Assembly of Abundant and Rare Bacterial and Fungal Communities in Different Typical Forest Types in the Zhongtiao Mountains
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
- (i)
- Mixed Planted Forest (MPF): The main tree species in the sample plot were Pinus tabuliformis, Quercus mongolica, Populus davidiana, Betula platyphylla, and Carpinus turczaninovii. A total of 32 trees were investigated in the plot. The average tree height in the sample plot was 13.80 ± 0.49 m, and the average diameter at breast height (DBH) in the sample plot was 12.45 ± 0.68 cm;
- (ii)
- Planted Forest of Pinus tabuliformis (PFP): The main tree species in the sample plot was Pinus tabuliformis. A total of 28 trees were investigated in the plot. The average tree height in the sample plot was 16.61 ± 4.62 m, and the average diameter at breast height (DBH) in the sample plot was 23.69 ± 3.11 cm;
- (iii)
- Mixed Planted Forest of Pinus tabuliformis and Quercus mongolica (MPPQ): The main tree species in the sample plot were Pinus tabuliformis and Quercus mongolica. A total of 44 trees were investigated in the plot. The average tree height in the sample plot was 15.17 ± 1.32 m, and the average diameter at breast height (DBH) in the sample plot was 21.94 ± 1.61 cm;
- (iv)
- Planted Forest of Quercus mongolica (PFQ): The main tree species in the sample plot was Quercus mongolica. A total of 44 trees were investigated in the plot. The average tree height in the sample plot was 10.87 ± 0.83 m, and the average diameter at breast height (DBH) in the sample plot was 16.03 ± 0.62 cm.
2.2. Soil Sample Collection and Determination
2.3. Data Analysis
3. Results
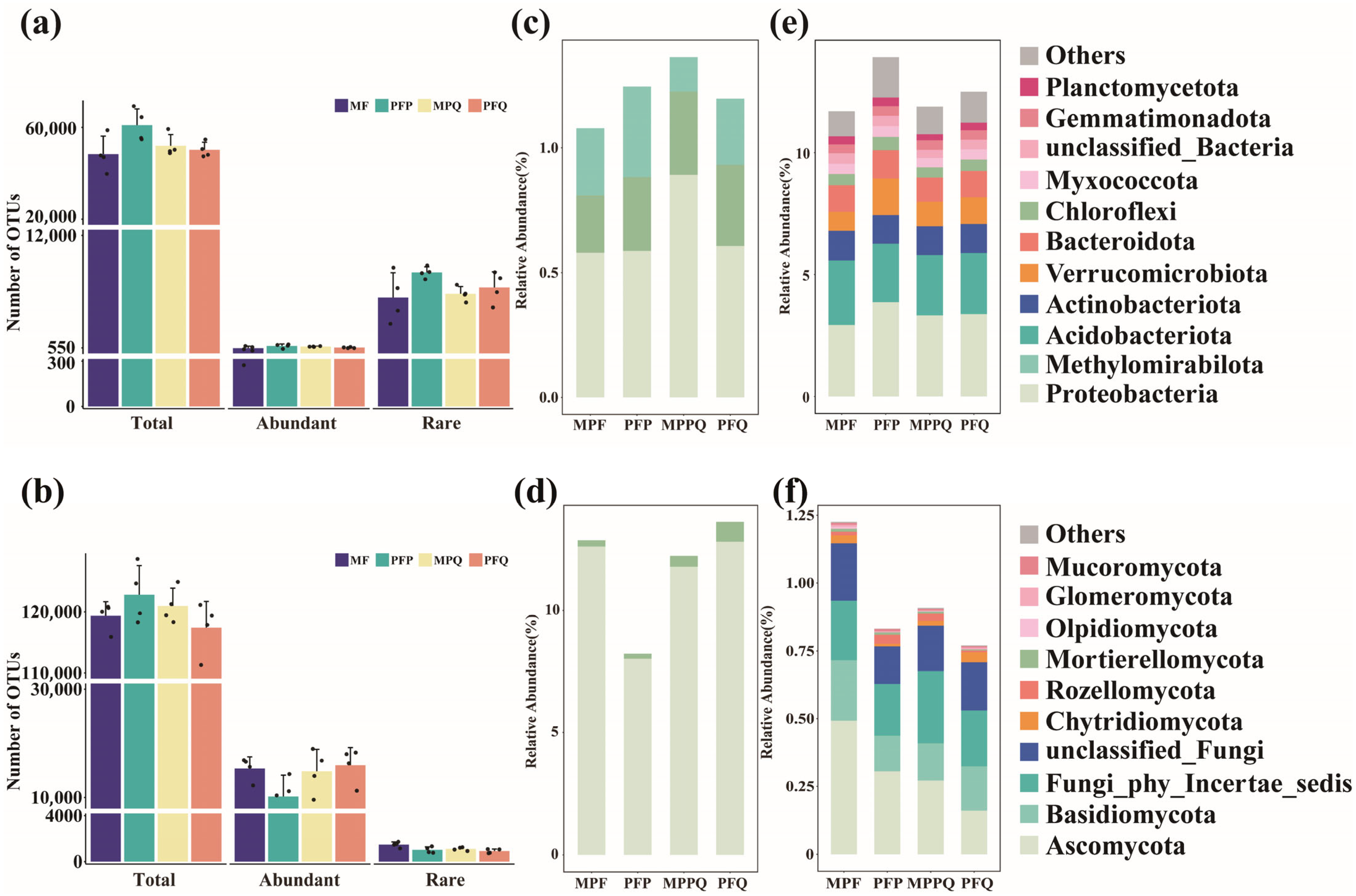
3.1. Composition of Abundant and Rare Microorganisms in Different Forest Types
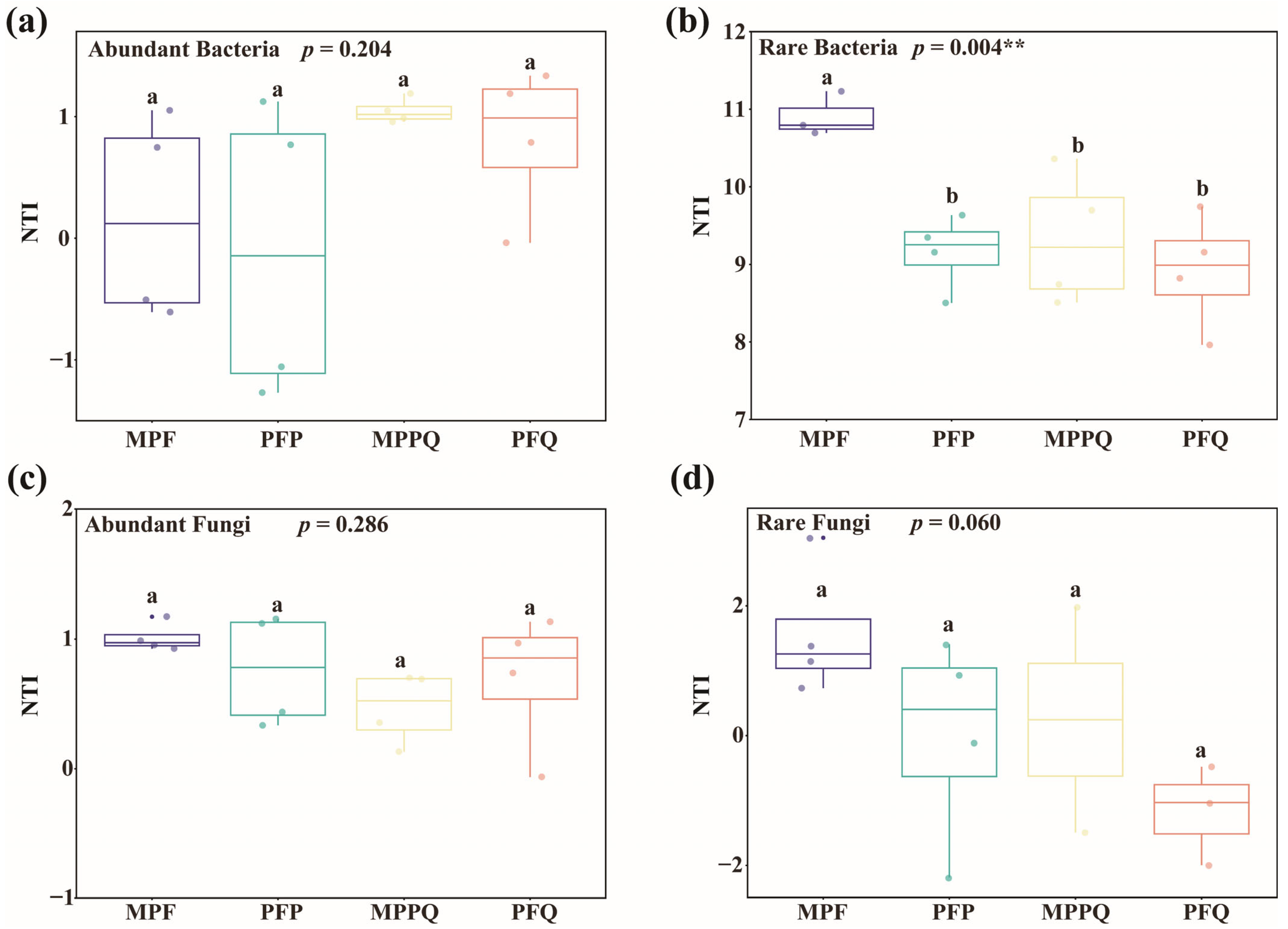
3.2. Diversity of Abundant and Rare Microbial Communities in Different Forest Types
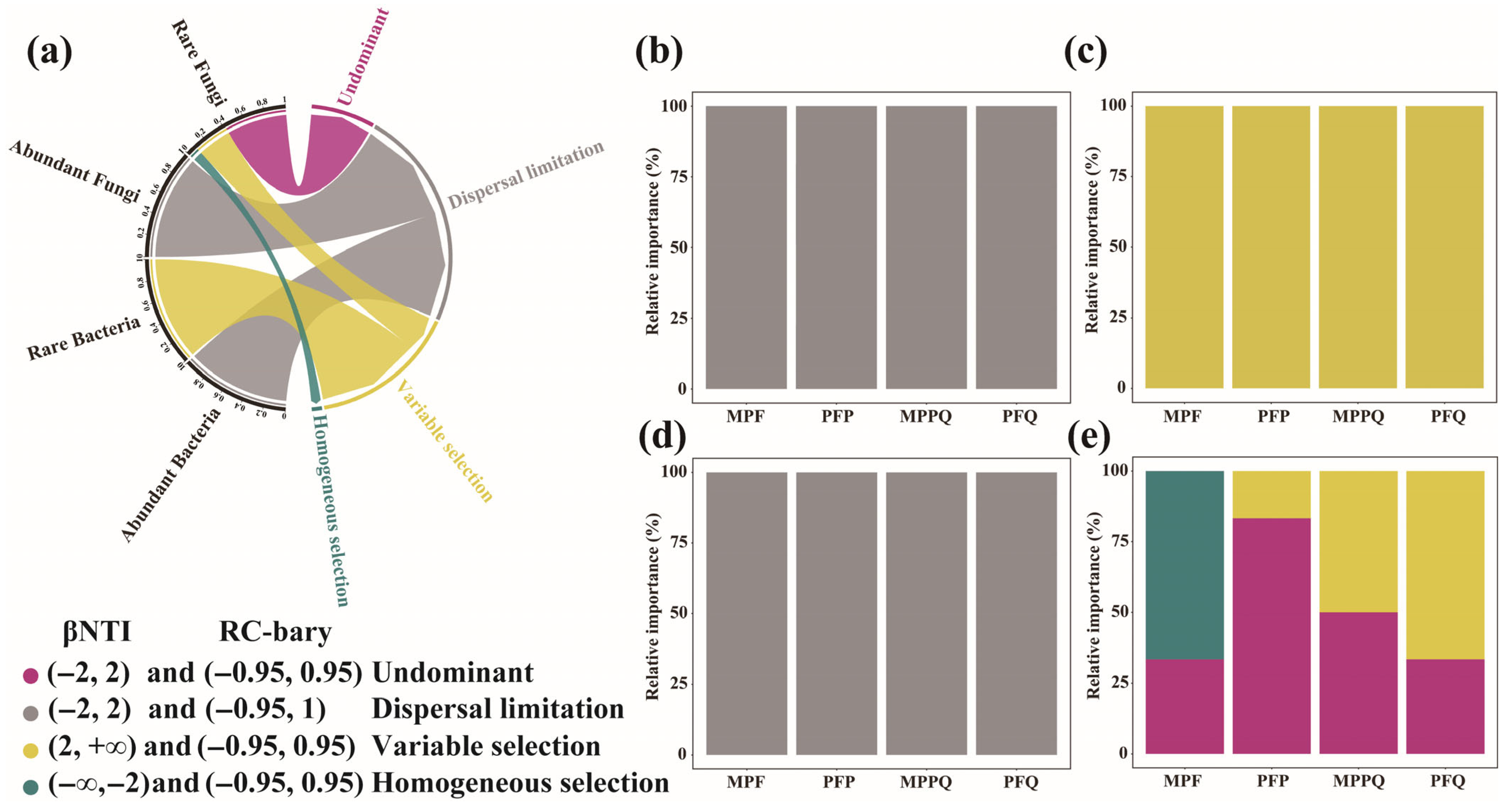
3.3. Assembly Processes of Abundant and Rare Microorganisms in Different Forest Types
4. Discussion
4.1. Differences in Abundant and Rare Microbial Community Composition and Diversity in Different Forest Types
4.2. Differences in the Assembly Processes of Abundant and Rare Microorganisms in Different Forest Types
