COVID-19 Vaccination Still Makes Sense: Insights on Pneumonia Risk and Hospitalization from a Large-Scale Study at an Academic Tertiary Center in Italy
Abstract
1. Introduction
2. Materials and Methods
Statistical Analysis
3. Results
COVID-19 Antigen Testing According to Vaccination Status
4. Discussion
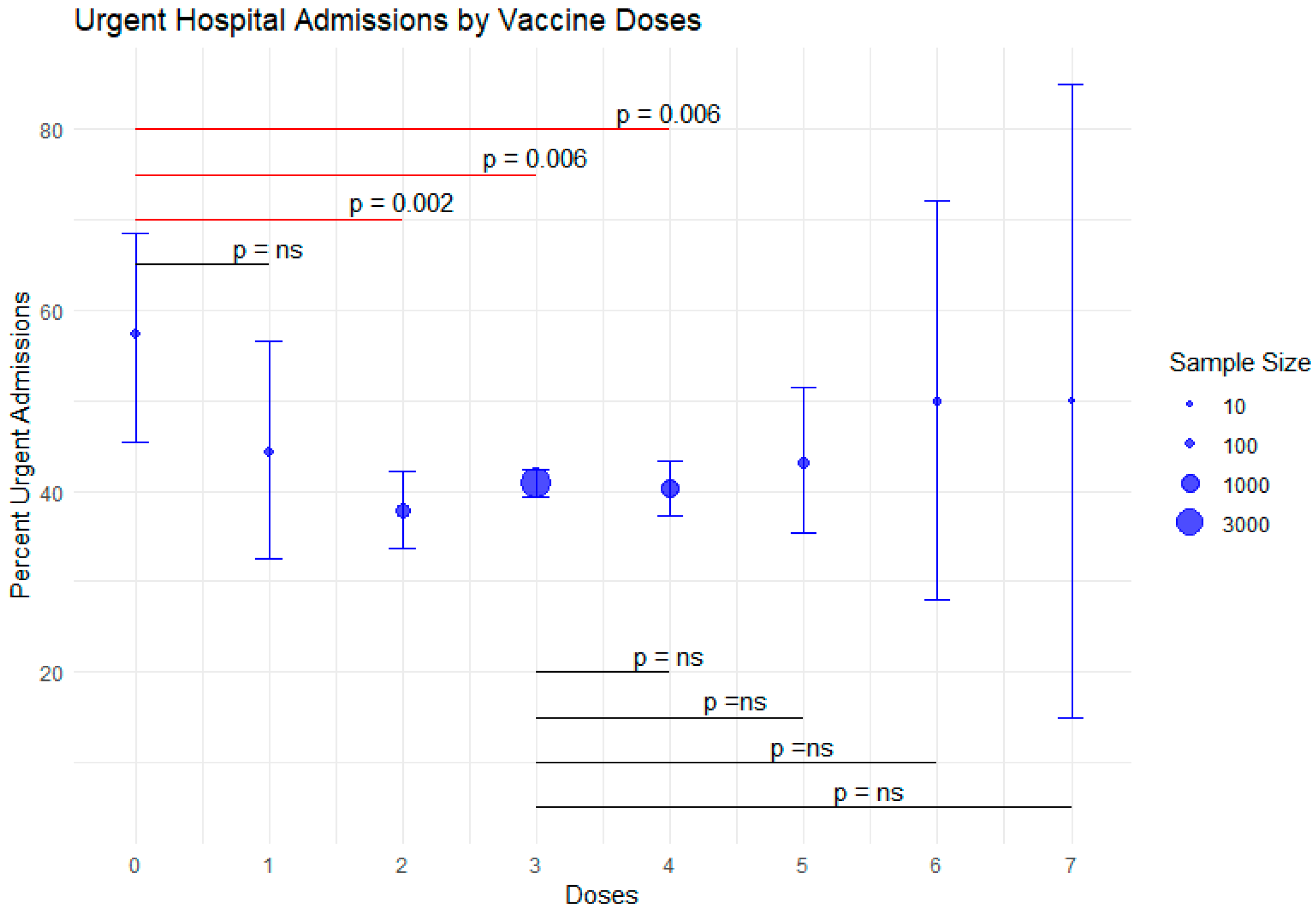
- Vaccination status is associated with the type of hospital admission required, with emergency hospital admission being more frequent in unvaccinated patients.
