Metformin Enhances Doxycycline Efficacy Against Pasteurella multocida: Evidence from In Vitro, In Vivo, and Morphological Studies
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains, Reagents, Cell Lines, and Culture Conditions
2.2. Minimum Inhibitory Concentration (MIC) Testing
2.3. Checkboard Assays
2.4. Time-Dependent Killing Curve
2.5. Safety Assessment
2.6. Development of Antibiotic Resistance
2.7. Animal Ethics and Husbandry
2.8. In Vivo Evaluation of Combination Therapy
2.9. Histopathological Examination
2.10. One-Step In Vitro Growth Curve
2.11. Detection of Membrane Permeability and Integrity, Proton Motive Force (PMF), and Drug Uptake
2.12. Quantitative Real-Time PCR (qRT-PCR) Assay
2.13. Bacterial Morphological Analysis
2.14. Statistical Analyses
3. Results
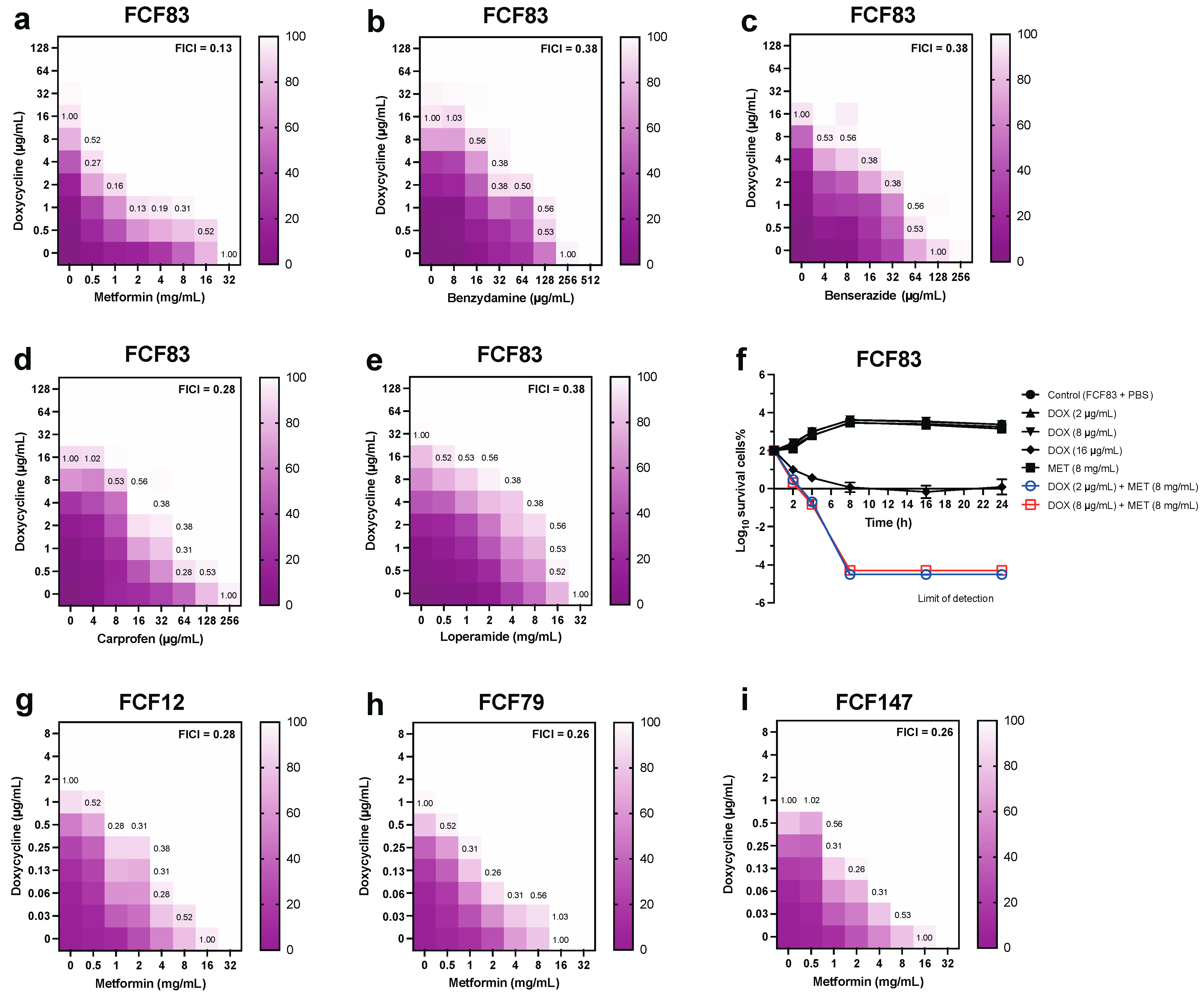
3.1. In Vitro Synergistic Antibacterial Activity of Drug Combinations Against Pm
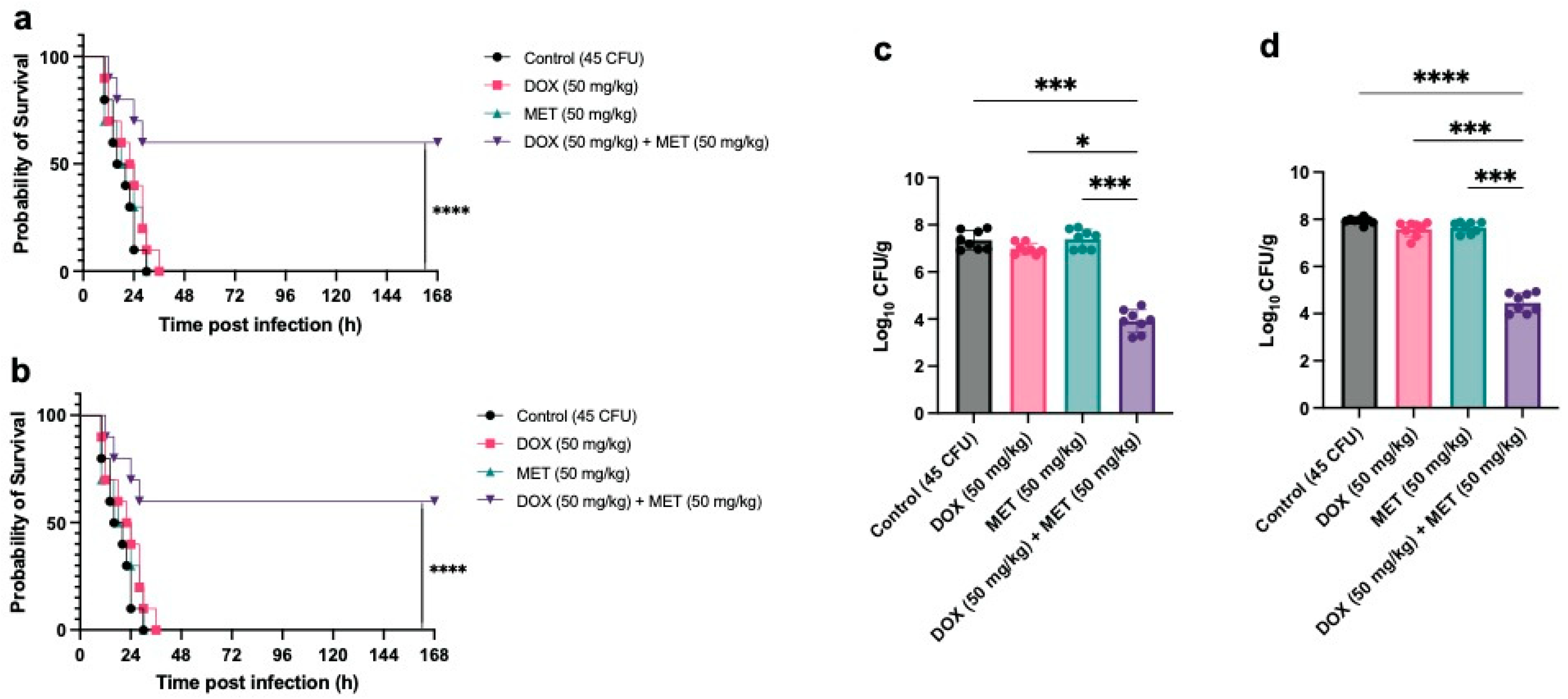
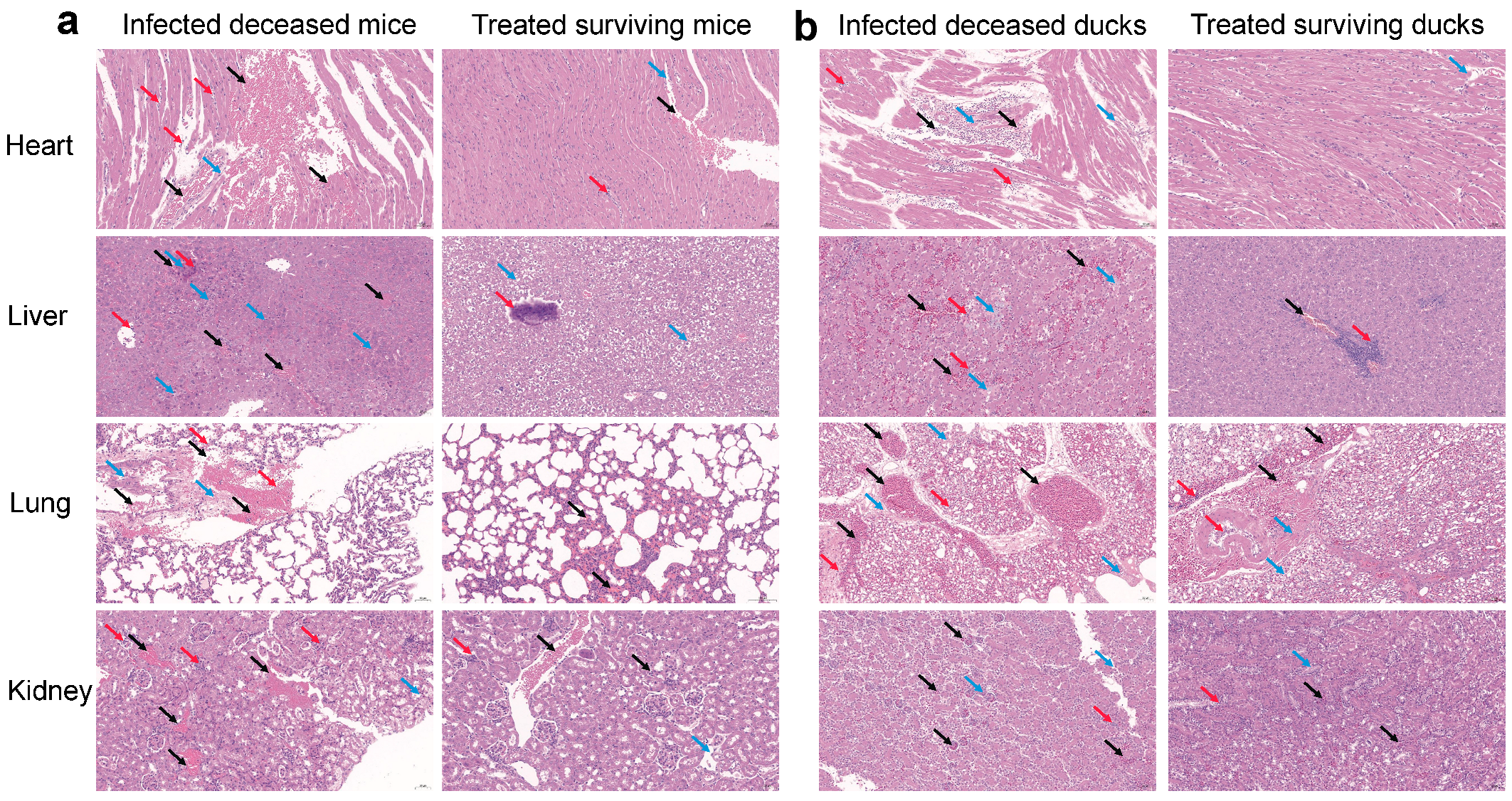
3.2. In Vivo Therapeutic Efficacy of Combined Metformin and Doxycycline Against Pm Infection
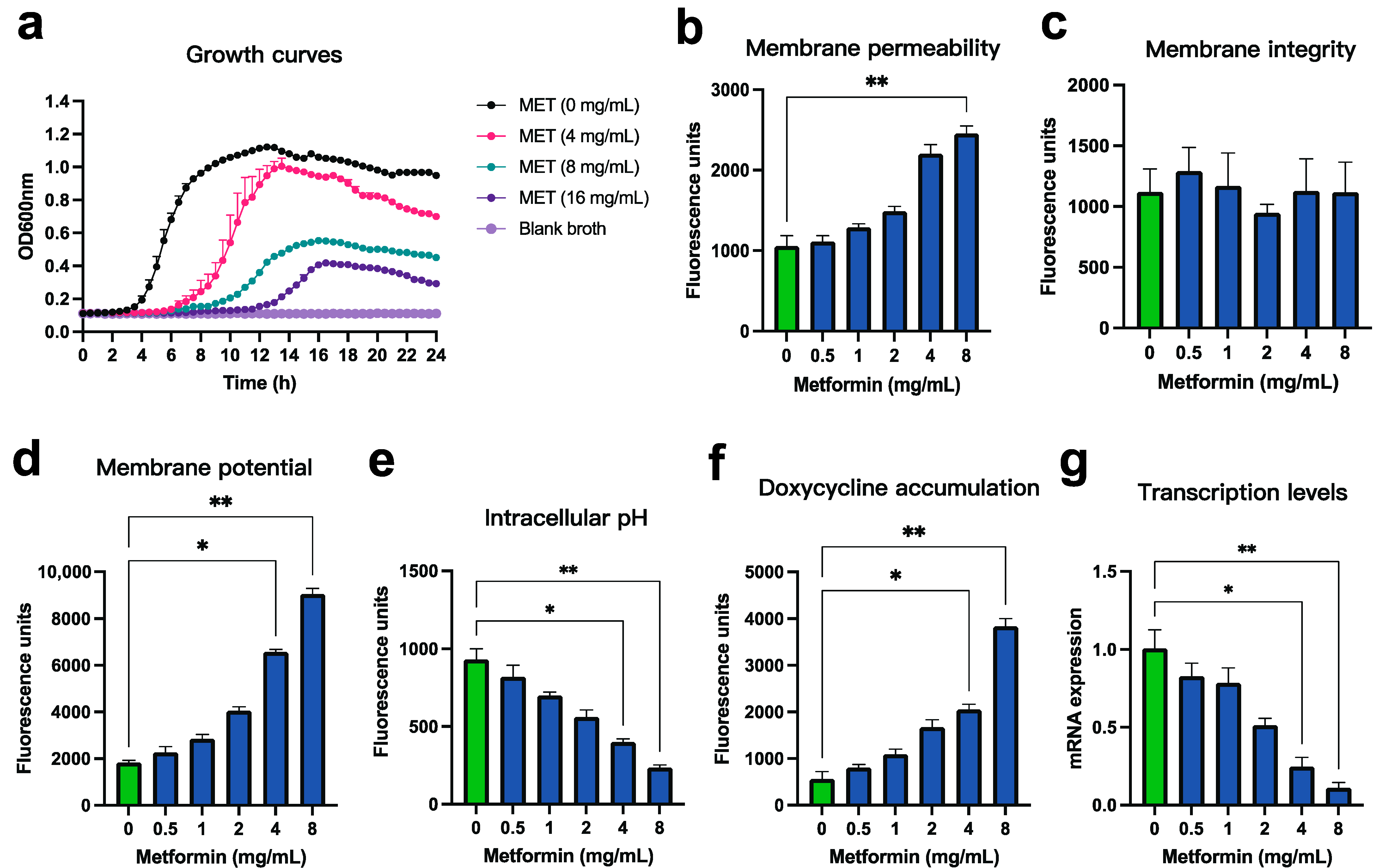
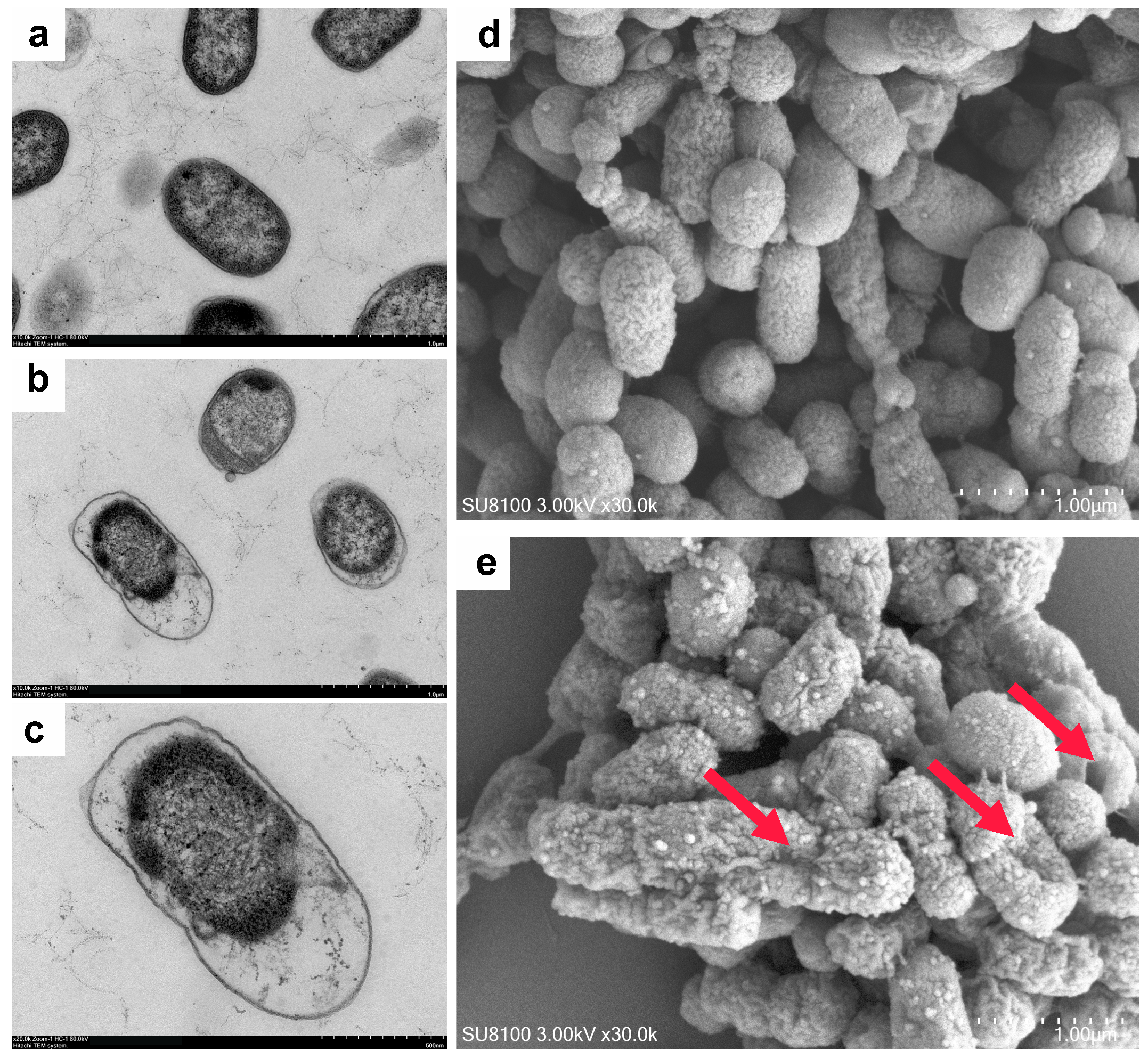
3.3. Mechanistic Verification of the Synergistic Effect Between Metformin and Doxycycline Against Pm
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Pm | Pasteurella multocida |
| PmA | Pasteurella multocida serotype A |
| LPS | Lipopolysaccharide |
| NPN | 1-N-phenylnaphthylamine |
| PI | propidium iodide |
| DiSC3(5) | 3,3-dipropylthiadicarbocyanine iodide |
| BCECF-AM | 2′,7′-Bis-(2-carboxyethyl)-5-(and-6)-carboxyfluorescein acetoxymethyl ester |
| MLST | Multi-locus sequence typing |
| FDA | U.S. Food and Drug Administration |
| PMF | Proton motive force |
| TSA | Tryptic soy agar |
