Abstract
Helicobacter pylori (H. pylori) is a common gastric pathogen and a leading cause of non-cardia gastric cancers. Known determinants can affect the diagnostic accuracy of invasive clinical methods for H. pylori diagnosis. The objective of this study was to determine the diagnostic accuracy of the CLOtest, a rapid urease test, and the histopathologic examination compared with polymerase chain reaction (PCR) in esophagogastroduodenoscopy patients from a population with high prevalence and other risk factors that may influence diagnostic accuracy. From 2018 to 2022, patients were recruited from a medical care center serving the southwestern Navajo Nation. Summary statistics were calculated using PCR as the comparator to the CLOtest and histopathologic examination. Among the 466 study participants, 27.1% (95% CI 22.9, 31.7%) tested positive for H. pylori using PCR to detect pathogen DNA. Sensitivity was lowest for the CLOtest (57.0%; 95% CI 45.8, 67.6) and highest for the combination the CLOtest and histopathology (72.2%; 95% CI 62.8, 80.4). Patient history of infection or possible GI bleeding influenced sensitivity by over 5%. In high H. pylori prevalence areas, emphasis should be placed on ensuring adequate treatment of suspected positive infections as false-positive results were rare. Including a more sensitive test might reduce the number of individuals falsely classified as H. pylori negative.
1. Introduction
Helicobacter pylori (H. pylori) is a common gastric pathogen and a leading cause of non-cardia gastric cancers [1]. The global prevalence of H. pylori ranges from 19% in developed countries to 88% in developing nations [2]. The United States estimated that H. pylori prevalence is 35%. However, a much higher prevalence is observed among Native Americans [2]. Among members of the Navajo Nation, active H. pylori prevalence has been estimated to be as high as 56–70%, which likely contributes to gastric cancer rates that are 3–4 times higher compared to non-Hispanic Whites in Arizona [3,4,5]. Gastric cancer is an outcome in approximately 9% of persons with H. pylori infection globally [6].
The detection of H. pylori infection is established via invasive and non-invasive techniques with varying sensitivities, specificities, and predictive values that fluctuate with disease prevalence, patient risk factors, or concurrent medication use. Common non-invasive diagnostic methods include the urea breath test (UBT), serology, and stool antigen assays. The UBT is among the most commonly used non-invasive diagnostic procedures, which identifies active infection by detecting a labeled carbon isotope in the patient’s expired air, produced as a byproduct of urea hydrolysis by H. pylori urease. Invasive diagnostic tests involve procuring gastric mucosal samples by swabbing, brushing, or during an esophagogastroduodenoscopy (EGD). Common clinical methods include the Rapid urease tests (RUTs, e.g., the Campylobacter-like organism test (CLOtest)) and histopathological evaluation. Additionally, polymerase chain reaction (PCR) testing can be used to detect H. pylori DNA, but it may require collaboration with research partners, limiting its use in clinical settings.
H. pylori detection tests have known determinants that impact their diagnostic accuracy. Rapid urease tests (e.g., a CLOtest) have been noted to return frequent false negative results with recent use of antibiotics, bismuth-containing compounds, proton pump inhibitors (PPIs), and the presence of intestinal metaplasia [7], atrophic gastritis [8], or upper gastrointestinal (GI) bleeding [9]. The sensitivity of histopathological analysis is similarly reduced by upper GI bleeding [10,11], atrophic gastritis [12], recent PPI therapy, or the presence of intestinal metaplasia [13]. Older studies compared RUTs with histopathology and showed somewhat improved sensitivity in the setting of upper GI bleeding [10,14,15]. Additionally, the accuracy of histopathologic evaluation depends on the skills of the microscopist or pathologist [13].
The Navajo Nation population exhibits unique environmental risk factors that may influence the accuracy of existing H. pylori diagnostic tests. Harris et al. [3] found a significant association between a lack of access to regulated water and a higher prevalence of H. pylori. Over 30% of Navajo members lack access to public water systems, and unregulated water sources may exceed the maximum contaminant levels of arsenic and uranium outlined in the Safe Drinking Act [16]. Chronic arsenic exposure has been tied to an increased risk for GI bleeding, inflammation, and malignancy [17,18]. Furthermore, to ensure stability of the enzyme and other components, CLOtest kits require storage at temperatures between 2 °C and 8 °C, which may not be reliably achievable under Northern Arizona climate conditions during the summer months [19].
Given the high prevalence of H. pylori among members of the Navajo Nation, it is imperative to ensure accurate and reliable diagnosis. Determining the true presence or absence of this carcinogenic organism is crucial for optimal treatment and improved health outcomes. This study aimed to evaluate the diagnostic accuracy of the CLOtest and histopathology in comparison to PCR testing among Navajo Nation members who were determined to require an esophagogastroduodenoscopy (EGD) test for medical reasons.
2. Materials and Methods
2.1. Setting and Subjects
For this cross-sectional study, we recruited 466 patients undergoing EGD at Winslow Indian Health Care Center (WIHCC) between 29 January 2018, and 10 August 2022. WIHCC provides medical care to eight chapters in the southwestern Navajo Nation. All adult patients scheduled for an EGD during the study period who reported Navajo as their tribal affiliation were eligible to participate. A packet of information about the study was provided during check-in to the clinic for the procedure, including copy of the consent form. The Navajo clinic nurse or the physician performing the procedure was present to answer questions. Individuals gave written informed consent. All participants provided informed consent for the abstraction of their medical records and the acquisition of two additional gastric biopsy samples during their elective EGD. After consent, samples were collected by the physician and medical record abstractions performed by the clinic nurse. Participants were only identified through a participant ID. Links between participant name and research data were not provided to the research lab for sample testing or data analysis.