- Positive or weakly positive COVID-19 swabs were considerably more prevalent in unvaccinated patients requiring hospitalization.
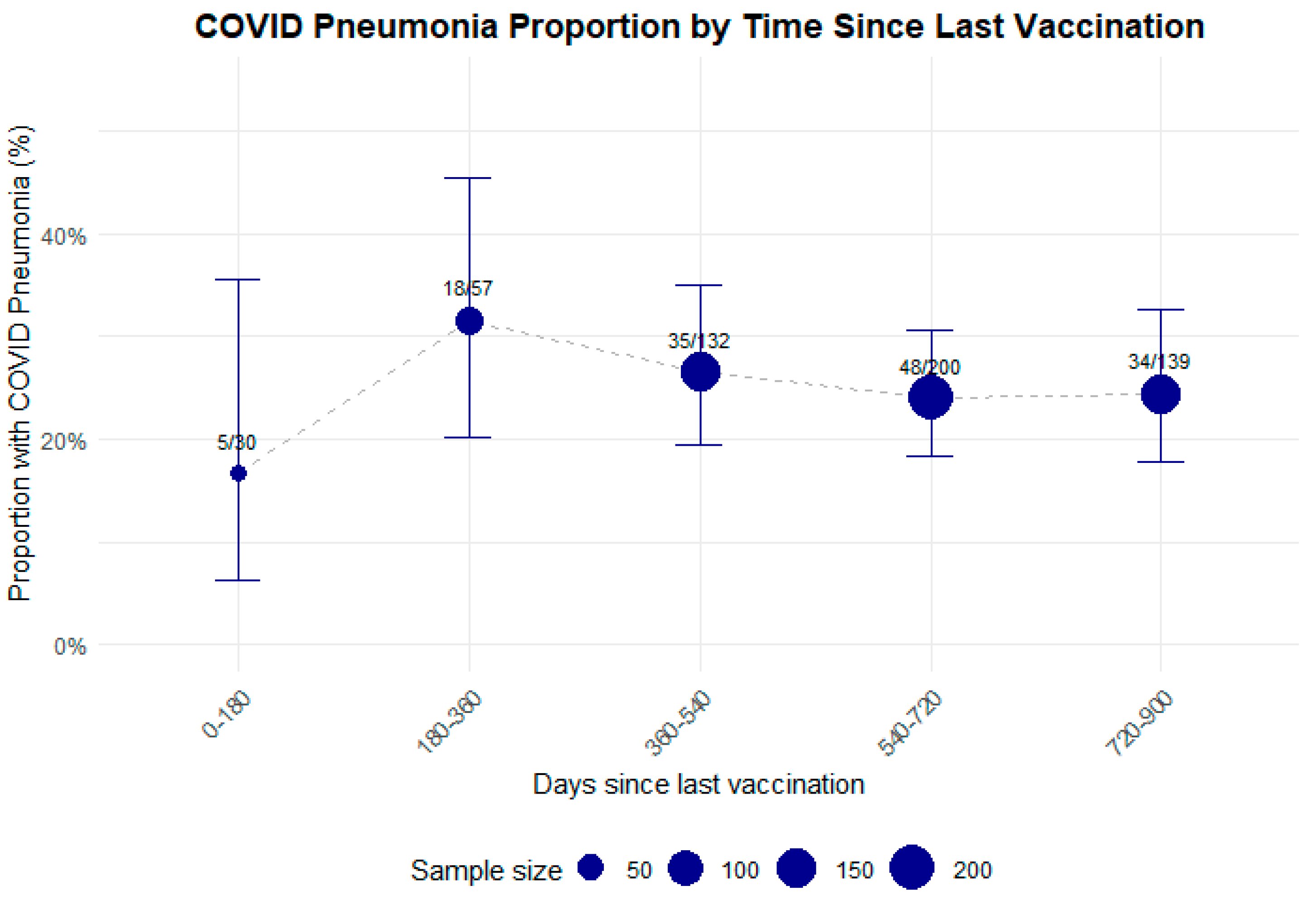
- The proportion of COVID-19 pneumonia and overall pneumonia is higher in hospitalized patients with no COVID-19 vaccination compared with vaccinated individuals, with no influence of timing since last vaccination dose.