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hartmann, M.; Six, J. Soil structure and microbiome functions in agroecosystems. Nat. Rev. Earth Environ. 2022, 4, 4–18. [Google Scholar] [CrossRef]
- Isobe, K.; Allison, S.D.; Khalili, B.; Martiny, A.C.; Martiny, J.B.H. Phylogenetic conservation of bacterial responses to soil nitrogen addition across continents. Nat. Commun. 2019, 10, 2499. [Google Scholar] [CrossRef] [PubMed]
- Bardgett, R.D.; van der Putten, W.H. Belowground biodiversity and ecosystem functioning. Nature 2014, 515, 505–511. [Google Scholar] [CrossRef]
- Zhou, J.; Xue, K.; Xie, J.; Deng, Y.; Wu, L.; Cheng, X.; Fei, S.; Deng, S.; He, Z.; Van Nostrand, J.D.; et al. Microbial mediation of carbon-cycle feedbacks to climate warming. Nat. Clim. Chang. 2011, 2, 106–110. [Google Scholar] [CrossRef]
- García-Palacios, P.; Crowther, T.W.; Dacal, M.; Hartley, I.P.; Reinsch, S.; Rinnan, R.; Rousk, J.; van den Hoogen, J.; Ye, J.-S.; Bradford, M.A. Evidence for large microbial-mediated losses of soil carbon under anthropogenic warming. Nat. Rev. Earth Environ. 2021, 2, 507–517, Erruatum in Nat. Rev. Earth Environ. 2021, 2, 585. [Google Scholar] [CrossRef]
- Wieder, W.R.; Bonan, G.B.; Allison, S.D. Global soil carbon projections are improved by modelling microbial processes. Nat. Clim. Chang. 2013, 3, 909–912. [Google Scholar] [CrossRef]
- Wang, K.; Zhang, Y.; Tang, Z.; Shangguan, Z.; Chang, F.; Jia, F.; Chen, Y.; He, X.; Shi, W.; Deng, L. Effects of grassland afforestation on structure and function of soil bacterial and fungal communities. Sci. Total Environ. 2019, 676, 396–406. [Google Scholar] [CrossRef]
- Bailey, V.L.; Smith, J.L.; Bolton, H., Jr. Fungal-to-bacterial ratios in soils investigated for enhanced C sequestration. Soil Biol. Biochem. 2002, 34, 997–1007. [Google Scholar] [CrossRef]
- Liang, Y.; Xiao, X.; Nuccio, E.E.; Yuan, M.; Zhang, N.; Xue, K.; Cohan, F.M.; Zhou, J.; Sun, B. Differentiation strategies of soil rare and abundant microbial taxa in response to changing climatic regimes. Environ. Microbiol. 2020, 22, 1327–1340. [Google Scholar] [CrossRef]
