; Guo, S. Metagenomic analysis of the soil microbial composition and salt tolerance mechanism in Yuncheng Salt Lake, Shanxi Province. Front. Microbiol. 2022, 13, 1004556. [Google Scholar] [CrossRef]
- Li, C.; Gao, X.; Liu, Y.; Wang, Y. Impact of anthropogenic activities on the enrichment of fluoride and salinity in groundwater in the Yuncheng Basin constrained by Cl/Br ratio, δ18O, δ2H, δ13C and δ7Li isotopes. J. Hydrol. 2019, 579, 124211. [Google Scholar] [CrossRef]
- Liu, A.; Li, Y.; Wang, Q.; Zhang, X.; Xiong, J.; Li, Y.; Lei, Y.; Sun, Y. Analysis of microbial diversity and community structure of rhizosphere soil of Cistanche salsa from different host plants. Front. Microbiol. 2022, 13, 971228. [Google Scholar] [CrossRef]
- Sun, S.; Zhang, J.; Cai, C.; Cai, Z.; Li, X.; Wang, R. Coupling of non-point source pollution and soil characteristics covered by Phyllostachys edulis stands in hilly water source area. J. Environ. Manag. 2020, 268, 110657. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Niu, Y.; Zhu, H.; Dong, K.; Wang, D.; Liu, F. Remediation of zinc-contaminated soils by using the two-step washing with citric acid and water-soluble chitosan. Chemosphere 2021, 282, 131092. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef] [PubMed]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Huang, J.; Han, M.; Fang, B.; Yang, J.; Xiao, H.; Zhang, X.; Han, J.; Yu, D.; Jiang, H.; Li, W. Aquiflexum lacus sp. nov., isolated from a lake sediment sample. Arch. Microbiol. 2021, 203, 2911–2917. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, Y.; Li, X. USEARCH 12: Open-source software for sequencing analysis in bioinformatics and microbiome. Imeta 2024, 3, e236. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Holmes, S.P. Exact sequence variants should replace operational taxonomic units in marker-gene data analysis. ISME J. 2017, 11, 2639–2643. [Google Scholar] [CrossRef]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.-H.; Ha, S.-M.; Kwon, S.; Lim, J.; Kim, Y.; Seo, H.; Chun, J. Introducing EzBioCloud: A taxonomically united database of 16S rRNA gene sequences and whole-genome assemblies. Int. J. Syst. Evol. Microbiol. 2017, 67, 1613–1617. [Google Scholar] [CrossRef] [PubMed]
- Di Benedetto, N.A.; Campaniello, D.; Bevilacqua, A.; Cataldi, M.P.; Sinigaglia, M.; Flagella, Z.; Corbo, M.R. Isolation, screening, and characterization of plant-growth-promoting bacteria from durum wheat rhizosphere to improve N and P nutrient use efficiency. Microorganisms 2019, 7, 541. [Google Scholar] [CrossRef] [PubMed]
- Shreshtha, K.; Prakash, A.; Pandey, P.K.; Pal, A.K.; Singh, J.; Tripathi, P.; Mitra, D.; Jaiswal, D.K.; Santos-Villalobos, S.d.L.; Tripathi, V. Isolation and characterization of plant growth promoting rhizobacteria from cacti root under drought condition. Curr. Res. Microb. Sci. 2025, 8, 100319. [Google Scholar] [CrossRef]
- Luo, D.; Shi, J.; Li, M.; Chen, J.; Wang, T.; Zhang, Q.; Yang, L.; Zhu, N.; Wang, Y. Consortium of phosphorus-solubilizing bacteria promotes maize growth and changes the microbial community composition of rhizosphere soil. Agronomy 2024, 14, 1535. [Google Scholar] [CrossRef]