4.1. Vaccination Status and Hospital Admission Type
4.2. Prevalence of Positive COVID-19 Testing
4.3. Prevalence of COVID-19 and Non-COVID-19 Pneumonia
4.4. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CCI | Charlson Comorbidity Index |
| CI | Confidence interval |
| IQR | Interquartile ranges |
| SD | Standard deviation |
References
- Suthar, A.B.; Wang, J.; Seffren, V.; Wiegand, R.E.; Griffing, S.; Zell, E. Public health impact of COVID-19 vaccines in the US: Observational study. BMJ 2022, 377, e069317. [Google Scholar] [CrossRef]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef]
- Malik, A.A.; McFadden, S.M.; Elharake, J.; Omer, S.B. Determinants of COVID-19 vaccine acceptance in the US. EClin. Med. 2020, 26, 100495. [Google Scholar] [CrossRef]
- Sadoff, J.; Gray, G.; Vandebosch, A.; Cárdenas, V.; Shukarev, G.; Grinsztejn, B.; Goepfert, P.A.; Truyers, C.; Van Dromme, I.; Spiessens, B.; et al. Final analysis of efficacy and safety of single-dose Ad26.COV2.S. N. Engl. J. Med. 2022, 386, 847–860. [Google Scholar] [CrossRef]
- European Parliament. Parliamentary Question. Withdrawal of the Marketing Authorisation for AstraZeneca’s Vaccine. E-001557/2024. Available online: https://www.europarl.europa.eu/doceo/document/E-10-2024-001557_EN.html?utm_source=chatgpt.com#def2 (accessed on 12 June 2025).
- Mascellino, M.T.; Di Timoteo, F.; De Angelis, M.; Oliva, A. Overview of the main anti-SARS-CoV-2 vaccines: Mechanism of action, efficacy and safety. Infect. Drug Resist. 2021, 14, 3459–3476. [Google Scholar] [CrossRef]
- Azzolini, E.; Levi, R.; Sarti, R.; Pozzi, C.; Mollura, M.; Mantovani, A.; Rescigno, M. Association between BNT162b2 vaccination and long COVID after infections not requiring hospitalization in health care workers. JAMA 2022, 328, 676–678. [Google Scholar] [CrossRef] [PubMed]
- Graña, C.; Ghosn, L.; Evrenoglou, T.; Jarde, A.; Minozzi, S.; Bergman, H.; Buckley, B.S.; Probyn, K.; Villanueva, G.; Henschke, N.; et al. Efficacy and safety of COVID-19 vaccines. Cochrane Database Syst. Rev. 2022, 12, CD015477. [Google Scholar] [CrossRef]
- Goldberg, Y.; Mandel, M.; Bar-On, Y.M.; Bodenheimer, O.; Freedman, L.; Haas, E.J.; Milo, R.; Alroy-Preis, S.; Ash, N.; Huppert, A. Waning immunity after the BNT162b2 vaccine in Israel. N. Engl. J. Med. 2021, 385, e85. [Google Scholar] [CrossRef] [PubMed]
- Lopez Bernal, J.; Andrews, N.; Gower, C.; Robertson, C.; Stowe, J.; Tessier, E.; Simmons, R.; Cottrell, S.; Roberts, R.; O'Doherty, M.; et al. Effectiveness of the Pfizer-BioNTech and Oxford-AstraZeneca vaccines on COVID-19 related symptoms, hospital admissions, and mortality in older adults in England: Test negative case-control study. BMJ 2021, 373, n1088. [Google Scholar] [CrossRef] [PubMed]
- World Health Organisation. WHO Ten Threats to Global Health in 2019. Available online: https://www.who.int/news-room/spotlight/ten-threats-to-global-health-in-2019 (accessed on 12 July 2025).
