Immobilization of Cd Through Biosorption by Bacillus altitudinis C10-4 and Remediation of Cd-Contaminated Soil
Abstract
1. Introduction
2. Materials and Methods
2.1. Minimum Inhibitory Concentration of Cd(II) and Multi-Metal Resistance
2.2. Bacterial Identification
2.3. Preparation of the Cells of Strain C10-4
2.4. Biosorption Experiments
2.4.1. Effect of Physicochemical Parameters on Biosorption of Cd(II) by Strain C10-4
2.4.2. Biosorption Isotherm and Kinetics
2.5. Characterization of Cd Immobilization by Strain C10-4
2.5.1. SEM-EDS, FTIR Analysis
2.5.2. Bioaccumulation of Cadmium in Strain C10-4
2.6. Bioremediation of Cd(II)-Contaminated Soils
2.6.1. Preparation of Cd(II)-Contaminated Soils
2.6.2. Bioremediation and Analysis of Cd(II)-Contaminated Soil
2.7. Statistical Analysis
3. Results and Discussion
3.1. MIC and Multi-Metal Resistance
3.2. Identification of Strain C10-4
3.3. Biosorption of Cadmium by Strain C10-4
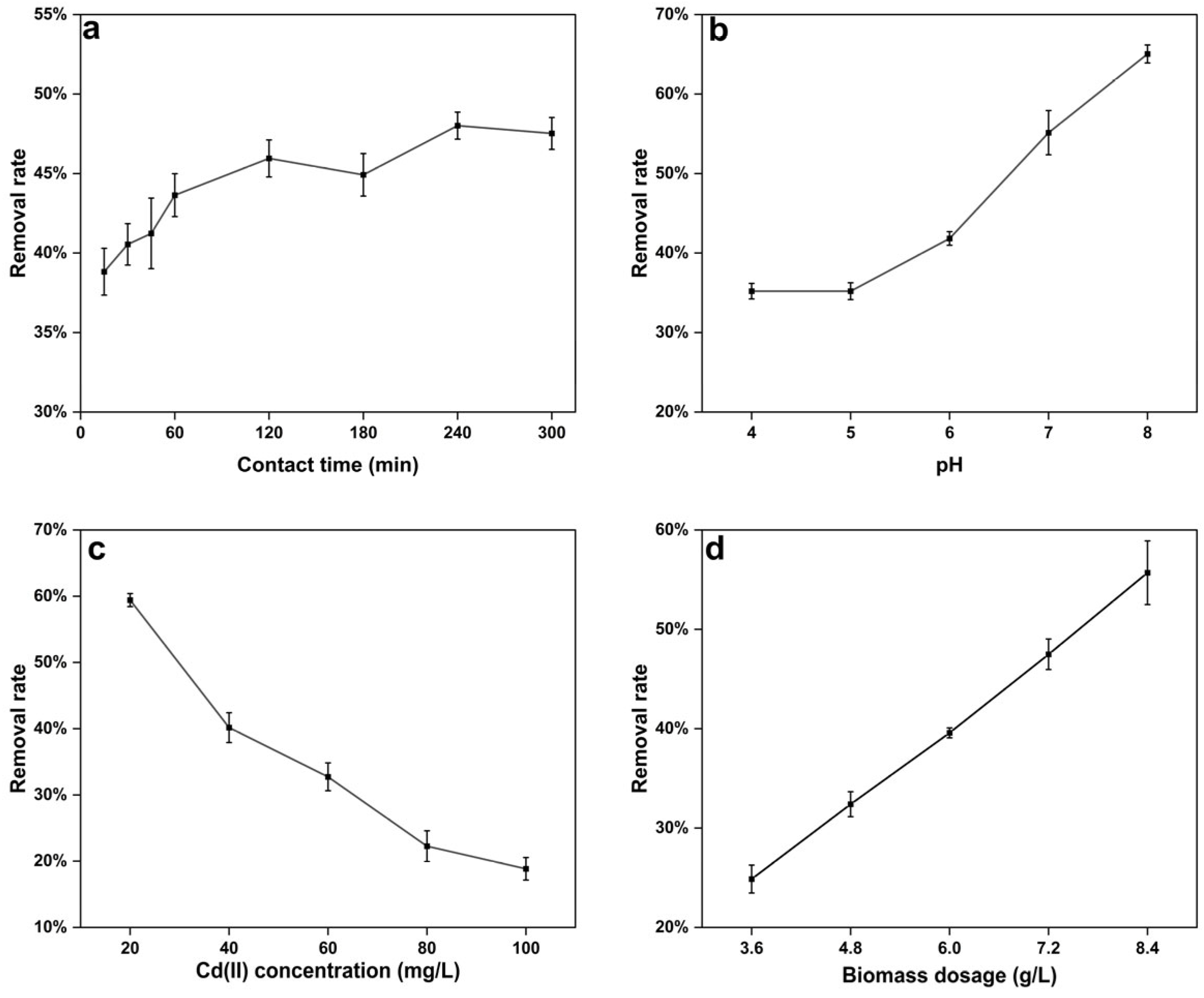
3.3.1. Effect of Factors on Biosorption of Cd(II) by Strain C10-4
- Effect of Contact Time
- 2.