| TSB | Tryptic soy broth |
| FBS | Fetal bovine serum |
| CHO | Chinese hamster ovary |
| DMEM | Dulbecco’s Modified Eagle’s Medium |
| MIC | Minimum inhibitory concentration |
| CA-MHB | Cation-adjusted Mueller–Hinton broth |
| OD | Optical density |
| FIC | Fractional inhibitory concentration |
| FICI | FIC index |
| PBS | Phosphate-buffered saline |
| CFU | Colony-forming unit |
| HEPES | 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid |
| TEM | Transmission electron microscopy |
| SEM | Scanning electron microscopy |
| cDNA | Complementary DNA |
| qRT-PCR | Quantitative real-time PCR |
| DOX | Doxycycline |
| MET | Metformin |
References
- Wilson, B.A.; Ho, M. Pasteurella multocida: From zoonosis to cellular microbiology. Clin. Microbiol. Rev. 2013, 26, 631–655. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; Wang, X.; Zhou, R.; Chen, H.; Wilson, B.A.; Wu, B. Pasteurella multocida: Genotypes and Genomics. Microbiol. Mol. Biol. Rev. 2019, 83, e00014-19. [Google Scholar] [CrossRef] [PubMed]
- Manohar, M.M.; Campbell, B.E.; Walduck, A.K.; Moore, R.J. Enhancement of live vaccines by co-delivery of immune modulating proteins. Vaccine 2022, 40, 5769–5780. [Google Scholar] [CrossRef] [PubMed]
- Guan, L.; Xue, Y.; Ding, W.; Zhao, Z. Biosynthesis and regulation mechanisms of the Pasteurella multocida capsule. Res. Vet. Sci. 2019, 127, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Tang, Y.; Dan, R.; Xie, M.; Zhang, T.; Li, P.; He, F.; Li, N.; Peng, Y. Pasteurella multocida activates apoptosis via the FAK-AKT-FOXO1 axis to cause pulmonary integrity loss, bacteremia, and eventually a cytokine storm. Vet. Res. 2024, 55, 46. [Google Scholar] [CrossRef] [PubMed]