2.2. Sample Testing
All biopsies underwent PCR testing for the detection of active H. pylori infection and the identification of virulence genes. Indeterminate results were excluded from the analysis. Three diagnostic tests were used to assess the status of H. pylori infection. WIHCC performed the CLO Rapid Urease Test (Avanos, GA, USA). One pair of gastric biopsy samples was sent to a laboratory contracted by WIHCC to perform histopathological examination and tested using immunohistochemical (IHC) staining; the precise techniques and methods may have varied depending on the pathologist’s clinical judgment. PCR testing was performed by Northern Arizona University (NAU) research partners between 29 January 2018, and 21 April 2023, and described elsewhere [20]. The two additional research biopsy samples were separately placed in 400 µL RNAlater (Sigma Chemical Co., St. Louis, MO, USA) filled tubes and then transported to the NAU laboratory. Pathogen DNA was isolated using the FastDNA Spin Kit (MP Biomedicals, LLC, Solon, OH, USA) and the FastPrep 24 instruments (MP Biomedicals LLC) as described by the manufacturer. Positive and negative controls were included in each reaction. In some cases, the ~519 bp-amplified product was separated by electrophoresis in 1% agarose gels, followed by SYBR Green staining and analysis under an ultraviolet (UV) light. The 16S rDNA gene was used with H. pylori 26695 (ATCC 700392) and H. pylori 60190 (ATCC 49503) strains as reference isolates.
2.3. Medical Record Abstraction
Medical record data—including demographic information, endoscopy indication, and previous H. pylori testing information—were abstracted by WIHCC staff members between 15 September 2018 and 22 August 2022. The medical record data and biopsy samples were de-identified and linked only by study participant ID, with the research team not having access to the linkage. Body Mass Index (BMI) was calculated from recorded height and weight values and classified by WHO categories [21].
2.4. Diagnostic Risk Factors
H. pylori population-specific prevalence, recent treatment for H. pylori infection, and the presence of blood in the gastric environment are established factors influencing the outcome of the diagnostic tests described above. For the estimation of predictive values, prevalence was estimated using published values for this population undergoing EGD [20], and the larger community from which these patients arise [3]. Samples were classified as being from patients with a suspected history of H. pylori infection when the record abstraction included treatment for the infection or when the indication for the EGD was listed as eradication check. Samples were classified as being from patients with suspected blood in the gastric environment-based signs or symptoms of GI bleeding in the record abstractions—blood in vomit or stool, a known upper GI bleed, or anemia significant enough to indicate endoscopy.
2.5. Data Analysis
We used real-time PCR as the gold standard, as it is highly sensitive and specific, and is recommended as a gold standard where laboratory capacity exists [22]. However, it is considered experimental and is not widely available for clinical use [23,24]. There is no consensus regarding a universal gold standard for comparing direct (e.g., histopathology, CLOtest, or culture) and indirect (antibody in stool or urine, UBT, or stool antigen tests) diagnostic testing [25]. As a clinical test-and-treat diagnostic tool, the noninvasive UBT is considered the gold standard and is appropriate for noninvasive confirmation of eradication or reinfection in high-prevalence populations [26,27,28]. Among invasive clinical diagnostic tools, histopathological examination is considered the gold standard for clinical diagnosis using biopsy samples [28].
Sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV), Receiver Operating Curves (ROC), percent agreement, and kappa coefficients were calculated separately for the CLOtest and histopathology against PCR as the gold standard [22]. We also evaluated the instances when a sample was positive, as well as when either the CLOtest or histopathology test was positive. We assumed 23% H. pylori prevalence in this population of patients undergoing EGD [20]. Analyses were stratified by risk factors that affect these diagnostic tests, specifically medical record notation of prior H. pylori infection history and presence of blood in the gastric environment. We did not further stratify by known risk factors such as age or sex.
Analyses were performed using Stata v17.0 (College Station, TX, USA). Demographic characteristics were compared by t-test and chi-square with Fisher’s exact to correct for small counts. The diagt command was used to summarize the diagnostic test performance. Cohen’s Kappa assessed interrater reliability between the tests according to guidelines suggested by Landis and Koch [29], where kappa < 0.00 indicated poor agreement, 0.00–0.20 was slight, 0.21–0.40 was fair, 0.41–0.60 was moderate, 0.61–0.80 was substantial, and 0.81–1.00 indicated almost perfect agreement. Only 117 samples would have been necessary to detect a medium effect at alpha = 0.05 and a power = 0.9.
2.6. Ethical Approvals
Prior to beginning sample collection, formal support tribal resolutions were obtained for this research from participating chapters, associated Navajo Agency Council areas, and Winslow Indian Health Care Center. The protocol and consent forms were approved by the Navajo Nation Human Research Review Board (NNHRRB; NNR-16-26). The study also was reviewed and approved by the NAU IRB (NAU: 819500-1). All data were deidentified with links broken prior to sending samples to NAU the study was deemed exempt. An earlier draft of this manuscript was approved by the NNHRRB 18 June 2024, with notification received 17 January 2025.
2.7. Patient and Public Involvement
The inception of this project was the observation of a clinical partner of the high rates of gastric cancer in his clinic and subsequent discussions about the diagnostic tests used to detect H. pylori, a known risk factor for developing gastric cancer. As part of all work on the Navajo Nation, we presented the project for approval and comments to community members at chapter meetings in the area before beginning the project. We also report the findings to the communities and continue to do so.