- Effect of pH
- 3.
- Effect of Cd(II) concentration
- 4.
- Effect of biomass dosage
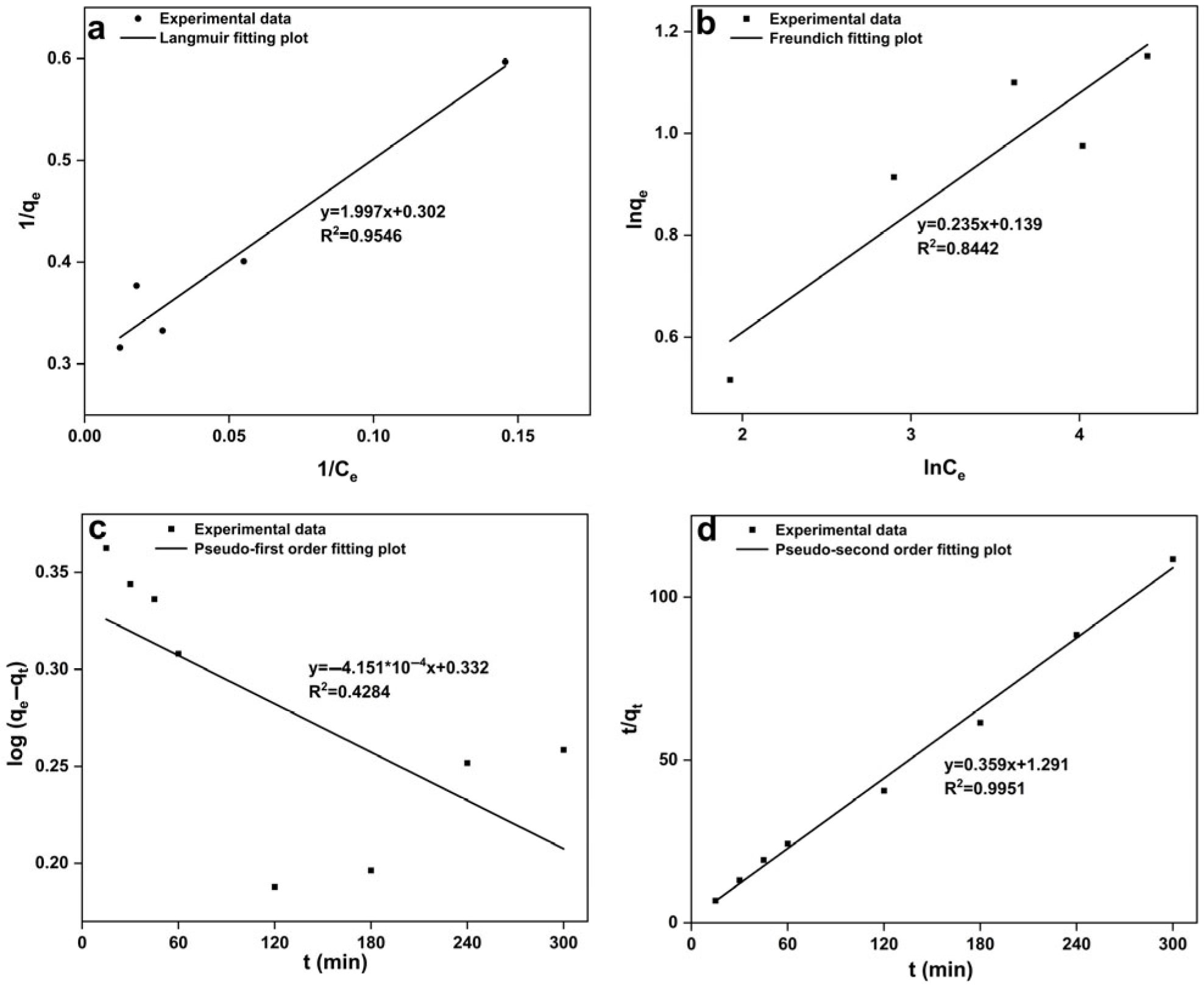
3.3.2. Modeling of Biosorption Isotherms and Kinetics
- Modeling of biosorption isotherm
- 2.