- Boccalini, S.; Cosma, C.; Monaci, P.; Guida, A.; Velpini, B.; Cerini, G.; Chiesi, F.; Bonanni, P.; Bechini, A. Exploring perceptions of vaccine safety: An Italian national survey on different COVID-19 vaccine formulations. Ann. Ist. Super. Sanità 2025, 61, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Marini, M.; Demichelis, A.; Menicagli, D.; Mancini, G.; Panizza, F.; Bilancini, E.; Cevolani, G. I want to be safe: Understanding the main drivers behind vaccination choice throughout the pandemic. BMC Public Health 2024, 24, 1111. [Google Scholar] [CrossRef]
- Goldman, R.D.; Hart, R.J.; Bone, J.N.; Seiler, M.; Olson, P.G.; Keitel, K.; Manzano, S.; Gualco, G.; Krupik, D.; Schroter, S.; et al. Adverse events among early caregivers’ COVID-19 vaccination correlated inversely with intention to vaccinate their children. Vaccine 2025, 55, 127001. [Google Scholar] [CrossRef]
- Patel, M.; Gokun, Y.; DeGraffinreid, C.; Washington, C.; Baltic, R.D.; Ringel, M.D.; Paskett, E.D.; Improving Access to Covid Testing in Ohio (IMPACT-Ohio) Group, Part of the National RADx-UP Initiative. COVID-19 vaccination uptake in Ohio: Analyzing the difference between metro and non-metro residents. BMC Public Health 2025, 25, 1103. [Google Scholar] [CrossRef]
- Paul, E.; Steptoe, A.; Fancourt, D. Attitudes towards vaccines and intention to vaccinate against COVID-19: Implications for public health communications. Lancet Reg. Health Eur. 2021, 1, 100012. [Google Scholar] [CrossRef]
- Zimmerman, T.; Shiroma, K.; Fleischmann, K.R.; Xie, B.; Jia, C.; Verma, N.; Lee, M.K. Misinformation and COVID-19 vaccine hesitancy. Vaccine 2022, 41, 136–144. [Google Scholar] [CrossRef]
- Tudoran, C.; Tudoran, M.; Lazureanu, V.E.; Marinescu, A.R.; Cut, T.G.; Oancea, C.; Pescariu, S.A.; Pop, G.N. Factors influencing the evolution of pulmonary hypertension in previously healthy subjects recovering from a SARS-CoV-2 infection. J. Clin. Med. 2021, 10, 5272. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nobakht, N.; Jang, C.; Grogan, T.; Fahim, P.; Kurtz, I.; Schaenman, J.; Wilson, J.; Kamgar, M.; RECOVID Investigators. RECOVID: Retrospective observational study of renal outcomes and long-term mortality in patients with COVID-19-associated AKI, a comparison between vaccinated and unvaccinated patients. Kidney Med. 2025, 7, 101020. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Trinh, N.T.; Jödicke, A.M.; Català, M.; Mercadé-Besora, N.; Hayati, S.; Lupattelli, A.; Prieto-Alhambra, D.; Nordeng, H.M. Effectiveness of COVID-19 vaccines to prevent long COVID: Data from Norway. Lancet Respir. Med. 2024, 12, e33–e34. [Google Scholar] [CrossRef] [PubMed]
- Saad-Roy, C.M.; Morris, S.E.; Boots, M.; Baker, R.E.; Lewis, B.L.; Farrar, J.; Marathe, M.V.; Graham, A.L.; Levin, S.A.; Wagner, C.E.; et al. Impact of waning immunity against SARS-CoV-2 severity exacerbated by vaccine hesitancy. PLoS Comput. Biol. 2024, 20, e1012211. [Google Scholar] [CrossRef] [PubMed]