3. Results
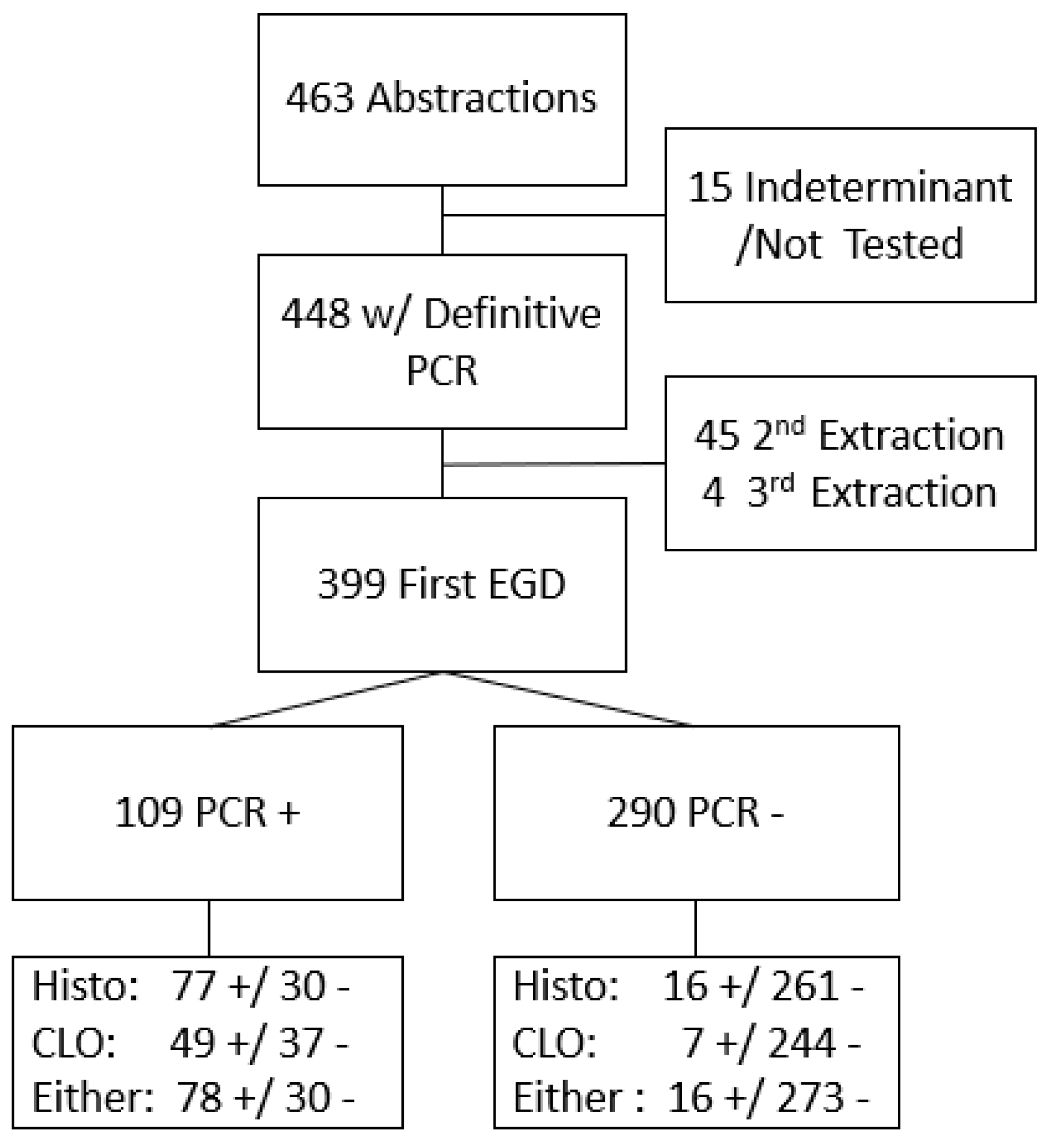
Among 463 participating patients with complete records and biopsy samples, PCR results were definitive for 4448 (15 had indeterminate results and were excluded). Of these 448 samples, 399 were identified as originating from their earliest endoscopy during the study period (Figure 1). Of these 399 patients, the CLOtest was performed in 337 cases, histopathologic examination in 384, and a combination of either the CLOtest or histopathology in 397 patients. H. pylori infection prevalence was 27.1% (95% CI 22.9, 31.7%) based on PCR results. Using histopathology results only, prevalence was similar (24.2%; 95% CI 20.2%, 28.8%). However, the prevalence estimate based on the CLOtest was lower (16.6%, 95% CI 13.0%, 21.0%). The US prevalence is estimated at 30%, and our prior work in this same population estimated the community prevalence of active infection to be 56% using UBT [3,30].
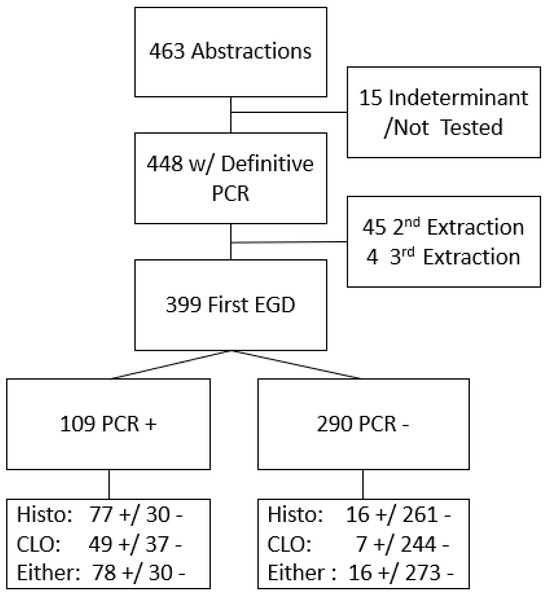
Figure 1.
Flow of participants through PCR and compared by Histopathology (Histo) or Campylobacter-like organism test (CLOtest) diagnostic testing result either alone or together. + indicates a positive result; - indicates a negative result.