- Jiang, N.; Chen, H.; Cheng, L.; Fu, Q.; Liu, R.; Liang, Q.; Fu, G.; Wan, C.; Huang, Y. Genomic analysis reveals the population structure and antimicrobial resistance of avian Pasteurella multocida in China. J. Antimicrob. Chemother. 2024, 79, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Zong, X.; Chen, G.; Zhang, Y.; Cao, Q.; Li, L.; Chen, H.; Peng, Z.; Tan, C. Isolation, Antimicrobial Susceptibility, and Genotypes of Three Pasteurellaeae Species Prevalent on Pig Farms in China Between 2021 and 2023. Microorganisms 2025, 13, 938. [Google Scholar] [CrossRef] [PubMed]
- Mader, R.; Eu, J.; Bourely, C.; Amat, J.P.; Broens, E.M.; Busani, L.; Callens, B.; Crespo-Robledo, P.; Damborg, P.; Filippitzi, M.E.; et al. Defining the scope of the European Antimicrobial Resistance Surveillance network in Veterinary medicine (EARS-Vet): A bottom-up and One Health approach. J. Antimicrob. Chemother. 2022, 77, 816–826. [Google Scholar] [CrossRef] [PubMed]
- Parmanik, A.; Das, S.; Kar, B.; Bose, A.; Dwivedi, G.R.; Pandey, M.M. Current Treatment Strategies Against Multidrug-Resistant Bacteria: A Review. Curr. Microbiol. 2022, 79, 388. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Fang, D.; Lv, Q.; Wang, Z.; Liu, Y. Targeted Therapeutic Strategies in the Battle Against Pathogenic Bacteria. Front. Pharmacol. 2021, 12, 673239. [Google Scholar] [CrossRef] [PubMed]
- Theuretzbacher, U.; Outterson, K.; Engel, A.; Karlen, A. The global preclinical antibacterial pipeline. Nat. Rev. Microbiol. 2020, 18, 275–285. [Google Scholar] [CrossRef] [PubMed]