- Cui, Y.; Bing, H.; Fang, L.; Wu, Y.; Yu, J.; Shen, G.; Jiang, M.; Wang, X.; Zhang, X. Diversity patterns of the rhizosphere and bulk soil microbial communities along an altitudinal gradient in an alpine ecosystem of the eastern Tibetan Plateau. Geoderma 2019, 338, 118–127. [Google Scholar] [CrossRef]
- Deng, Q.; Zhang, T.; Xie, D.; Yang, Y. Rhizosphere microbial communities are significantly affected by optimized phosphorus management in a slope farming system. Front. Microbiol. 2021, 12, 739844. [Google Scholar] [CrossRef]
- Ding, J.; Zhou, G.; Chen, X.; Xu, W.; Gao, X.; Zhang, Y.; Jiang, B.; Li, H.; Wang, K. Analysis of microbial diversity and community structure of rhizosphere soil of three astragalus species grown in special high-cold environment of northwestern Yunnan, China. Microorganisms 2024, 12, 539. [Google Scholar] [CrossRef]
- Steinegger, M.; Soeding, J. MMseqs2 enables sensitive protein sequence searching for the analysis of massive data sets. Nat. Biotechnol. 2017, 35, 1026–1028. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11 molecular evolutionary genetics analysis version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Letunic, I.; Bork, P. Interactive Tree of Life (iTOL) v6: Recent updates to the phylogenetic tree display and annotation tool. Nucleic Acids Res. 2024, 52, W78–W82. [Google Scholar] [CrossRef] [PubMed]
- Lorenzi, A.S.; Chia, M.A. Cyanobacteria’s power trio: Auxin, siderophores, and nitrogen fixation to foster thriving agriculture. World J. Microbiol. Biotechnol. 2024, 40, 381. [Google Scholar] [CrossRef] [PubMed]
- Tan, W.; Zhao, X.; Dang, Q.; Cui, D.; Xi, B. Microbially reducible extent of solid-phase humic substances is governed by their physico-chemical protection in soils: Evidence from electrochemical measurements. Sci. Total Environ. 2020, 708, 134683. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, X.; Li, H.; Zhang, R.; Liu, X.; Nan, F.; Liu, Q.; Lv, J.; Feng, J.; Ma, C. Evaluating the role of recalcitrant dissolved organic matter in bacterial community dynamics in urbanized freshwater ecosystems. Sci. Total Environ. 2024, 957, 177475. [Google Scholar] [CrossRef]
- Guo, B.; Liu, Y.; Liu, P.; Cai, R.; Zheng, Q.; Shi, Q.; He, C. Glacial snow and ice contribute differentially to the dissolved organic matter in the runoff of Qiangyong Glacier, Tibetan Plateau. J. Hydrol. 2025, 652, 132600. [Google Scholar] [CrossRef]
- Korzhenkov, A.A.; Toshchakov, S.V.; Bargiela, R.; Gibbard, H.; Ferrer, M.; Teplyuk, A.V.; Jones, D.L.; Kublanov, I.V.; Golyshin, P.N.; Golyshina, O.V. Archaea dominate the microbial community in an ecosystem with low-to-moderate temperature and extreme acidity. Microbiome 2019, 7, 11. [Google Scholar] [CrossRef]
- Wang, X.; Sun, R.; Tian, Y.; Guo, K.; Sun, H.; Liu, X.; Chu, H.; Liu, B. Long-term phytoremediation of coastal saline soil reveals plant species-specific patterns of microbial community recruitment. Msystems 2020, 5, 14. [Google Scholar] [CrossRef]