- Wu, W.; Logares, R.; Huang, B.; Hsieh, C.H. Abundant and rare picoeukaryotic sub-communities present contrasting patterns in the epipelagic waters of marginal seas in the northwestern Pacific Ocean. Environ. Microbiol. 2017, 19, 287–300. [Google Scholar] [CrossRef]
- Jiao, S.; Lu, Y. Abundant fungi adapt to broader environmental gradients than rare fungi in agricultural fields. Glob. Change Biol. 2020, 26, 4506–4520. [Google Scholar] [CrossRef]
- Delgado-Baquerizo, M.; Bardgett, R.D.; Vitousek, P.M.; Maestre, F.T.; Williams, M.A.; Eldridge, D.J.; Lambers, H.; Neuhauser, S.; Gallardo, A.; Garcia-Velazquez, L.; et al. Changes in belowground biodiversity during ecosystem development. Proc. Natl. Acad. Sci. USA 2019, 116, 6891–6896. [Google Scholar] [CrossRef] [PubMed]
- Pedros-Alio, C. The rare bacterial biosphere. Annu. Rev. Mar. Sci. 2012, 4, 449–466. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Wang, L.; Song, X.; Li, X.; Ma, J.; Chen, F. Changes in abundant and rare microbial taxa that dominated the formation of soil carbon pool during short-term dryland-to-paddy conversion. Carbon Res. 2023, 2, 26. [Google Scholar] [CrossRef]
- Liu, X.; Wang, H.; Wu, Y.; Bi, Q.; Ding, K.; Lin, X. Manure application effects on subsoils: Abundant taxa initiate the diversity reduction of rare bacteria and community functional alterations. Soil Biol. Biochem. 2022, 174, 108816. [Google Scholar] [CrossRef]
- Jiao, S.; Wang, J.; Wei, G.; Chen, W.; Lu, Y. Dominant role of abundant rather than rare bacterial taxa in maintaining agro-soil microbiomes under environmental disturbances. Chemosphere 2019, 235, 248–259. [Google Scholar] [CrossRef]
- Chen, Q.-L.; Ding, J.; Zhu, D.; Hu, H.-W.; Delgado-Baquerizo, M.; Ma, Y.-B.; He, J.-Z.; Zhu, Y.-G. Rare microbial taxa as the major drivers of ecosystem multifunctionality in long-term fertilized soils. Soil Biol. Biochem. 2020, 141, 107686. [Google Scholar] [CrossRef]
- Banerjee, S.; van der Heijden, M.G.A. Soil microbiomes and one health. Nat. Rev. Microbiol. 2023, 21, 6–20. [Google Scholar] [CrossRef]
- Rochefort, A.; Simonin, M.; Marais, C.; Guillerm-Erckelboudt, A.Y.; Barret, M.; Sarniguet, A. Transmission of Seed and Soil Microbiota to Seedling. mSystems 2021, 6, e0044621. [Google Scholar] [CrossRef]