- Thaker, M.; Spanogiannopoulos, P.; Wright, G.D. The tetracycline resistome. Cell. Mol. Life Sci. 2010, 67, 419–431. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Tong, Z.; Shi, J.; Wang, Z.; Liu, Y. Bacterial proton motive force as an unprecedented target to control antimicrobial resistance. Med. Res. Rev. 2023, 43, 1068–1090. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Shi, J.; Wang, D.; Kong, P.; Wang, Z.; Liu, Y. Antimicrobial peptides as promising antibiotic adjuvants to combat drug-resistant pathogens. Crit. Rev. Microbiol. 2024, 50, 267–284. [Google Scholar] [CrossRef] [PubMed]
- Magnowska, Z.; Jana, B.; Brochmann, R.P.; Hesketh, A.; Lametsch, R.; De Gobba, C.; Guardabassi, L. Carprofen-induced depletion of proton motive force reverses TetK-mediated doxycycline resistance in methicillin-resistant Staphylococcus pseudintermedius. Sci. Rep. 2019, 9, 17834. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Tong, Z.; Shi, J.; Jia, Y.; Deng, T.; Wang, Z. Reversion of antibiotic resistance in multidrug-resistant pathogens using non-antibiotic pharmaceutical benzydamine. Commun. Biol. 2021, 4, 1328. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Jia, Y.; Yang, K.; Li, R.; Xiao, X.; Zhu, K.; Wang, Z. Metformin Restores Tetracyclines Susceptibility against Multidrug Resistant Bacteria. Adv. Sci. 2020, 7, 1902227. [Google Scholar] [CrossRef] [PubMed]
- Ejim, L.; Farha, M.A.; Falconer, S.B.; Wildenhain, J.; Coombes, B.K.; Tyers, M.; Brown, E.D.; Wright, G.D. Combinations of antibiotics and nonantibiotic drugs enhance antimicrobial efficacy. Nat. Chem. Biol. 2011, 7, 348–350. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Huan, Q.; Huang, Y.; Liu, Y.; Li, R.; Xu, X.; Wang, Z. Metformin Reverses tmexCD1-toprJ1- and tet(A)-Mediated High-Level Tigecycline Resistance in K. pneumoniae. Antibiotics 2022, 11, 162. [Google Scholar] [CrossRef] [PubMed]