- Darbandi, A.; Koupaei, M.; Kiani, P.; Ghanavati, R.; Najafi, P.; Hosseini, J.; Shokouhamiri, M.R.; Asadi, A.; Parsapour, R. Acceptance-hesitancy of COVID-19 vaccination and factors affecting it in adults: Systematic review study. Immun. Inflamm. Dis. 2024, 12, e70076. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Duś-Ilnicka, I.; Mazur, M.; Rybińska, A.; Radwan-Oczko, M.; Jurczyszyn, K.; Paradowska-Stolarz, A. SARS-CoV-2 IgG seropositivity post-vaccination among dental professionals: A prospective study. BMC Infect. Dis. 2023, 23, 539. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Choi, J.H.; Kim, Y.R.; Heo, S.T.; Oh, H.; Kim, M.; Lee, H.R.; Yoo, J.R. Healthcare workers in South Korea maintain a SARS-CoV-2 antibody response six months after receiving a second dose of the BNT162b2 mRNA vaccine. Front. Immunol. 2022, 13, 827306. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pascucci, D.; Lontano, A.; Marziali, E.; Vetrugno, G.; Moscato, U.; Vaccination Team of Hospital Hygiene Unit; Laurenti, P.; Group authorship. Assessing vaccine coverage and delivery strategies for influenza and COVID-19 among Italian healthcare workers: A 2015–2023 case study. Hum. Vaccin. Immunother. 2025, 21, 2493027. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Eiden, A.L.; Drakeley, S.; Modi, K.; Mackie, D.; Bhatti, A.; DiFranzo, A. Attitudes and beliefs of healthcare providers toward vaccination in the United States: A cross-sectional online survey. Vaccine 2024, 42, 126437, Erratum in: Vaccine 2025, 53, 127054. [Google Scholar] [CrossRef] [PubMed]
- Annandale, G.; Kola-Palmer, S.; Duke, É. The complex landscape of vaccine hesitancy and hesitant adopters: Quantitative predictors and thematic insights into COVID-19 vaccine attitudes. Hum. Vaccin. Immunother. 2025, 21, 2511350. [Google Scholar] [CrossRef] [PubMed]
- Yadav, G.; Dandu, H.; Malhotra, H.S.; Jain, A.; Radera, S.; Agrawal, V.; Verma, A.K.; Prakash, R.; Yadav, S.; Kumar, N.; et al. COVID-19 vaccination and utility of booster dose: A community-based cross-sectional study. Vaccine 2025, 60, 127325. [Google Scholar] [CrossRef] [PubMed]
- Spitzer, A.; Angel, Y.; Marudi, O.; Zeltser, D.; Saiag, E.; Goldshmidt, H.; Goldiner, I.; Stark, M.; Halutz, O.; Gamzu, R.; et al. Association of a third dose of BNT162b2 vaccine with incidence of SARS-CoV-2 infection among health care workers in Israel. JAMA 2022, 327, 341–349. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Saha, A.; Ghosh Roy, S.; Dwivedi, R.; Tripathi, P.; Kumar, K.; Nambiar, S.M.; Pathak, R. Beyond the pandemic era: Recent advances and efficacy of SARS-CoV-2 vaccines against emerging variants of concern. Vaccines 2025, 13, 424. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Begum, T.; Efstathiou, N.; Bailey, C.; Guo, P. Cultural and social attitudes towards COVID-19 vaccination and factors associated with vaccine acceptance in adults across the globe: A systematic review. Vaccine 2024, 42, 125993. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, E.B.; Daoust, J.F.; Vikse, J.; Nelson, V. “Until I know it’s safe for me”: The role of timing in COVID-19 vaccine decision-making and vaccine hesitancy. Vaccines 2021, 9, 1417. [Google Scholar] [CrossRef]
- Fogolari, M.; Francesconi, M.; De Florio, L.; Giovanetti, M.; Veralli, R.; De Flora, C.; Maruotti, A.; Scarpa, F.; Spoto, S.; Sambuco, F.; et al. SARS-CoV-2 variants in COVID-19 disease: A focus on disease severity and vaccine immunity in patients admitted to the emergency department. J. Pers. Med. 2022, 12, 2001. [Google Scholar] [CrossRef]