- Modeling of biosorption kinetics
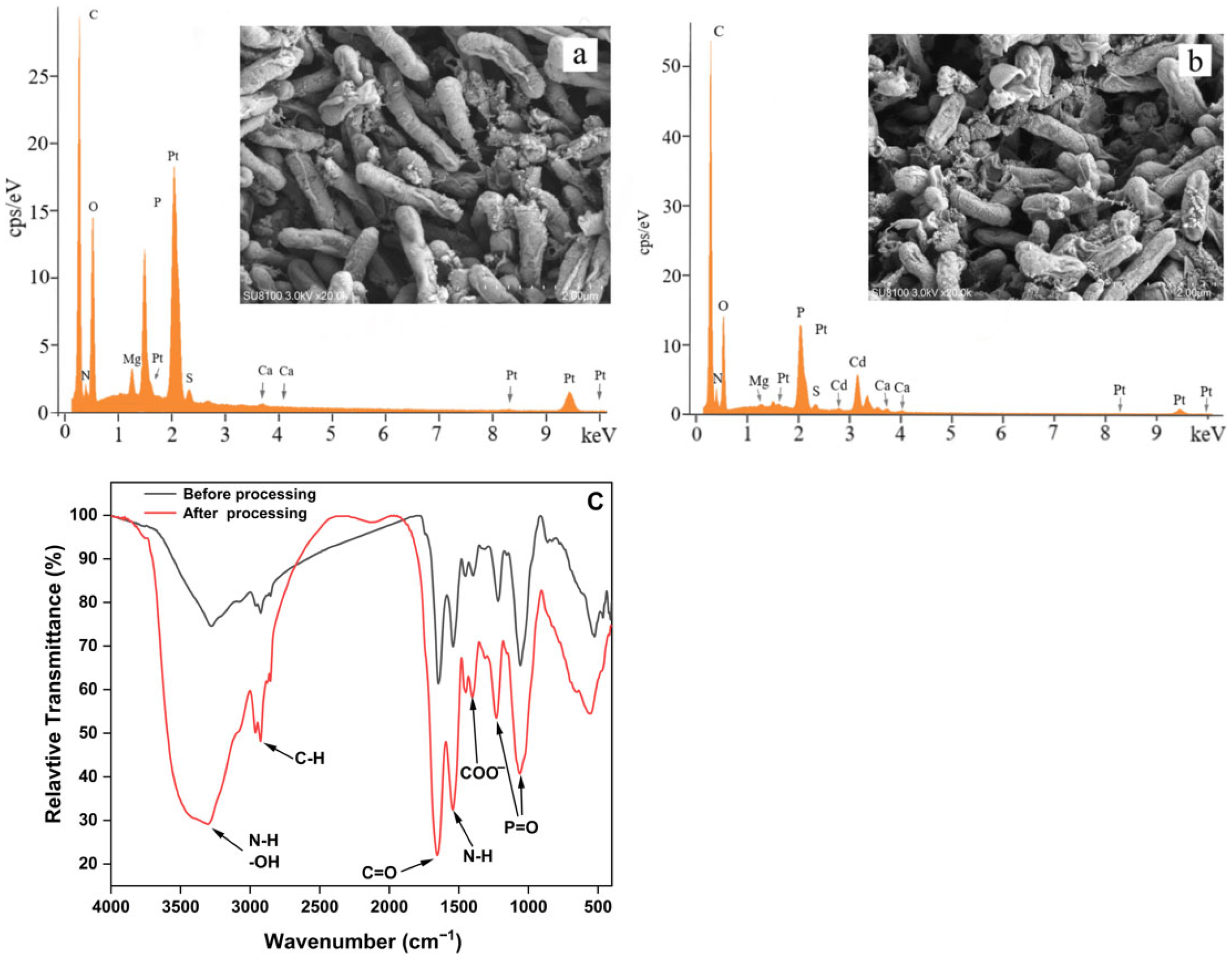
3.4. Mechanism of Cd Immobilization by Strain C10-4
3.4.1. SEM and EDS Analysis
3.4.2. FTIR Analysis
3.4.3. Distribution of Cd in Different Parts of Strain C10-4
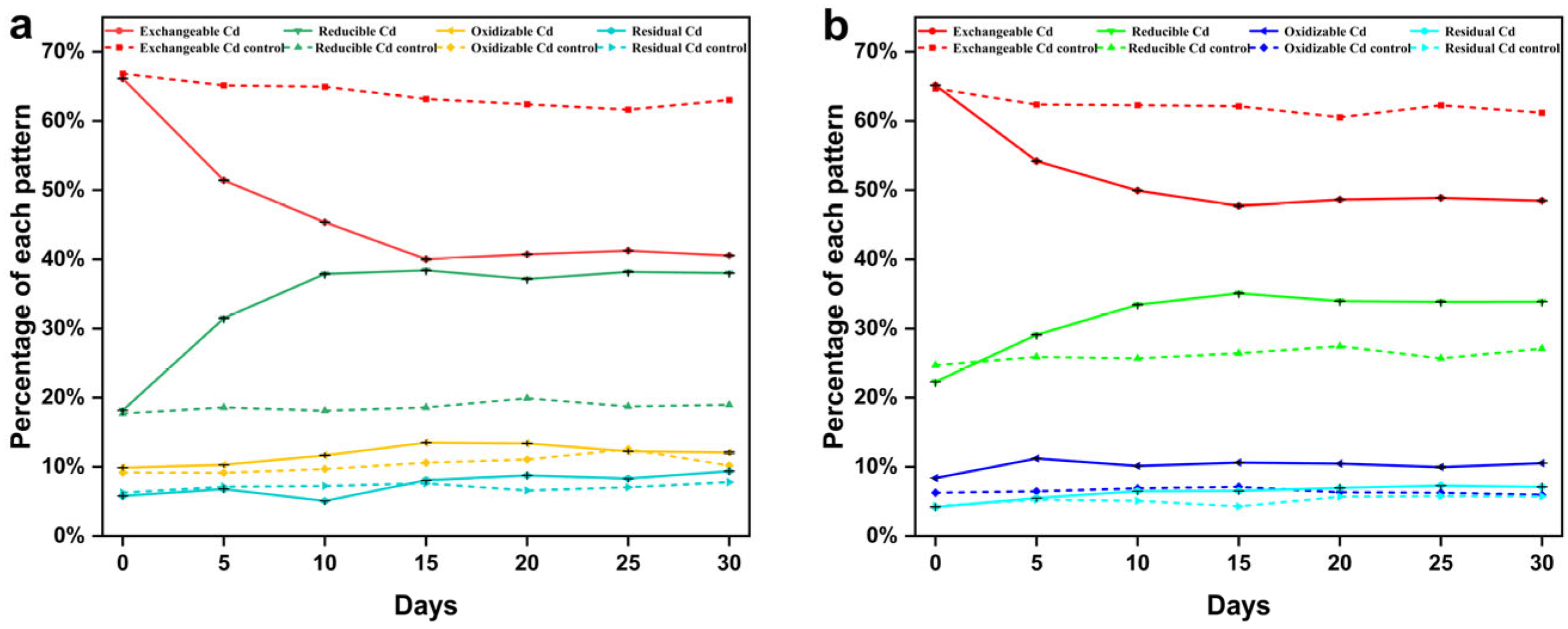
3.5. Analysis of the Bioremediation of Cd(II)-Contaminated Soil
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- The China Ecological and Environmental Status Bulletin. 2019–2021. Available online: https://www.mee.gov.cn/hjzl/sthjzk/zghjzkgb/ (accessed on 16 July 2025).
- Huttunen-Saarivirta, E.; Korpiniemi, H.; Kuokkala, V.T.; Paajanen, H. Corrosion of cadmium plating by runway de-icing chemicals: Study of surface phenomena and comparison of corrosion tests. Surf. Coat. Technol. 2013, 232, 101–115. [Google Scholar] [CrossRef]
- Mo, D.; Hu, L.; Zeng, G.; Chen, G.; Wan, J.; Yu, Z.; Huang, Z.; He, K.; Zhang, C.; Cheng, M. Cadmium-containing quantum dots: Properties, applications, and toxicity. Appl. Microbiol. Biotechnol. 2017, 101, 2713–2733. [Google Scholar] [CrossRef] [PubMed]
- Turner, A. Cadmium pigments in consumer products and their health risks. Sci. Total Environ. 2019, 657, 1409–1418. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Luo, X.; Zhang, Q.; Duan, X.; Yuan, Y.; Zheng, S. Contributions of selenium-oxidizing bacteria to selenium biofortification and cadmium bioremediation in a native seleniferous Cd-polluted sandy loam soil. Ecotoxicol. Environ. Saf. 2024, 272, 116081. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Li, F.; Cui, J.; Yang, S.; Fang, L. Simultaneous removal of Cd(II) and As(III) by graphene-like biochar-supported zero-valent iron from irrigation waters under aerobic conditions: Synergistic effects and mechanisms. J. Hazard. Mater. 2020, 395, 122623. [Google Scholar] [CrossRef] [PubMed]
- IARC. Agents Classified by the IARC Monographs. 2018. Available online: https://monographs.iarc.fr/agents-classified-by-the-iarc (accessed on 21 June 2024).