- Webb, C.O.; Ackerly, D.D.; McPeek, M.A.; Donoghue, M.J. Phylogenies and Community Ecology. Annu. Rev. Ecol. Syst. 2002, 33, 475–505. [Google Scholar] [CrossRef]
- Chase, J.M.; Myers, J.A. Disentangling the importance of ecological niches from stochastic processes across scales. Philos. Trans. R. Soc. B Biol. Sci. 2011, 366, 2351–2363. [Google Scholar] [CrossRef]
- Stegen, J.C.; Lin, X.; Konopka, A.E.; Fredrickson, J.K. Stochastic and deterministic assembly processes in subsurface microbial communities. ISME J. 2012, 6, 1653–1664. [Google Scholar] [CrossRef] [PubMed]
- Meyer, K.M.; Memiaghe, H.; Korte, L.; Kenfack, D.; Alonso, A.; Bohannan, B.J.M. Why do microbes exhibit weak biogeographic patterns? ISME J. 2018, 12, 1404–1413. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Chen, R.; Stegen, J.C.; Guo, Z.; Zhang, J.; Li, Z.; Lin, X. Two key features influencing community assembly processes at regional scale: Initial state and degree of change in environmental conditions. Mol. Ecol. 2018, 27, 5238–5251. [Google Scholar] [CrossRef]
- Tripathi, B.M.; Stegen, J.C.; Kim, M.; Dong, K.; Adams, J.M.; Lee, Y.K. Soil pH mediates the balance between stochastic and deterministic assembly of bacteria. ISME J. 2018, 12, 1072–1083. [Google Scholar] [CrossRef]
- Liu, L.; Yang, J.; Yu, Z.; Wilkinson, D.M. The biogeography of abundant and rare bacterioplankton in the lakes and reservoirs of China. ISME J. 2015, 9, 2068–2077. [Google Scholar] [CrossRef]
- Wan, W.; Gadd, G.M.; Yang, Y.; Yuan, W.; Gu, J.; Ye, L.; Liu, W. Environmental adaptation is stronger for abundant rather than rare microorganisms in wetland soils from the Qinghai-Tibet Plateau. Mol. Ecol. 2021, 30, 2390–2403. [Google Scholar] [CrossRef]
- Wang, H.; Tian, D.; Liu, H.; Wang, Z.; He, Y.; Lu, J.; Zhu, Y.; Wei, S.; Wang, H.; Wu, L.; et al. Rare rather than abundant taxa of soil bacteria and fungi regulate soil multifunctionality in Eucalyptus plantations. Catena 2024, 245, 108303. [Google Scholar] [CrossRef]
- Chen, R.; Zhu, J.; Jiang, L.; Liu, L.; Gao, C.; Chen, B.; Xu, D.; Liu, J.; He, Z. Soil Phosphorus Availability Controls Deterministic and Stochastic Processes of Soil Microbial Community along an Elevational Gradient in Subtropical Forests. Forests 2023, 14, 1475. [Google Scholar] [CrossRef]