- Hossain, S.; Rafi, R.H.; Ripa, F.A.; Khan, M.R.I.; Hosen, M.E.; Molla, M.K.I.; Faruqe, M.O.; Al-Bari, M.A.A.; Das, S. Modulating the antibacterial effect of the existing antibiotics along with repurposing drug metformin. Arch. Microbiol. 2024, 206, 190. [Google Scholar] [CrossRef] [PubMed]
- Bognar, B.; Spohn, R.; Lazar, V. Drug combinations targeting antibiotic resistance. NPJ Antimicrob. Resist. 2024, 2, 29. [Google Scholar] [CrossRef] [PubMed]
- Jiang, N.; Chen, H.; Wang, W.; Liang, Q.; Fu, Q.; Liu, R.; Fu, G.; Wan, C.; Huang, Y.; Cheng, L. Characterization of a Capsule-Deficient Pasteurella multocida Isolated from Cygnus melancoryphus: Genomic, Phenotypic, and Virulence Insights. Microorganisms 2025, 13, 1024. [Google Scholar] [CrossRef] [PubMed]
- Institute, C.L.S. Performance Standards for Antimicrobial Susceptibility Testing; Clinical and Laboratory Standards Institute (CLSI): Wayne, PA, USA, 2024. [Google Scholar]
- MacNair, C.R.; Stokes, J.M.; Carfrae, L.A.; Fiebig-Comyn, A.A.; Coombes, B.K.; Mulvey, M.R.; Brown, E.D. Overcoming mcr-1 mediated colistin resistance with colistin in combination with other antibiotics. Nat. Commun. 2018, 9, 458. [Google Scholar] [CrossRef] [PubMed]
- Maisuria, V.B.; Okshevsky, M.; Deziel, E.; Tufenkji, N. Proanthocyanidin Interferes with Intrinsic Antibiotic Resistance Mechanisms of Gram-Negative Bacteria. Adv. Sci. 2019, 6, 1802333. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Liu, Y.; Li, P.; Wu, X.; Xia, Y.; Zhang, D.; Li, N.; Peng, Y.; Zhu, G.; Hardeland, R.; et al. Melatonin inhibits Gram-negative pathogens by targeting citrate synthase. Sci. China Life Sci. 2022, 65, 1430–1444. [Google Scholar] [CrossRef] [PubMed]