- Shi, G.L.; Zhu, S.; Bai, S.N.; Xia, Y.; Lou, L.Q.; Cai, Q.S. The transportation and accumulation of arsenic, cadmium, and phosphorus in 12 wheat cultivars and their relationships with each other. J. Hazard. Mater. 2015, 299, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Anetor, J.I. Rising environmental cadmium levels in developing countries: Threat to genome stability and health. Niger. J. Physiol. Sci. 2012, 27, 103–115. [Google Scholar] [CrossRef] [PubMed]
- Rani, A.; Kumar, A.; Lal, A.; Pant, M. Cellular mechanisms of cadmium-induced toxicity: A review. Int. J. Environ. Health Res. 2014, 24, 378–399. [Google Scholar] [CrossRef] [PubMed]
- Rinaldi, M.; Micali, A.; Marini, H.; Adamo, E.B.; Puzzolo, D.; Pisani, A.; Trichilo, V.; Altavilla, D.; Squadrito, F.; Minutoli, L. Cadmium, organ toxicity and therapeutic approaches: A review on brain, kidney and testis damage. Curr. Med. Chem. 2017, 24, 3879–3893. [Google Scholar] [CrossRef] [PubMed]
- Cabral Pinto, M.M.S.; Ordens, C.M.; Condesso de Melo, M.T.; Inácio, M.; Almeida, A.; Pinto, E.; Ferreira da Silva, E.A. An inter-disciplinary approach to evaluate human health risks due to long-term exposure to contaminated groundwater near a chemical complex. Expo. Health 2020, 12, 199–214. [Google Scholar] [CrossRef]
- Kumar, A.; Subrahmanyam, G.; Mondal, R.; Cabral-Pinto, M.M.S.; Shabnam, A.A.; Jigyasu, D.K.; Malyan, S.K.; Fagodiya, R.K.; Khan, S.A.; Kumar, A.; et al. Bio-remediation approaches for alleviation of cadmium contamination in natural resources. Chemosphere 2021, 268, 128855. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Yan, P.; Yu, H.; Yang, S.; Xu, J.; Liu, X. Spatiotemporal modeling of soil heavy metals and early warnings from scenarios-based prediction. Chemosphere 2020, 255, 126908. [Google Scholar] [CrossRef] [PubMed]
- Kurniawan, T.A.; Chan, G.Y.; Lo, W.H.; Babel, S. Physico–chemical treatment techniques for wastewater laden with heavy metals. Chem. Eng. J. 2006, 118, 83–98. [Google Scholar] [CrossRef]
- Li, J.; Liu, Y.R.; Zhang, L.M.; He, J.Z. Sorption mechanism and distribution of cadmium by different microbial species. J. Environ. Manag. 2019, 237, 552–559. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Guo, Z.; Peng, C.; Anaman, R.; Zhang, P. Immobilization of Cd in the soil of mining areas by Fe-Mn oxidizing bacteria. Sci. Total Environ. 2023, 873, 162306. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Teng, Z.; Wang, G.; Luo, W.; Guo, Y.; Ji, X.; Hu, W.; Li, M. Anaerobic syntrophic system composed of phosphate solubilizing bacteria and dissimilatory iron reducing bacteria induces cadmium immobilization via secondary mineralization. J. Hazard. Mater. 2023, 446, 130702. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.; Wu, S.; Zhou, Z.; Wang, G. Microbial Cd(II) and Cr(VI) resistance mechanisms and application in bioremediation. J. Hazard. Mater. 2021, 401, 123685. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Li, Y.; Peng, S.Y.; Wei, L.; Zhang, B.; Deng, X.Y.; Zhong, M.; Cheng, X. Cadmium biosorption and mechanism investigation using two cadmium-tolerant microorganisms isolated from rhizosphere soil of rice. J. Hazard. Mater. 2024, 470, 134134. [Google Scholar] [CrossRef] [PubMed]
- Peng, W.; Li, X.; Song, J.; Jiang, W.; Liu, Y.; Fan, W. Bioremediation of cadmium- and zinc-contaminated soil using Rhodobacter sphaeroides. Chemosphere 2018, 197, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Han, H.; Wu, X.; Hui, R.; Xia, X.; Chen, Z.; Yao, L.; Yang, J. Synergistic effects of Cd-loving Bacillus sp. N3 and iron oxides on immobilizing Cd and reducing wheat uptake of Cd. Environ. Pollut. 2022, 305, 119303. [Google Scholar] [CrossRef] [PubMed]
- Saxena, A.K.; Kumar, M.; Chakdar, H.; Anuroopa, N.; Bagyaraj, D.J. Bacillus species in soil as a natural resource for plant health and nutrition. J. Appl. Microbiol. 2020, 128, 1583–1594. [Google Scholar] [CrossRef] [PubMed]