- Wu, X.; Deane, D.C.; Xing, H.; Yang, J.; Chen, J.; Liu, X.; Dong, S.; He, F.; Liu, Y.; Lü, X.-T. Structure and functions of soil microbial communities and tree composition are more closely associated with keystone microbes than rare microbes in a subtropical forest. J. Plant Ecol. 2025, 18, rtae105. [Google Scholar] [CrossRef]
- Mori, H.; Maruyama, F.; Kato, H.; Toyoda, A.; Dozono, A.; Ohtsubo, Y.; Nagata, Y.; Fujiyama, A.; Tsuda, M.; Kurokawa, K. Design and Experimental Application of a Novel Non-Degenerate Universal Primer Set that Amplifies Prokaryotic 16S rRNA Genes with a Low Possibility to Amplify Eukaryotic rRNA Genes. DNA Res. 2013, 21, 217–227. [Google Scholar] [CrossRef] [PubMed]
- Xiong, W.; Zhao, Q.; Zhao, J.; Xun, W.; Li, R.; Zhang, R.; Wu, H.; Shen, Q. Different Continuous Cropping Spans Significantly Affect Microbial Community Membership and Structure in a Vanilla-Grown Soil as Revealed by Deep Pyrosequencing. Microb. Ecol. 2014, 70, 209–218. [Google Scholar] [CrossRef]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef]
- Koljalg, U.; Nilsson, R.H.; Abarenkov, K.; Tedersoo, L.; Taylor, A.F.; Bahram, M.; Bates, S.T.; Bruns, T.D.; Bengtsson-Palme, J.; Callaghan, T.M.; et al. Towards a unified paradigm for sequence-based identification of fungi. Mol. Ecol. 2013, 22, 5271–5277. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Zhao, Z.; Lv, F.; Wang, R.; Wang, Z.; Zhao, Z.; Li, Z.; Zhai, B. Assembly of abundant and rare bacterial and fungal sub-communities in different soil aggregate sizes in an apple orchard treated with cover crop and fertilizer. Soil Biol. Biochem. 2021, 156, 108222. [Google Scholar] [CrossRef]
- Mei, Z.; Wu, C.; Shi, S.; Zhang, H.; Zhu, Z.; Chen, J.; Ge, T. Loss of protistan diversity weakens soil phosphorus availability. Appl. Soil. Ecol. 2025, 208, 105976. [Google Scholar] [CrossRef]
- Zhao, Z.; Ma, Y.; Feng, T.; Kong, X.; Wang, Z.; Zheng, W.; Zhai, B. Assembly processes of abundant and rare microbial communities in orchard soil under a cover crop at different periods. Geoderma 2022, 406, 115543. [Google Scholar] [CrossRef]