Table 1 presents a comparison of participants by demographic and clinical indicators. Patients testing negative for H. pylori were older (57.6 years) than those testing positive (52.5 years). This effect was more pronounced among patients with a suspected history of H. pylori infection (59.0 years versus 52.6 years for those testing negative and positive, respectively, p < 0.01). Males had a higher percentage of positive test results (p < 0.01).

Table 1.
Demographic characteristics and symptoms of Navajo patients undergoing their first endoscopy by H. pylori status using PCR sequencing.
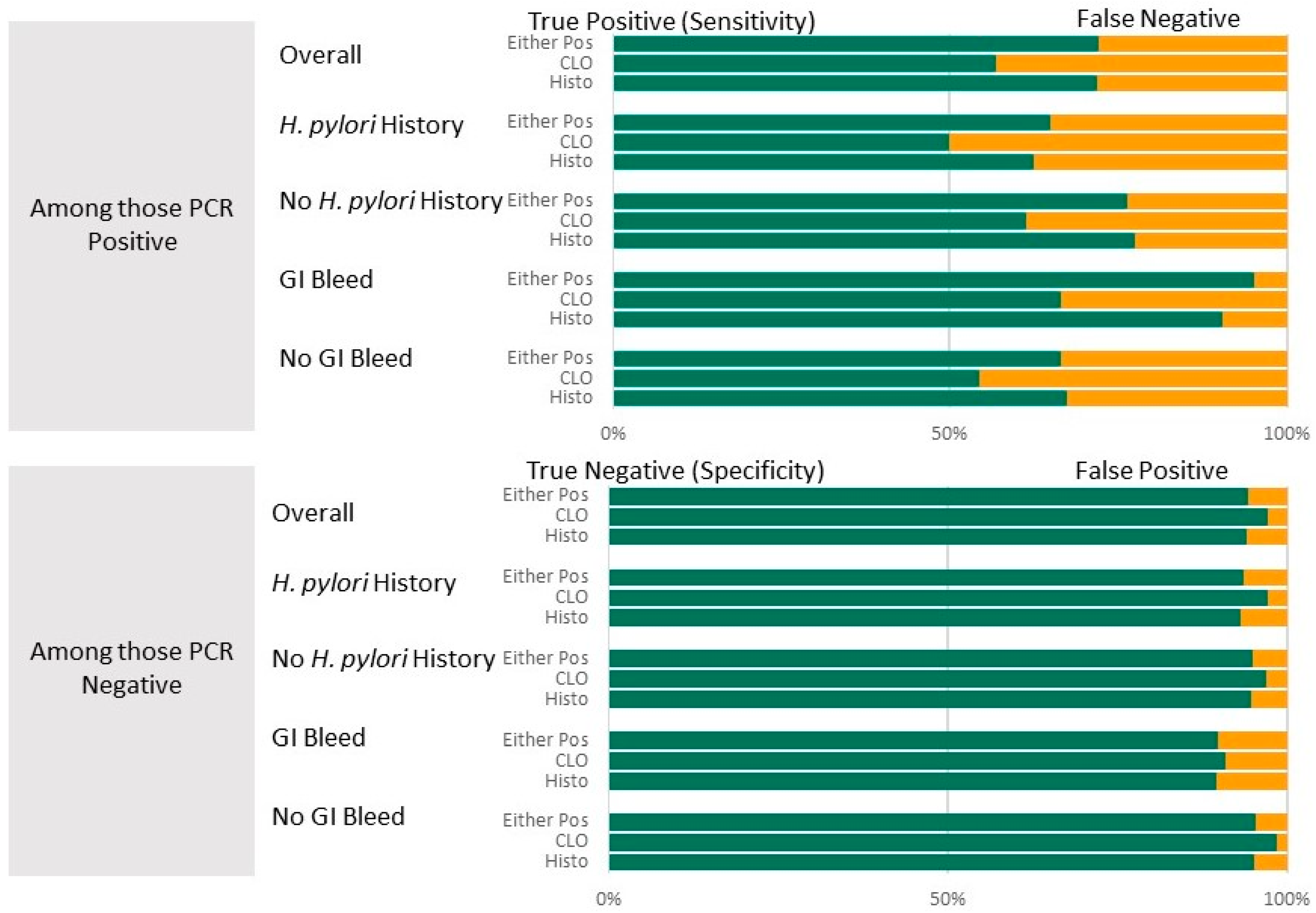
Table 2 presents summary statistics for diagnostic test results (CLOtest, histopathology, and combination). The table summarizes diagnostic accuracy for all participants. It is then stratified by whether or not a history of H. pylori was suspected and whether or not a suspicion of GI bleed was an indication for the EGD. Both the CLOtest and histopathology, individually and in combination, have high specificity (94.2–97.2%) and high NPV (88.3–91.9%) compared to the PCR results. Sensitivity was lowest for the CLOtest (57.0%) and highest for the combination (72.2%), while PPV was lowest for histopathology (78.8%) and highest for the CLOtest (85.9%). These results are visualized in Figure 2. There was substantial agreement between the clinical and PCR results, with Kappa values between 0.61 and 0.70.

Table 2.
Summary test performance statistics for three H. pylori diagnostic tests assuming an underlying prevalence of 23%. PCR of the H. pylori 16S gene used as the gold standard.
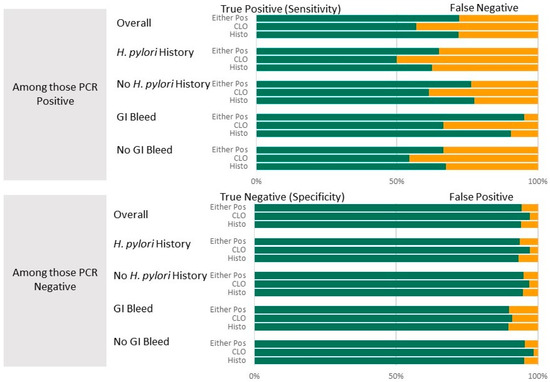
Figure 2.