- Masadeh, M.M.; Alzoubi, K.H.; Masadeh, M.M.; Aburashed, Z.O. Metformin as a Potential Adjuvant Antimicrobial Agent Against Multidrug Resistant Bacteria. Clin. Pharmacol. 2021, 13, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.; Sun, X.; Yang, J.; Yang, L.; Li, M.; Wang, Y.; Jiao, H.; Li, G. Metformin reverse minocycline to inhibit minocycline-resistant Acinetobacter baumannii by destroy the outer membrane and enhance membrane potential in vitro. BMC Microbiol. 2022, 22, 215. [Google Scholar] [CrossRef] [PubMed]
- Arredondo, A.; Alvarez, G.; Nart, J.; Mor, C.; Blanc, V.; Leon, R. Detection and expression analysis of tet(B) in Streptococcus oralis. J. Oral Microbiol. 2019, 11, 1643204. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Li, P.; Xue, Z.; Qi, Y.; Li, X.; Zhu, D.; Ma, H.; Kong, L. RecA inhibitor epicatechin prolongs the development of fluoroquinolone resistance in Pasteurella multocida. Int. J. Biol. Macromol. 2024, 255, 128026. [Google Scholar] [CrossRef] [PubMed]
- Ezraty, B.; Vergnes, A.; Banzhaf, M.; Duverger, Y.; Huguenot, A.; Brochado, A.R.; Su, S.Y.; Espinosa, L.; Loiseau, L.; Py, B.; et al. Fe-S cluster biosynthesis controls uptake of aminoglycosides in a ROS-less death pathway. Science 2013, 340, 1583–1587. [Google Scholar] [CrossRef] [PubMed]
- Allison, K.R.; Brynildsen, M.P.; Collins, J.J. Metabolite-enabled eradication of bacterial persisters by aminoglycosides. Nature 2011, 473, 216–220. [Google Scholar] [CrossRef] [PubMed]
- Knowler, W.C.; Barrett-Connor, E.; Fowler, S.E.; Hamman, R.F.; Lachin, J.M.; Walker, E.A.; Nathan, D.M.; Diabetes Prevention Program Research, G. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N. Engl. J. Med. 2002, 346, 393–403. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Shi, J.; Tong, Z.; Jia, Y.; Yang, K.; Wang, Z. Potent Broad-Spectrum Antibacterial Activity of Amphiphilic Peptides against Multidrug-Resistant Bacteria. Microorganisms 2020, 8, 1398. [Google Scholar] [CrossRef] [PubMed]