- Wróbel, M.; Śliwakowski, W.; Kowalczyk, P.; Kramkowski, K.; Dobrzyński, J. Bioremediation of heavy metals by the genus Bacillus. Int. J. Environ. Res. Public Health 2023, 20, 4964. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Dang, Z.; Guo, C.L.; Lu, G.N.; Gu, R.R.; Liu, H.J.; Zhang, H. Biosorption of Cd(II) by live and dead cells of Bacillus cereus RC-1 isolated from cadmium-contaminated soil. Colloids Surf. B Biointerfaces 2013, 107, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Nazli, F.; Jamil, M.; Hussain, A.; Hussain, T. Exopolysaccharides and indole-3-acetic acid producing Bacillus safensis strain FN13 potential candidate for phytostabilization of heavy metals. Environ. Monit. Assess. 2020, 192, 738. [Google Scholar] [CrossRef] [PubMed]
- Li, W.L.; Wang, J.F.; Lv, Y.; Dong, H.J.; Wang, L.L.; He, T.; Li, Q.S. Improving cadmium mobilization by phosphate-solubilizing bacteria via regulating organic acids metabolism with potassium. Chemosphere 2020, 244, 125475. [Google Scholar] [CrossRef] [PubMed]
- Saran, A.; Imperato, V.; Fernandez, L.; Gkorezis, P.; d’Haen, J.; Merini, L.J.; Vangronsveld, J.; Thijs, S. Phytostabilization of polluted military soil supported by bioaugmentation with PGP-trace element tolerant bacteria isolated from Helianthus petiolaris. Agronomy 2020, 10, 204. [Google Scholar] [CrossRef]
- Liu, H.; Hong, Z.; Lin, J.; Huang, D.; Ma, L.Q.; Xu, J.; Dai, Z. Bacterial coculture enhanced Cd sorption and as bioreduction in co-contaminated systems. J. Hazard. Mater. 2023, 444 Pt A, 130376. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.S.; Patel, S.; Saini, N.; Chen, S. Robust demarcation of 17 distinct Bacillus species clades, proposed as novel Bacillaceae genera, by phylogenomics and comparative genomic analyses: Description of Robertmurraya kyonggiensis sp. nov. and proposal for an emended genus Bacillus limiting it only to the members of the Subtilis and Cereus clades of species. Int. J. Syst. Evol. Microbiol. 2020, 70, 5753–5798. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Ren, J.; Fang, L.; Sun, K.; He, W. The role and mechanism of Bacillus megaterium strain A14 in inhibiting the cadmium uptake by peanut plants in acidic red soil. J. Appl. Microbiol. 2024, 135, lxae120. [Google Scholar] [CrossRef] [PubMed]
- Mohapatra, R.K.; Nayak, M.; Parhi, P.K.; Pandey, S.; Thatoi, H.; Panda, C.R.; Choi, Y. Biosorption performance and mechanism insights of live and dead biomass of halophilic Bacillus altitudinis strain CdRPSD103 for removal of Cd(II) from aqueous solution. Int. Biodeterior. Biodegrad. 2024, 191, 105811. [Google Scholar] [CrossRef]
- Liu, S.; Huang, Y.; Zheng, Q.; Zhan, M.; Hu, Z.; Ji, H.; Zhu, D.; Zhao, X. Cd-Resistant plant growth-promoting rhizobacteria Bacillus siamensis R27 absorbed Cd and reduced Cd accumulation in Lettuce (Lactuca sativa L.). Microorganisms 2024, 12, 2321. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhai, L.; Yao, S.; Cao, Y.; Cao, Y.; Zhang, X.; Su, J.; Ge, Y.; Zhao, R.; Cheng, C. Brachybacterium hainanense sp. nov., isolated from noni (Morinda citrifolia L.) branch. Int. J. Syst. Evol. Microbiol. 2015, 65, 4196–4201. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.Z.; Cai, M.Y. Determinative Manual for Routine Bacteriology; Scientific Press: Beijing, China, 2001. [Google Scholar]
- Glaeser, S.P.; Kämpfer, P. Multilocus sequence analysis (MLSA) in prokaryotic taxonomy. Syst. Appl. Microbiol. 2015, 38, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar] [CrossRef] [PubMed]
- Kimura, M. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 1980, 16, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Felsenstein, J. Confidence limits on phylogenies: An approach using the bootstrap. Evolution 1985, 39, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Aryal, M.; Liakopoulou-Kyriakides, M. Bioremoval of heavy metals by bacterial biomass. Environ. Monit. Assess. 2015, 187, 4173. [Google Scholar] [CrossRef] [PubMed]
- Limcharoensuk, T.; Sooksawat, N.; Sumarnrote, A.; Awutpet, T.; Kruatrachue, M.; Pokethitiyook, P.; Auesukaree, C. Bioaccumulation and biosorption of Cd2+ and Zn2+ by bacteria isolated from a zinc mine in Thailand. Ecotoxicol. Environ. Saf. 2015, 122, 322–330. [Google Scholar] [CrossRef] [PubMed]
- Paul, M.L.; Samuel, J.; Chandrasekaran, N.; Mukherjee, A. Comparative kinetics, equilibrium, thermodynamic and mechanistic studies on biosorption of hexavalent chromium by live and heat killed biomass of Acinetobacter junii VITSUKMW2, an indigenous chromite mine isolate. Chem. Eng. J. 2012, 187, 104–113. [Google Scholar] [CrossRef]