Diagnostic accuracy (top panel: Sensitivity shown in green (with false negatives shown in yellow; lower Panel: Specificity shown in green with false positives shown in yellow)) of the CLOtest and histopathology diagnostic tests overall and by suspected history of H. pylori or GI bleed as EGD indicators. PCR test results were the gold standard.
Stratifying by suspected history of H. pylori. Among those with a suspected history of H. pylori infection in the medical record, the ability to detect non-infected individuals remained high (specificity 93.3–97.3%), while the ability to detect individuals with an infection was reduced (sensitivity decreased by 7–9.5%) compared to the overall rate. Predictive values also remained near 80%, although slightly reduced from the overall predictive values for patients with a history of infection: PPV for a positive histopathology decreased by 5.1%, and by 4.4% for the combination. In contrast, compared with the overall, when restricting the comparison to those without a history of H. pylori, the test statistics improved (sensitivity by 4.3–5.6%) or remained nearly the same (average change for specificity, NPV, and PPV was 1.1%; Table 2).
Stratifying by Suspected GI bleed as EGD indication. Among those with suspicion of GI bleed as an indication for the EGD, the ability to detect an infected individual increased compared with the overall ability for each test individually. The greatest increase was improved sensitivity by 23% when using the combination, but histopathology alone yielded an 18% improvement. Detecting a non-infected individual decreased by about 5% across all tests. However, few (<70) patients were identified as having a possible GI bleed, and the resulting confidence intervals are wide. In contrast, compared with the overall, when restricting comparisons to those without GI bleed, sensitivity decreased by about 4%. Specificities, PPV, and NPVs remained within 1–2% of the overall, except for PPV, which increased by 5.9%.
Demographic and clinical characteristics of false positives and false negatives. A comparison of demographics between those correctly detected and those missed as cases (sensitivity, Table S1) revealed that the mean age of the 29 participants incorrectly identified as negative was higher, at 57.6 years, compared to the 50.7 years of the 76 participants correctly identified as positive by histopathology (p = 0.04). Likewise, the average age of the 36 patients with false-negative results was higher than the mean for the 49 participants correctly identified as positive by the CLOtest (59.6 vs. 49.6, p < 0.01). Although the trends were similar, there were no significant demographic differences between true negatives and false positives by either histopathology or the CLOtest (Table S2).
4. Discussion
This cross-sectional study aimed to assess two routine invasive clinical diagnostic tests, the CLOtest and histopathology, in comparison to PCR for identifying H. pylori infection among patients undergoing scheduled EGD within an American Indian-serving clinical setting. Among those patients for whom this was their first EGD within the study period, 27.1% were positive for H. pylori by PCR. This is lower than the prevalence of the source population. However, this was expected as the individuals included in the study may have been undergoing treatment for GI conditions (41.4% of participants indicated prior H. pylori infection). As others have shown [31], H. pylori was less common among female patients (23% positive compared with 37% of males). We found no differences by BMI or smoking status.
The diagnostic tests had a higher capacity to correctly identify negative patients (specificity) and a moderate ability to identify infected patients (sensitivity). The highest agreement (TP + TN)/total: 88.4%) was achieved when the CLOtest or histopathology was used to indicate infection. This means, in a hypothetical setting where the prevalence is nearly 25%, both tests are used. Still, only one needs to be positive to diagnose H. pylori infection; among 1000 patients, 70 infected patients would be incorrectly identified as negative. In contrast, 41 patients without the infection would be incorrectly marked as having the infection. The lowest false positive rate (i.e., chance of treating a patient who is not infected) occurs when using the CLOtest alone. Still, the CLOtest alone also returns the highest false negative (108 missed diagnoses in our hypothetical population). Our results suggest that false-positive results are rare with these tests, meaning emphasis should be placed on ensuring adequate treatment of a suspected positive H. pylori infection.
Sensitivity decreased across both diagnostic tests among patients with a suspected history of H. pylori, which is consistent with prior studies finding that treatment with PPIs and antibiotics can impede H. pylori detection [13]. The American College of Gastroenterology recommends two weeks of withheld therapy before testing for eradication [32]. While the data abstraction process did not clarify the timing of each patient’s prior H. pylori infection to ascertain if or when they ceased PPI or antibiotic therapy, the observed decrease in sensitivity is expected.
In contrast to prior studies, sensitivity increased for the CLOtest and histopathology among those with a possible GI bleed. Tu et al. [10] found CLOtests and histopathology were less sensitive than non-invasive tests like the UBT among patients with peptic ulcer bleeding (CLOtest: 45.5%, histopathology: 77.2%, and UBT: 95.4%). Further, they found that blood in the antrum had the strongest effect on the CLOtest sensitivity. Sensitivities were higher among those with a suspected GI bleed as an endoscopy indication for the CLOtest and histopathology (66.7% and 90.5%, respectively) than those without GI bleeding (54.4% and 67.4%, respectively). However, we defined a GI bleed indication as anemia significant enough to warrant EGD or blood in the stool or vomit. Specific underlying features to more clearly define this symptomatology were limited. It must be noted that the number of patients with this indication was small (n < 70).
Guidelines for treatment of H. pylori include 7 or 14 days of a combination of antibiotics plus a PPI, with a recommendation to consider probiotics [32]. Further, GI upset is commonly reported as an association with treatment, and 21% of respondents in a national survey did not complete H. pylori treatment as a result [33]. Given that H. pylori antibiotic resistance rates are rising [34], the high prevalence of H. pylori and diagnostic specificity suggest a lower risk of unwarranted treatments. Regional antibiotic susceptibility data for H. pylori are scarce [32]; although high presence of antibiotic resistance mutations in amplified H. pylori DNA has been identified in this population (38% for clarithromycin and 94% for metronidazole) [35]. Inquiring about previous antibiotic use may help clinicians select treatment regimens more effectively [36]. Our results indicate that H. pylori is more frequently underdiagnosed (low sensitivity) than falsely diagnosed (high specificity). These findings suggest that clinicians within the Navajo population should favor treating patients with diagnostic indications. Furthermore, ensuring strong patient support and follow-up to promote compliance with antibiotic therapy remains critical to mitigating the risks of undertreated infections and antibiotic resistance.