- Morovatshoar, R.; Hushmandi, K.; Orouei, S.; Saadat, S.H.; Raesi, R. Investigating the trend of demographic changes, mortality, clinical and paraclinical findings of patients hospitalized in the Corona ward, before and after the start of general vaccination of COVID-19. BMC Infect. Dis. 2024, 24, 488. [Google Scholar] [CrossRef]
- Haas, E.J.; Angulo, F.J.; McLaughlin, J.M.; Anis, E.; Singer, S.R.; Khan, F.; Brooks, N.; Smaja, M.; Mircus, G.; Pan, K.; et al. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: An observational study using national surveillance data. Lancet 2021, 397, 1819–1829. [Google Scholar] [CrossRef] [PubMed]
- Krause, P.R.; Fleming, T.R.; Peto, R.; Longini, I.M.; Figueroa, J.P.; Sterne, J.A.C.; Cravioto, A.; Rees, H.; Higgins, J.P.T.; Boutron, I.; et al. Considerations in boosting COVID-19 vaccine immune responses. Lancet 2021, 398, 1377–1380. [Google Scholar] [CrossRef]
- Perez-Alos, L.; Hansen, C.B.; Almagro Armenteros, J.J.; Madsen, J.R.; Heftdal, L.D.; Hasselbalch, R.B.; Pries-Heje, M.M.; Bayarri-Olmos, R.; Jarlhelt, I.; Hamm, S.R.; et al. Previous immunity shapes immune responses to SARS-CoV-2 booster vaccination and Omicron breakthrough infection risk. Nat. Commun. 2023, 14, 5624. [Google Scholar] [CrossRef] [PubMed]
- Reinholm, A.; Maljanen, S.; Jalkanen, P.; Altan, E.; Tauriainen, S.; Belik, M.; Skon, M.; Haveri, A.; Osterlund, P.; Iakubovskaia, A.; et al. Neutralizing antibodies after the third COVID-19 vaccination in healthcare workers with or without breakthrough infection. Commun. Med. 2024, 4, 28. [Google Scholar] [CrossRef]
- Barouch, S.E.; Chicz, T.M.; Blanc, R.; Barbati, D.R.; Parker, L.J.; Tong, X.; Li, W.; McNamara, R.P. Concurrent administration of COVID-19 and influenza vaccines enhances spike-specific antibody responses. Open Forum Infect. Dis. 2024, 11, ofae144. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Awadalla, M.; AlRawi, H.Z.; Henawi, R.A.; Barnawi, F.; Alkadi, H.; Alyami, A.; Alsughayir, A.; Alsaif, A.S.; Mubarak, A.; Alturaiki, W.; et al. Humoral and cellular immune durability of different COVID-19 vaccine platforms following homologous/heterologous boosters: One-year post vaccination. Front. Immunol. 2025, 16, 1526444. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Doyon-Plourde, P.; Przepiorkowski, J.; Young, K.; Zhao, L.; Sinilaite, A. Intraseasonal waning immunity of seasonal influenza vaccine—A systematic review and meta-analysis. Vaccine 2023, 41, 4462–4471. [Google Scholar] [CrossRef]
- Srivastava, K.; Carreno, J.M.; Gleason, C.; Monahan, B.; Singh, G.; Abbad, A.; Tcheou, J.; Raskin, A.; Kleiner, G.; van Bakel, H.; et al. SARS-CoV-2-infection- and vaccine-induced antibody responses are long lasting with an initial waning phase followed by a stabilization phase. Immunity 2024, 57, 587–599.e584. [Google Scholar] [CrossRef]
- Gazit, S.; Saciuk, Y.; Perez, G.; Peretz, A.; Pitzer, V.E.; Patalon, T. Short term, relative effectiveness of four doses versus three doses of BNT162b2 vaccine in people aged 60 years and older in Israel: Retrospective, test negative, case-control study. BMJ 2022, 377, e071113. [Google Scholar] [CrossRef]