- Mohapatra, R.K.; Parhi, P.K.; Pandey, S.; Bindhani, B.K.; Thatoi, H.; Panda, C.R. Active and passive biosorption of Pb(II) using live and dead biomass of marine bacterium Bacillus xiamenensis PbRPSD202: Kinetics and isotherm studies. J. Environ. Manag. 2019, 247, 121–134. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Tian, H.; Yang, J.; Shi, P.; Lou, Q.; Ni, Z.; Peng, X. Multivariate analyses and evaluation of heavy metals by chemometric BCR sequential extraction method in surface sediments from Lingdingyang Bay, South China. Sustainability 2015, 7, 4938–4951. [Google Scholar] [CrossRef]
- Liu, H.; Huang, H.; Liang, K.; Lin, K.; Shangguan, Y.; Xu, H. Characterization of a cadmium-resistant functional bacteria (Burkholderia sp. SRB-1) and mechanism analysis at physiochemical and genetic level. Environ. Sci. Pollut. Res. Int. 2023, 30, 78408–78422. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Xing, Y.; Liu, S.; Hao, X.; Chen, W.; Huang, Q. Characterization of Cd2+ biosorption by Pseudomonas sp. strain 375, a novel biosorbent isolated from soil polluted with heavy metals in Southern China. Chemosphere 2020, 240, 124893. [Google Scholar] [CrossRef] [PubMed]
- Qin, W.; Zhao, J.; Yu, X.; Liu, X.; Chu, X.; Tian, J.; Wu, N. Improving cadmium resistance in Escherichia coli through continuous genome evolution. Front. Microbiol. 2019, 10, 278. [Google Scholar] [CrossRef] [PubMed]
- Khan, Z.; Rehman, A.; Hussain, S.Z.; Nisar, M.A.; Zulfiqar, S.; Shakoori, A.R. Cadmium resistance and uptake by bacterium, Salmonella enterica 43C, isolated from industrial effluent. AMB Express 2016, 6, 54. [Google Scholar] [CrossRef] [PubMed]
- Chun, J.; Oren, A.; Ventosa, A.; Christensen, H.; Arahal, D.R.; da Costa, M.S.; Rooney, A.P.; Yi, H.; Xu, X.W.; De Meyer, S.; et al. Proposed minimal standards for the use of genome data for the taxonomy of prokaryotes. Int. J. Syst. Evol. Microbiol. 2018, 68, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Hegazy, G.E.; Soliman, N.A.; Ossman, M.E.; Abdel-Fattah, Y.R.; Moawad, M.N. Isotherm and kinetic studies of cadmium biosorption and its adsorption behaviour in multi-metals solution using dead and immobilized archaeal cells. Sci. Rep. 2023, 13, 2550. [Google Scholar] [CrossRef] [PubMed]
- Xia, L.; Xu, X.; Zhu, W.; Huang, Q.; Chen, W. A comparative study on the biosorption of Cd2+ onto Paecilomyces lilacinus XLA and Mucoromycote sp. XLC. Int. J. Mol. Sci. 2015, 16, 15670–15687. [Google Scholar] [CrossRef] [PubMed]
- Khadivinia, E.; Sharafi, H.; Hadi, F.; Zahiri, H.S.; Modiri, S.; Tohidi, A.; Mousavi, A.; Salmanian, A.H.; Noghabi, K.A. Cadmium biosorption by a glyphosate-degrading bacterium, a novel biosorbent isolated from pesticide-contaminated agricultural soils. J. Ind. Eng. Chem. 2014, 20, 4304–4310. [Google Scholar] [CrossRef]
- Masoudzadeh, N.; Zakeri, F.; Lotfabad, T.B.; Sharafi, H.; Masoomi, F.; Zahiri, H.S.; Ahmadian, G.; Noghabi, K.A. Biosorption of cadmium by Brevundimonas sp. ZF12 strain, a novel biosorbent isolated from hot-spring waters in high background radiation areas. J. Hazard. Mater. 2011, 197, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Tunali, S.; Çabuk, A.; Akar, T. Removal of lead and copper ions from aqueous solutions by bacterial strain isolated from soil. Chem. Eng. J. 2006, 115, 203–211. [Google Scholar] [CrossRef]
- Liu, S.Y.; Pu, Q.; Mo, T.J.; Peng, G.; Sun, Y.; Zhang, Y.; Wang, J.; Li, Y.; Xu, H.J. The mechanism of immobilization of Cd (II) by phosphate-solubilizing bacteria Bacillus sp. B19. Water Air Soil Pollut. 2023, 234, 543. [Google Scholar] [CrossRef]
- Kailasam, S.; Sundaramanickam, A.; Kanth, S.V. Isotherms and kinetics of multi-heavy metal sorption by marine phosphate-solubilizing bacteria from seagrass meadow. Int. J. Environ. Sci. Technol. 2024, 21, 5731–5742. [Google Scholar] [CrossRef]
- Ren, G.; Jin, Y.; Zhang, C.; Gu, H.; Qu, J. Characteristics of Bacillus sp. PZ-1 and its biosorption to Pb(II). Ecotoxicol. Environ. Saf. 2015, 117, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
- Freundlich, H.M.F. Über die adsorption in losungen. Z. Phys. Chem. 1906, 57, 385–470. [Google Scholar] [CrossRef]