Our study has limitations. Although WIHCC is the region’s primary clinic for Navajo citizens, this study included a sample of patients who underwent endoscopy and elected to participate in this study. Thus, it may not be representative of the community with respect to symptomology, treatment, and access to care. Additionally, the record abstraction and medication review were not comprehensive for potential treatments in other locations and whether medications were described in the record. These issues may have led to some misclassification regarding suspected H. pylori history or GI bleeding. However, we included only patient data from their first visit to minimize the inclusion of patients who may have been post-H. pylori treatment. Another possible limitation may have been the collection of a single antral and fundal specimen for the research arm of this study. For the assessment of H. pylori, atrophic gastritis, and gastric intestinal metaplasia, the Updated Sydney System Biopsy Protocol relies on five gastric biopsies (two from the antrum, one from the incisura angularis, and two from the body) [37]. While this may have contributed to lower overall sensitivity rates, it is unlikely that the differences observed with suspected GI bleeding and prior H. pylori infection were affected.
Despite these limitations, our study demonstrates that, compared with PCR, two commonly used invasive diagnostic methods exhibit high specificity but low sensitivity for H. pylori detection among patients in this clinical setting. This lower sensitivity is most pronounced among older patients. Specifically, we identified a statistically significant decrease in mean age among patients with true positive test results compared to those with false negative results with both histopathology and a CLOtest. The Maastricht VI/Florence Consensus Report recommends invasive diagnostic testing for H. pylori among patients over 50 years of age with symptomatic dyspepsia due to their increased risk of gastric cancer [38]. Accordingly, adherence to this recommendation is likely to yield insufficient H. pylori detection among patients in whom these invasive methods are most strongly indicated and may be contributing to the elevated prevalence of H. pylori infection and gastric cancer within the Navajo Nation [3,4,5]. One possible contributing factor to this difference may be that older patients exhibit a greater lifetime exposure to heavy metals in a region wherein over 30% of inhabitants lack access to regulated water systems. However, further research is needed to elucidate this possible connection and to identify other factors [16]. Per the Maastricht recommendations, complementary testing with non-invasive methods, including serology or UBT, should be pursued in patients with high clinical suspicion for H. pylori infection, even if invasive testing yielded negative findings. Our results also suggest that invasive testing exhibits high specificity for H. pylori diagnosis, which, when understood in the context of the Navajo population’s 3–4 times higher disease prevalence compared to non-Hispanic Whites, warrants both diagnostic and therapeutic vigilance on behalf of clinicians to ensure that adequate detection and treatment are undertaken.
5. Conclusions
The high specificity of these tests (i.e., false positive results are rare) indicates that emphasis should be placed on ensuring adequate treatment of a suspected positive infection within the Navajo population, given the high H. pylori prevalence and limited diagnostic sensitivity. Including a more sensitive test might reduce the number of individuals falsely classified as H. pylori negative, especially older patients in whom invasive testing is most indicated. This test limitation should be considered when setting up clinical approaches to serving this population and reducing the burden of gastric disease and gastric cancer.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/microorganisms13081920/s1, Table S1: Demographic and clinical characteristics of false positives and false negatives; Table S2: Comparison of Positive and Negative Predictive Values.
Author Contributions
Conceptualization, F.P.M., P.R.S., R.B.H. and H.E.B.; methodology, F.P.M., H.E.B., R.B.H. and J.G.; data validation, R.D., L.P., H.E.B. and F.P.M.; formal analysis, H.E.B., P.R.S., J.G. and R.B.H.; investigation, F.P.M., R.D. and L.P.; resources, F.P.M., R.D., L.P., H.E.B. and R.B.H.; data curation, H.E.B. and F.P.M.; writing—original draft preparation, H.E.B., R.B.H., J.G. and J.J.; writing—review and editing, F.P.M., R.D., L.P., J.G. and P.R.S.; supervision, F.P.M. and R.B.H.; project administration, H.E.B. and F.P.M.; funding acquisition, R.B.H., F.P.M. and P.R.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Cancer Institute of the National Institutes of Health under the awards U54CA143924 (UACC) and U54CA143925 (NAU) for the Partnership of Native American Cancer Prevention.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board of Northern Arizona University (819500-1; 7 March 2016) and the Navajo Nation Human Research Review Board (NNR-16.263; 13 June 2018). All personnel associated with the study completed all required human subject training modules.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the Navajo Nation Human Research Review Board because they are the property of the Navajo Nation.