- Dong, L.; Li, M.X.; Li, S.; Yue, L.X.; Ali, M.; Han, J.R.; Lian, W.H.; Hu, C.J.; Lin, Z.L.; Shi, G.Y.; et al. Aridity drives the variability of desert soil microbiomes across north-western China. Sci. Total Environ. 2024, 907, 168048. [Google Scholar] [CrossRef] [PubMed]
- Stegen, J.C.; Lin, X.; Fredrickson, J.K.; Konopka, A.E. Estimating and mapping ecological processes influencing microbial community assembly. Front. Microbiol. 2015, 6, 370. [Google Scholar] [CrossRef]
- Dukunde, A.; Schneider, D.; Schmidt, M.; Veldkamp, E.; Daniel, R. Tree Species Shape Soil Bacterial Community Structure and Function in Temperate Deciduous Forests. Front. Microbiol. 2019, 10, 1519. [Google Scholar] [CrossRef]
- Ding, L.J.; Ren, X.Y.; Zhou, Z.Z.; Zhu, D.; Zhu, Y.G. Forest-to-Cropland Conversion Reshapes Microbial Hierarchical Interactions and Degrades Ecosystem Multifunctionality at a National Scale. Environ. Sci. Technol. 2024, 58, 11027–11040. [Google Scholar] [CrossRef]
- Carrasco, J.; Preston, G.M. Growing edible mushrooms: A conversation between bacteria and fungi. Environ. Microbiol. 2020, 22, 858–872. [Google Scholar] [CrossRef]
- Liu, D.; Fei, Y.H.; Peng, Y.; Zhu, S.; Lu, J.; Luo, Y.; Chen, Z.; Jiang, Y.; Wang, S.; Tang, Y.T.; et al. Genotype of pioneer plant Miscanthus is not a key factor in the structure of rhizosphere bacterial community in heavy metal polluted sites. J. Hazard. Mater. 2024, 477, 135242. [Google Scholar] [CrossRef] [PubMed]
- Ho, A.; Di Lonardo, D.P.; Bodelier, P.L. Revisiting life strategy concepts in environmental microbial ecology. FEMS Microbiol. Ecol. 2017, 93, fix006. [Google Scholar] [CrossRef]
- Wu, L.; Ren, C.; Jiang, H.; Zhang, W.; Chen, N.; Zhao, X.; Wei, G.; Shu, D. Land abandonment transforms soil microbiome stability and functional profiles in apple orchards of the Chinese Losses Plateau. Sci. Total Environ. 2024, 906, 167556. [Google Scholar] [CrossRef]
- Uroz, S.; Buée, M.; Deveau, A.; Mieszkin, S.; Martin, F. Ecology of the forest microbiome: Highlights of temperate and boreal ecosystems. Soil Biol. Biochem. 2016, 103, 471–488. [Google Scholar] [CrossRef]
- Cheng, H.; Zhou, X.; Dong, R.; Wang, X.; Liu, G.; Li, Q. Natural vegetation regeneration facilitated soil organic carbon sequestration and microbial community stability in the degraded karst ecosystem. Catena 2023, 222, 106856. [Google Scholar] [CrossRef]