- Vallianou, N.G.; Stratigou, T.; Tsagarakis, S. Metformin and gut microbiota: Their interactions and their impact on diabetes. Hormones 2019, 18, 141–144. [Google Scholar] [CrossRef] [PubMed]
- Hegazy, W.A.H.; Khayat, M.T.; Ibrahim, T.S.; Nassar, M.S.; Bakhrebah, M.A.; Abdulaal, W.H.; Alhakamy, N.A.; Bendary, M.M. Repurposing Anti-diabetic Drugs to Cripple Quorum Sensing in Pseudomonas aeruginosa. Microorganisms 2020, 8, 1285. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Wei, Y.; Li, H.; Shi, D.; Yang, D.; Yin, J.; Zhou, S.; Chen, T.; Li, J.; Jin, M. Emerging pollutant metformin in water promotes the development of multiple-antibiotic resistance in Escherichia coli via chromosome mutagenesis. J. Hazard. Mater. 2022, 430, 128474. [Google Scholar] [CrossRef] [PubMed]
- Singhal, A.; Jie, L.; Kumar, P.; Hong, G.S.; Leow, M.K.; Paleja, B.; Tsenova, L.; Kurepina, N.; Chen, J.; Zolezzi, F.; et al. Metformin as adjunct antituberculosis therapy. Sci. Transl. Med. 2014, 6, 263ra159. [Google Scholar] [CrossRef] [PubMed]
- Mediaas, S.D.; Haug, M.; Louet, C.; Wahl, S.G.F.; Gidon, A.; Flo, T.H. Metformin improves Mycobacterium avium infection by strengthening macrophage antimicrobial functions. Front. Immunol. 2024, 15, 1463224. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.; Kim, A.H.; Lee, Y.; Ji, S.C.; Cho, J.Y.; Yu, K.S.; Chung, J.Y. Effects of vancomycin-induced gut microbiome alteration on the pharmacodynamics of metformin in healthy male subjects. Clin. Transl. Sci. 2021, 14, 1955–1966. [Google Scholar] [CrossRef] [PubMed]