- Saiag, E.; Alcalay, Y.; Marudi, O.; Orr-Urtreger, A.; Hagin, D. Cellular and humoral immune response to the fourth Pfizer-BioNTech COVID-19 vaccine dose in individuals aged 60 years and older. Vaccine 2023, 41, 914–921. [Google Scholar] [CrossRef]
- Altarawneh, H.N.; Chemaitelly, H.; Ayoub, H.H.; Hasan, M.R.; Coyle, P.; Yassine, H.M.; Al-Khatib, H.A.; Smatti, M.K.; Al-Kanaani, Z.; Al-Kuwari, E.; et al. Protective effect of previous SARS-CoV-2 infection against Omicron BA.4 and BA.5 subvariants. N. Engl. J. Med. 2022, 387, 1620–1622. [Google Scholar] [CrossRef]
- Becerril-Gaitan, A.; Vaca-Cartagena, B.F.; Ferrigno, A.S.; Mesa-Chavez, F.; Barrientos-Gutiérrez, T.; Tagliamento, M.; Lambertini, M.; Villarreal-Garza, C. Immunogenicity and risk of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) infection after Coronavirus Disease 2019 (COVID-19) vaccination in patients with cancer: A systematic review and meta-analysis. Eur. J. Cancer 2022, 160, 243–260. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Palalay, H.; Vyas, R.; Tafuto, B. Real-world effectiveness of mRNA COVID-19 vaccines in the elderly during the Delta and Omicron variants: Systematic review. World J. Meta-Anal. 2023, 11, 167–180. [Google Scholar] [CrossRef] [PubMed]
- Doherty, T.M.; Weinberger, B.; Didierlaurent, A.; Lambert, P.H. Age-related changes in the immune system and challenges for the development of age-specific vaccines. Ann. Med. 2025, 57. [Google Scholar] [CrossRef]
- Andrews, N.; Tessier, E.; Stowe, J.; Gower, C.; Kirsebom, F.; Simmons, R.; Gallagher, E.; Thelwall, S.; Groves, N.; Dabrera, G.; et al. Duration of protection against mild and severe disease by COVID-19 vaccines. N. Engl. J. Med. 2022, 386, 340–350. [Google Scholar] [CrossRef]
- Dodge, M.C.; Ellervik, C.; Kataria, Y. A meta-analysis of Severe Acute Respiratory Syndrome Coronavirus 2 anti-spike immunoglobulin G antibody durability up to 9 months after full vaccination in adults. Clin. Lab. Med. 2025, 45, 111–136. [Google Scholar] [CrossRef] [PubMed]
- Bigdelou, B.; Sepand, M.R.; Najafikhoshnoo, S.; Negrete, J.A.T.; Sharaf, M.; Ho, J.Q.; Sullivan, I.; Chauhan, P.; Etter, M.; Shekarian, T.; et al. COVID-19 and preexisting comorbidities: Risks, synergies, and clinical outcomes. Front. Immunol. 2022, 13, 890517. [Google Scholar] [CrossRef]
- Janc, J.; Woźniak, A.; Leśnik, P.; Łysenko, L. Does cognitive function impairment affect the duration of hospitalization and in-hospital mortality in geriatric patients hospitalized for COVID-19? PLoS ONE 2023, 18, e0284977. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ministero della Salute. Circolare n. 21209 del 08 Aprile 2022; Ministero della Salute: Roma, Italy, 2022. Available online: https://arcs.sanita.fvg.it/media/uploads/2022/04/19/CIRCOLARE%20TRASMISSIONE%20NOTA%20SECOND%20%20BOOSTER.PDF (accessed on 17 July 2025).
- Istituto Superiore di Sanità. Bollettino di Sorveglianza Integrata COVID-19—23 Novembre 2022; Istituto Superiore di Sanità: Roma, Italy, 2022; Available online: https://www.epicentro.iss.it/coronavirus/bollettino/Bollettino-sorveglianza-integrata-COVID-19_23-novembre-2022.pdf (accessed on 17 July 2025).