- Hartshornea, R.S.; Reardonb, C.L.; Rossc, D.; Nuesterd, J.; Clarkea, T.A.; Gatesa, A.J.; Millsa, P.C.; Fredricksonb, J.K.; Zacharab, J.M.; Shib, L.; et al. Characterization of an electron conduit between bacteria and the extracellular environment. Proc. Natl. Acad. Sci. USA 2009, 106, 22169–22174. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Liu, W.; Deng, Y.; Jiang, Y.H.; Xue, K.; He, Z.; Van Nostrand, J.D.; Wu, L.; Yang, Y.; Wang, A. Stochastic assembly leads to alternative communities with distinct functions in a bioreactor microbial community. mBio 2013, 4, e00584. [Google Scholar] [CrossRef]
- Chase, J.M. Stochastic Community Assembly Causes Higher Biodiversity in More Productive Environments. Science 2010, 328, 1388–1391. [Google Scholar] [CrossRef] [PubMed]
- Cui, W.; Li, R.; Fan, Z.; Wu, L.; Zhao, X.; Wei, G.; Shu, D. Weak environmental adaptation of rare phylotypes sustaining soil multi-element cycles in response to decades-long fertilization. Sci. Total Environ. 2023, 871, 162063. [Google Scholar] [CrossRef]
- Debray, R.; Herbert, R.A.; Jaffe, A.L.; Crits-Christoph, A.; Power, M.E.; Koskella, B. Priority effects in microbiome assembly. Nat. Rev. Microbiol. 2022, 20, 109–121. [Google Scholar] [CrossRef]
- Zhou, J.; Ning, D. Stochastic Community Assembly: Does It Matter in Microbial Ecology? Microbiol. Mol. Biol. Rev. 2017, 81, e00002–e00017. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Dini-Andreote, F.; Falcão Salles, J. Community Assembly Processes of the Microbial Rare Biosphere. Trends Microbiol. 2018, 26, 738–747. [Google Scholar] [CrossRef] [PubMed]
- Rinnan, R.; Michelsen, A.; BÅÅTh, E.; Jonasson, S. Fifteen years of climate change manipulations alter soil microbial communities in a subarctic heath ecosystem. Glob. Change Biol. 2006, 13, 28–39. [Google Scholar] [CrossRef]
- Schmidt, S.K.; Nemergut, D.R.; Darcy, J.L.; Lynch, R. Do bacterial and fungal communities assemble differently during primary succession? Mol. Ecol. 2014, 23, 254–258. [Google Scholar] [CrossRef]