Acknowledgments
We thank the participating tribal governmental entities for their support and review of this work, the board of directors at WIHCC, who generously allowed us to secure gastric biopsies from their patients, the participating patients, and the nursing and surgical staff, who assisted with sample collection and patient consenting. We also thank the original Winslow Indian Health Care Center staff for their willingness to start this project.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Wroblewski, L.E.; Peek, R.M.; Wilson, K.T. Helicobacter pylori and gastric cancer: Factors that modulate disease risk. Clin. Microbiol. Rev. 2010, 23, 713–739. [Google Scholar] [CrossRef]
- Hooi, J.K.Y.; Lai, W.Y.; Ng, W.K.; Suen, M.M.Y.; Underwood, F.E.; Tanyingoh, D.; Malfertheiner, P.; Graham, D.Y.; Wong, V.W.S.; Wu, J.C.Y.; et al. Global Prevalence of Helicobacter pylori Infection: Systematic Review and Meta-Analysis. Gastroenterology 2017, 153, 420–429. [Google Scholar] [CrossRef]
- Harris, R.B.; Brown, H.E.; Begay, R.L.; Sanderson, P.R.; Chief, C.; Monroy, F.P.; Oren, E. Helicobacter pylori prevalence and risk factors in three rural Indigenous communities of Northern Arizona. Int. J. Environ. Res. Public Health 2022, 19, 797. [Google Scholar] [CrossRef]
- Navajo Epidemiology Center. Cancer Among the Navajo 2005–2013; Navajo Epidemiology Center: Window Rock, AZ, USA, 2018. [Google Scholar]
- Stancioiu, F.; Ahmed, S.H.; Buschor, R. Helicobacter pylori: Findings in a Native American Population. IHS Prim. Care Provid. 2005, 30, 59–63. [Google Scholar]
- Shirani, M.; Pakzad, R.; Haddadi, M.H.; Akrami, S.; Asadi, A.; Kazemian, H.; Moradi, M.; Kaviar, V.H.; Zomorodi, A.R.; Khoshnood, S.; et al. The global prevalence of gastric cancer in Helicobacter pylori-infected individuals: A systematic review and meta-analysis. BMC Infect. Dis. 2023, 23, 1–30. [Google Scholar] [CrossRef]
- Uotani, T.; Graham, D.Y. Diagnosis of Helicobacter pylori using the rapid urease test. Ann. Transl. Med. 2015, 3, 9. [Google Scholar]
- Lan, H.C.; Chen, T.S.; Li, A.F.Y.; Chang, F.Y.; Lin, H.C. Additional corpus biopsy enhances the detection of Helicobacter pylori infection in a background of gastritis with atrophy. BMC Gastroenterol. 2012, 12, 182. [Google Scholar] [CrossRef]
- Choi, Y.J.; Kim, N.; Lim, J.; Jo, S.Y.; Shin, C.M.; Lee, H.S.; Park, Y.S.; Hwang, J.-H.; Kim, J.-W.; Jeong, S.-H.; et al. Accuracy of diagnostic tests for Helicobacter pylori in patients with peptic ulcer bleeding. Helicobacter 2012, 17, 77–85. [Google Scholar] [CrossRef]
- Tu, T.C.; Lee, C.L.; Wu, C.H.; Chen, T.K.; Chan, C.C.; Huang, S.H.; Lee, S.C. Comparison of invasive and noninvasive tests for detecting Helicobacter pylori infection in bleeding peptic ulcers. Gastrointest. Endosc. 1999, 49 Pt 1, 302–306. [Google Scholar] [CrossRef]
- Gisbert, J.P.; Abraira, V. Accuracy of Helicobacter pylori diagnostic tests in patients with bleeding peptic ulcer: A systematic review and meta-analysis. Am. J. Gastroenterol. 2006, 101, 848–863. [Google Scholar] [CrossRef]
- Kokkola, A.; Rautelin, H.; Puolakkainen, P.; Sipponen, P.; Färkkilä, M.; Haapiainen, R.; Kosunen, T.U. Diagnosis of Helicobacter pylori infection in patients with atrophic gastritis: Comparison of histology, 13C-urea breath test, and serology. Scand. J. Gastroenterol. 2000, 35, 138–141. [Google Scholar] [CrossRef]
- Lee, J.Y.; Kim, N. Diagnosis of Helicobacter pylori by invasive test: Histology. Ann. Transl. Med. 2015, 3, 10. [Google Scholar]
- Griñó, P.; Pascual, S.; Such, J.; Casellas, J.A.; Niveiro, M.; Andreu, M.; Pérez-Mateo, P. Comparison of diagnostic methods for Helicobacter pylori infection in patients with upper gastrointestinal bleeding. Scand. J. Gastroenterol. 2001, 36, 1254–1258. [Google Scholar] [CrossRef]
- Archimandritis, A.; Tzivras, M.; Sougioultzis, S.; Papaparaskevas, I.; Apostolopoulos, P.; Avlami, A.; Davaris, P. Rapid urease test is less sensitive than histology in diagnosing Helicobacter pylori infection in patients with non-variceal upper gastrointestinal bleeding. J. Gastroenterol. Hepatol. 2000, 15, 369–373. [Google Scholar] [CrossRef]
- Hoover, J.; Gonzales, M.; Shuey, C.; Barney, Y.; Lewis, J. Elevated arsenic and uranium concentrations in unregulated water sources on the Navajo Nation, USA. Expo. Health 2017, 9, 113–124. [Google Scholar] [CrossRef]
- Jeong, C.H.; Seok, J.S.; Petriello, M.C.; Han, S.G. Arsenic downregulates tight junction claudin proteins through p38 and NF-κB in intestinal epithelial cell line, HT-29. Toxicology 2017, 379, 31–39. [Google Scholar] [CrossRef]
- Rahman, M.M.; Chowdhury, U.K.; Mukherjee, S.C.; Mondal, B.K.; Paul, K.; Lodh, D.; Biswas, B.K.; Chanda, C.R.; Basu, G.K.; Saha, K.C.; et al. Chronic Arsenic Toxicity in Bangladesh and West Bengal, India—A Review and Commentary. J. Toxicol. Clin. Toxicol. 2001, 39, 683–700. [Google Scholar] [CrossRef]
- Weather. Navajo National Monument, AZ Climate Averages, Monthly Weather Conditions [Internet]. Available online: https://www.weatherworld.com/climate-averages/az/navajo+national+monument.html (accessed on 18 March 2024).