- Xu, J.; Sun, L.; Sun, Z.; Xing, X.; Li, J.; Lu, X.; Huang, Y.; Ren, Q. Pontibacter beigongshangensis sp. nov., isolated from the mash of wine. Curr. Microbiol. 2019, 76, 1525–1530. [Google Scholar] [CrossRef]
- Cai, M.; Wang, L.; Cai, H.; Li, Y.; Wang, Y.; Tang, Y.; Wu, X. Salinarimonas ramus sp. nov. and Tessaracoccus oleiagri sp. nov., isolated from a crude oil-contaminated saline soil. Int. J. Syst. Evol. Microbiol. 2011, 61, 1767–1775. [Google Scholar] [CrossRef]
- Kim, S.-J.; Lim, J.-M.; Hamada, M.; Ahn, J.-H.; Weon, H.-Y.; Suzuki, K.-i.; Ahn, T.-Y.; Kwon, S.-W. Marmoricola solisilvae sp. nov. and Marmoricola terrae sp. nov., isolated from soil and emended description of the genus Marmoricola. Int. J. Syst. Evol. Microbiol. 2015, 65, 1825–1830. [Google Scholar] [CrossRef]
- Etesami, H.; Beattie, G.A. Mining halophytes for plant growth-promoting halotolerant bacteria to enhance the salinity tolerance of non-halophytic crops. Front. Microbiol. 2018, 9, 148. [Google Scholar] [CrossRef]
- Bodur, S.O.; Samuel, S.O.; Polat, M.F.; Aycan, M.; Asiloglu, R. Protists exhibit community-level adaptation and functional redundancy under gradient soil salinity. Sci. Total Environ. 2025, 981, 179606. [Google Scholar] [CrossRef]
| Samples | pH | EC (mS/cm) | TOC (mg/kg) | TP (mg/kg) | TN (mg/kg) |
|---|---|---|---|---|---|
| SGG | 8.12 ± 0.01 d | 6.48 ± 0.16 d | 358.70 ± 15.02 b | 46.13 ± 0.46 b | 247.38 ± 20.41 bc |
| SGR | 8.56 ± 0.03 a | 21.12 ± 0.20 a | 350.28 ± 3.53 b | 52.53 ± 2.31 b | 221.19 ± 6.44 a |
| ACA | 8.29 ± 0.02 b | 5.12 ± 0.20 e | 195.91 ± 5.63 c | 49.87 ± 15.79 b | 233.57 ± 15.57 ac |
| CVA | 8.20 ± 0.05 c | 13.04 ± 0.17 c | 1550.00 ± 6.28 a | 76.67 ± 3.70 a | 285.00 ± 4.69 a |
| LBR | 8.16 ± 0.03 cd | 17.12 ± 0.16 b | 192.50 ± 12.35 c | 58.13 ± 5.33 b | 258.81 ± 11.89 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Xu, L.; Qi, X.; Huang, J.; Han, M.; Wang, C.; Li, X.; Jiang, H. Microbial Assembly and Stress-Tolerance Mechanisms in Salt-Adapted Plants Along the Shore of a Salt Lake: Implications for Saline–Alkaline Soil Remediation. Microorganisms 2025, 13, 1942. https://doi.org/10.3390/microorganisms13081942
Wang X, Xu L, Qi X, Huang J, Han M, Wang C, Li X, Jiang H. Microbial Assembly and Stress-Tolerance Mechanisms in Salt-Adapted Plants Along the Shore of a Salt Lake: Implications for Saline–Alkaline Soil Remediation. Microorganisms. 2025; 13(8):1942. https://doi.org/10.3390/microorganisms13081942
Chicago/Turabian StyleWang, Xiaodong, Liu Xu, Xinyu Qi, Jianrong Huang, Mingxian Han, Chuanxu Wang, Xin Li, and Hongchen Jiang. 2025. "Microbial Assembly and Stress-Tolerance Mechanisms in Salt-Adapted Plants Along the Shore of a Salt Lake: Implications for Saline–Alkaline Soil Remediation" Microorganisms 13, no. 8: 1942. https://doi.org/10.3390/microorganisms13081942
APA StyleWang, X., Xu, L., Qi, X., Huang, J., Han, M., Wang, C., Li, X., & Jiang, H. (2025). Microbial Assembly and Stress-Tolerance Mechanisms in Salt-Adapted Plants Along the Shore of a Salt Lake: Implications for Saline–Alkaline Soil Remediation. Microorganisms, 13(8), 1942. https://doi.org/10.3390/microorganisms13081942