- Mor, A.; Petersen, I.; Sorensen, H.T.; Thomsen, R.W. Metformin and other glucose-lowering drug initiation and rates of community-based antibiotic use and hospital-treated infections in patients with type 2 diabetes: A Danish nationwide population-based cohort study. BMJ Open 2016, 6, e011523. [Google Scholar] [CrossRef] [PubMed]
- Shih, C.J.; Wu, Y.L.; Chao, P.W.; Kuo, S.C.; Yang, C.Y.; Li, S.Y.; Ou, S.M.; Chen, Y.T. Association between Use of Oral Anti-Diabetic Drugs and the Risk of Sepsis: A Nested Case-Control Study. Sci. Rep. 2015, 5, 15260. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, N.; Wang, W.; Liang, Q.; Fu, Q.; Liu, R.; Fu, G.; Wan, C.; Cheng, L.; Huang, Y.; Chen, H. Metformin Enhances Doxycycline Efficacy Against Pasteurella multocida: Evidence from In Vitro, In Vivo, and Morphological Studies. Microorganisms 2025, 13, 1724. https://doi.org/10.3390/microorganisms13081724
Jiang N, Wang W, Liang Q, Fu Q, Liu R, Fu G, Wan C, Cheng L, Huang Y, Chen H. Metformin Enhances Doxycycline Efficacy Against Pasteurella multocida: Evidence from In Vitro, In Vivo, and Morphological Studies. Microorganisms. 2025; 13(8):1724. https://doi.org/10.3390/microorganisms13081724
Chicago/Turabian StyleJiang, Nansong, Weiwei Wang, Qizhang Liang, Qiuling Fu, Rongchang Liu, Guanghua Fu, Chunhe Wan, Longfei Cheng, Yu Huang, and Hongmei Chen. 2025. "Metformin Enhances Doxycycline Efficacy Against Pasteurella multocida: Evidence from In Vitro, In Vivo, and Morphological Studies" Microorganisms 13, no. 8: 1724. https://doi.org/10.3390/microorganisms13081724
APA StyleJiang, N., Wang, W., Liang, Q., Fu, Q., Liu, R., Fu, G., Wan, C., Cheng, L., Huang, Y., & Chen, H. (2025). Metformin Enhances Doxycycline Efficacy Against Pasteurella multocida: Evidence from In Vitro, In Vivo, and Morphological Studies. Microorganisms, 13(8), 1724. https://doi.org/10.3390/microorganisms13081724