- Monroy, F.P.; Brown, H.E.; Sanderson, P.R.; Jarrin, G.; Mbegbu, M.; Kyman, S.; Harris, R.B. Helicobacter pylori in Native Americans in Northern Arizona. Diseases 2022, 10, 19. [Google Scholar] [CrossRef]
- WHO. A Healthy Lifestyle—WHO Recommendations [Internet]. 2024. Available online: https://www.who.int/europe/news-room/fact-sheets/item/a-healthy-lifestyle---who-recommendations (accessed on 2 May 2024).
- Patel, S.K.; Pratap, C.B.; Jain, A.K.; Gulati, A.K.; Nath, G. Diagnosis of Helicobacter pylori: What should be the gold standard? World J. Gastroenterol. WJG 2014, 20, 12847. [Google Scholar] [CrossRef]
- Hunt, R.; Xiao, S.; Megraud, F.; Leon-Barua, R.; Bazzoli, F.; van der Merwe, S.; Vaz Coelho, L.G.; Fock, M.; Fedail, S.; Cohen, H.; et al. World Gastroenterology Organisation Global Guideline Helicobacter pylori in Developing Countries. J. Dig. Dis. 2011, 12, 319–329. [Google Scholar] [CrossRef]
- Chey, W.D.; Wong, B.C.Y. American College of Gastroenterology guideline on the management of Helicobacter pylori infection. Am. J. Gastroenterol. 2007, 102, 1808–1825. [Google Scholar] [CrossRef]
- Miftahussurur, M.; Yamaoka, Y. Diagnostic Methods of Helicobacter pylori Infection for Epidemiological Studies: Critical Importance of Indirect Test Validation. BioMed Res. Int. 2016, 2016, 4819423. [Google Scholar] [CrossRef]
- Bruden, D.L.; Bruce, M.G.; Miernyk, K.M.; Morris, J.; Hurlburt, D.; Hennessy, T.W.; Peters, H.; Sacco, F.; Parkinson, A.J.; McMahon, B.J. Diagnostic accuracy of tests for Helicobacter pylori in an Alaska Native population. World J. Gastroenterol. 2011, 17, 4682–4688. [Google Scholar] [CrossRef]
- Mounsey, A.; Leonard, E.A. Noninvasive Diagnostic Tests for Helicobacter pylori Infection. Am. Fam. Physician 2019, 100, 16–17. [Google Scholar]
- Cardos, A.I.; Maghiar, A.; Zaha, D.C.; Pop, O.; Fritea, L.; Miere, F.; Cavalu, S. Evolution of Diagnostic Methods for Helicobacter pylori Infections: From Traditional Tests to High Technology, Advanced Sensitivity and Discrimination Tools. Diagnostics 2022, 12, 508. [Google Scholar] [CrossRef]
- Landis, J.R.; Koch, G.G. The measurement of observer agreement for categorical data. Biometrics 1977, 33, 159. [Google Scholar] [CrossRef]
- Nagy, P.; Johansson, S.; Molloy-Bland, M. Systematic review of time trends in the prevalence of Helicobacter pylori infection in China and the USA. Gut Pathog. 2016, 8, 8. [Google Scholar] [CrossRef]
- Ferro, A.; Morais, S.; Pelucchi, C.; Dierssen-Sotos, T.; Martín, V.; López-Carrillo, L.; Malekzadeh, R.; Tsugane, S.; Hamada, G.; Hidaka, A.; et al. Sex differences in the prevalence of Helicobacter pylori infection. Eur. J. Gastroenterol. Hepatol. 2019, 31, 593–598. [Google Scholar] [CrossRef]
- Chey, W.D.; Leontiadis, G.I.; Howden, C.W.; Moss, S.F. ACG Clinical Guideline: Treatment of Helicobacter pylori Infection. Am. J. Gastroenterol. 2017, 112, 212–238. [Google Scholar] [CrossRef]
- Bailey, K.S.; Brown, H.E.; Lekic, V.; Pradeep, K.; Merchant, J.L.; Harris, R.B. Helicobacter pylori treatment knowledge, access and barriers: A cross-sectional study. Helicobacter 2023, 28, e12954. [Google Scholar] [CrossRef]
- Hulten, K.G.; Lamberth, L.B.; Kalfus, I.N.; Graham, D.Y. National and Regional US Antibiotic Resistance to Helicobacter pylori: Lessons From a Clinical Trial. Gastroenterology 2021, 161, 342–344.e1. [Google Scholar] [CrossRef] [PubMed]
- Monroy, F.P.; Brown, H.E.; Acevedo-Solis, C.M.; Rodriguez-Galaviz, A.; Dholakia, R.; Pauli, L.; Harris, R.B. Antibiotic resistance rates for Helicobacter pylori in rural Arizona: A molecular-based study. Microorganisms 2023, 11, 2290. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.G.; Park, R.W.; Shin, S.J.; Yoon, D.; Kang, J.K.; Hwang, J.C.; Kim, S.S.; Kim, J.H.; Lee, K.M. The relationship between the failure to eradicate Helicobacter pylori and previous antibiotics use. Dig. Liver Dis. 2016, 48, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Dixon, M.F.; Genta, R.M.; Yardley, J.H.; Correa, P.; Batts, K.P.; Dahms, B.B.; Filipe, M.I.; Haggitt, R.C.; Haot, J.; Hui, P.K.; et al. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am. J. Surg. Pathol. 1996, 20, 1161–1181. [Google Scholar] [CrossRef] [PubMed]
- Malfertheiner, P.; Megraud, F.; Rokkas, T.; Gisbert, J.P.; Liou, J.M.; Schulz, C.; Gasbarrini, A.; Hunt, R.H.; Leja, M.; O’Morain, C.; et al. Management of Helicobacter pylori infection: The Maastricht VI/Florence consensus report. Gut 2022, 71, 1724–1762. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).