- Baran, M.F.; Duz, M.Z. Removal of cadmium (II) in the aqueous solutions by biosorption of Bacillus licheniformis isolated from soil in the area of Tigris River. Int. J. Environ. Anal. Chem. 2019, 101, 533–548. [Google Scholar] [CrossRef]
- Kulkarni, R.M.; Shetty, K.V.; Srinikethan, G. Cadmium (II) and nickel (II) biosorption by Bacillus laterosporus (MTCC 1628). J. Taiwan Inst. Chem. Eng. 2014, 45, 1628–1635. [Google Scholar] [CrossRef]
- Rezaee, A.; Ahmady-Asbchin, S. Removal of toxic metal Cd (II) by Serratia bozhouensis CdIW2 using in moving bed biofilm reactor (MBBR). J. Environ. Manag. 2023, 344, 118361. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Wang, J.; Zhang, L. Two cadmium-resistant strains of agricultural soil effective in remediating soil cadmium pollution. J. Environ. Chem. Eng. 2023, 11, 111189. [Google Scholar] [CrossRef]
- Deepika, K.V.; Raghuram, M.; Kariali, E.; Bramhachari, P.V. Biological responses of symbiotic Rhizobium radiobacter strain VBCK1062 to the arsenic contaminated rhizosphere soils of mung bean. Ecotoxicol. Environ. Saf. 2016, 134 Pt 1, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Jia, Q.; Jing, W.; Dahms, H.U.; Wang, L. Screening strains for microbial biosorption technology of cadmium. Chemosphere 2020, 251, 126428. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.W.; Ho, H.C.; Yao, C.L.; Tseng, T.Y.; Kao, C.M.; Chen, S.C. Bioremediation potential of cadmium by recombinant Escherichia coli surface expressing metallothionein MTT5 from Tetrahymena thermophila. Chemosphere 2023, 310, 136850. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Liao, M.; Lu, X.; Xu, N.; Xie, X.; Gao, W. Unveiling the performance of a novel alkalizing bacterium Enterobacter sp. LYX-2 in immobilization of available Cd. J. Environ. Sci. 2024, 137, 245–257. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Wang, W.; Peng, Y.; Zhou, F.; He, D.; Wang, J.; Chang, Y.; Yang, J.; Zhou, J.; Wang, W.; et al. Effects of simulated Cd depositon on soil Cd availability, microbial response, and crop cd uptake in the passivation-remediation process of Cd-contaminated purple soil. Sci. Total Environ. 2019, 683, 782–792. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Luo, Y.; Zeng, G.; Wu, X.; Wu, B.; Li, X.; Xu, H. Characteristics and in situ remediation effects of heavy metal immobilizing bacteria on cadmium and nickel co-contaminated soil. Ecotoxicol. Environ. Saf. 2020, 192, 110294. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Yuan, K.; Peng, Q.; Lv, R.; Zheng, Y. Stenotrophomonas strain CD2 reduces cadmium accumulation in Brassica rapa L. Front. Sustain. Food Syst. 2024, 8, 1362265. [Google Scholar] [CrossRef]
- Wu, S.; Zhou, Z.; Zhu, L.; Zhong, L.; Dong, Y.; Wang, G.; Shi, K. Cd immobilization mechanisms in a Pseudomonas strain and its application in soil Cd remediation. J. Hazard. Mater. 2022, 425, 127919. [Google Scholar] [CrossRef] [PubMed]
- Zeng, F.; Ali, S.; Zhang, H.; Ouyang, Y.; Qiu, B.; Wu, F.; Zhang, G. The influence of pH and organic matter content in paddy soil on heavy metal availability and their uptake by rice plants. Environ. Pollut. 2011, 159, 84–91. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, T.; Zhang, C.; Hu, X.; Wang, T.; Lyu, Z.; Sun, L. Immobilization of Cd Through Biosorption by Bacillus altitudinis C10-4 and Remediation of Cd-Contaminated Soil. Microorganisms 2025, 13, 1798. https://doi.org/10.3390/microorganisms13081798
Gao T, Zhang C, Hu X, Wang T, Lyu Z, Sun L. Immobilization of Cd Through Biosorption by Bacillus altitudinis C10-4 and Remediation of Cd-Contaminated Soil. Microorganisms. 2025; 13(8):1798. https://doi.org/10.3390/microorganisms13081798
Chicago/Turabian StyleGao, Tianyu, Chenlu Zhang, Xueqiang Hu, Tianqi Wang, Zhitang Lyu, and Lei Sun. 2025. "Immobilization of Cd Through Biosorption by Bacillus altitudinis C10-4 and Remediation of Cd-Contaminated Soil" Microorganisms 13, no. 8: 1798. https://doi.org/10.3390/microorganisms13081798
APA StyleGao, T., Zhang, C., Hu, X., Wang, T., Lyu, Z., & Sun, L. (2025). Immobilization of Cd Through Biosorption by Bacillus altitudinis C10-4 and Remediation of Cd-Contaminated Soil. Microorganisms, 13(8), 1798. https://doi.org/10.3390/microorganisms13081798