| Overall | 0 | 1–2 | 3 | ≥4 | P for Difference | |
|---|---|---|---|---|---|---|
| N * | 5749 | 75 | 590 | 1458 | 623 | |
| Age (mean (SD)) | 66.52 (15.75) | 70.32 (14.07) | 58.83 (17.39) | 69.17 (15.55) | 73.70 (14.38) | <0.001 |
| Sex = Male (n, %) | 3289 (57.2) | 33 (44.0) | 319 (54.1) | 863 (59.2) | 358 (57.5) | 0.764 |
| CCI * (mean (SD)) | 1.84 (2.27) | 2.75 (3.02) | 1.62 (2.23) | 2.46 (2.57) | 2.40 (2.31) | 0.881 |
| Hypertension (n, %) | 3007 (52.3) | 45 (60.0) | 213 (36.1) | 836 (57.3) | 408 (65.5) | <0.001 |
| COVID-19 (n, %) | <0.001 | |||||
| Negative | 1458 (68.9) | 0 (0.0) | 162 (78.6) | 1458 (100.0) | 0 (0.0) | |
| Positive | 623 (29.5) | 68 (90.7) | 43 (20.9) | 0 (0.0) | 623 (100.0) | |
| Weak | 34 (1.6) | 7 (9.3) | 1 (0.5) | 0 (0.0) | 0 (0.0) | |
| Pneumonia (n,%) | 389 (6.8) | 17 (22.7) | 35 (5.9) | 202 (13.9) | 151 (24.2) | <0.001 |
| COVID-19 Pneumonia (n, %) | 157 (43.7) | 17 (100.0) | 10 (33.3) |
| Vaccine Doses | Frequency | Emergency Admission (%) | 95% CI Emergency Admission | Standard Admission (%) | 95% CI Standard Admission |
|---|---|---|---|---|---|
| 0 | 75 | 57.3 | [45.4, 68.5] | 42.7 | [31.5, 54.6] |
| 1 | 70 | 44.3 | [32.6, 56.6] | 55.7 | [43.4, 67.4] |
| 2 | 520 | 37.9 | [33.7, 42.2] | 62.1 | [57.8, 66.3] |
| 3 | 3885 | 40.8 | [39.3, 42.4] | 59.2 | [57.6, 60.7] |
| 4 | 1024 | 40.3 | [37.3, 43.4] | 59.7 | [56.6, 62.7] |
| 5 | 155 | 43.2 | [35.4, 51.4] | 56.8 | [48.6, 64.6] |
| 6 | 16 | 50 | [28.0, 72.0] | 50 | [28.0, 72.0] |
| 7+ | 4 | 50 | [15.0, 85.0] | 50 | [15.0, 85.0] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azzolini, E.; Lupo Pasinetti, B.; Voza, A.; Desai, A.; Bartoletti, M.; Aliberti, S.; Greco, M. COVID-19 Vaccination Still Makes Sense: Insights on Pneumonia Risk and Hospitalization from a Large-Scale Study at an Academic Tertiary Center in Italy. Microorganisms 2025, 13, 1744. https://doi.org/10.3390/microorganisms13081744
Azzolini E, Lupo Pasinetti B, Voza A, Desai A, Bartoletti M, Aliberti S, Greco M. COVID-19 Vaccination Still Makes Sense: Insights on Pneumonia Risk and Hospitalization from a Large-Scale Study at an Academic Tertiary Center in Italy. Microorganisms. 2025; 13(8):1744. https://doi.org/10.3390/microorganisms13081744
Chicago/Turabian StyleAzzolini, Elena, Brenda Lupo Pasinetti, Antonio Voza, Antonio Desai, Michele Bartoletti, Stefano Aliberti, and Massimiliano Greco. 2025. "COVID-19 Vaccination Still Makes Sense: Insights on Pneumonia Risk and Hospitalization from a Large-Scale Study at an Academic Tertiary Center in Italy" Microorganisms 13, no. 8: 1744. https://doi.org/10.3390/microorganisms13081744
APA StyleAzzolini, E., Lupo Pasinetti, B., Voza, A., Desai, A., Bartoletti, M., Aliberti, S., & Greco, M. (2025). COVID-19 Vaccination Still Makes Sense: Insights on Pneumonia Risk and Hospitalization from a Large-Scale Study at an Academic Tertiary Center in Italy. Microorganisms, 13(8), 1744. https://doi.org/10.3390/microorganisms13081744