- Ren, J.; Liu, X.; Yang, W.; Yang, X.; Li, W.; Xia, Q.; Li, J.; Gao, Z.; Yang, Z. Rhizosphere soil properties, microbial community, and enzyme activities: Short-term responses to partial substitution of chemical fertilizer with organic manure. J. Environ. Manag. 2021, 299, 113650. [Google Scholar] [CrossRef]
- Maciel Rabelo Pereira, C.; López-García, Á.; da Silva, D.K.A.; Costa Maia, L.; Guldberg Frøslev, T.; Kjøller, R.; Rosendahl, S. Tropical forest type influences community assembly processes in arbuscular mycorrhizal fungi. J. Biogeogr. 2019, 47, 434–444. [Google Scholar] [CrossRef]
- Huo, X.; Ren, C.; Wang, D.; Wu, R.; Wang, Y.; Li, Z.; Huang, D.; Qi, H. Microbial community assembly and its influencing factors of secondary forests in Qinling Mountains. Soil Biol. Biochem. 2023, 184, 109075. [Google Scholar] [CrossRef]
- Wang, X.; Wiegand, T.; Kraft, N.J.B.; Swenson, N.G.; Davies, J.S.; Hao, Z.; Howe, R.; Lin, Y.; Ma, K.; Mi, X.; et al. Stochastic dilution effects weaken deterministic effects of niche-based processes in species rich forests. Ecology 2016, 97, 347–360. [Google Scholar] [CrossRef]
- Peay, K.G.; Baraloto, C.; Fine, P.V. Strong coupling of plant and fungal community structure across western Amazonian rainforests. ISME J. 2013, 7, 1852–1861. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Wang, R.; Zhang, M. Assembly of Abundant and Rare Bacterial and Fungal Communities in Different Typical Forest Types in the Zhongtiao Mountains. Microorganisms 2025, 13, 1911. https://doi.org/10.3390/microorganisms13081911
Li Z, Wang R, Zhang M. Assembly of Abundant and Rare Bacterial and Fungal Communities in Different Typical Forest Types in the Zhongtiao Mountains. Microorganisms. 2025; 13(8):1911. https://doi.org/10.3390/microorganisms13081911
Chicago/Turabian StyleLi, Zixing, Ran Wang, and Mengtao Zhang. 2025. "Assembly of Abundant and Rare Bacterial and Fungal Communities in Different Typical Forest Types in the Zhongtiao Mountains" Microorganisms 13, no. 8: 1911. https://doi.org/10.3390/microorganisms13081911
APA StyleLi, Z., Wang, R., & Zhang, M. (2025). Assembly of Abundant and Rare Bacterial and Fungal Communities in Different Typical Forest Types in the Zhongtiao Mountains. Microorganisms, 13(8), 1911. https://doi.org/10.3390/microorganisms13081911




