Impact of Sugarcane–Pumpkin Intercropping on Soil Microbial Diversity
Abstract
1. Introduction
2. Materials and Methods
2.1. Field Plots and Experimental Design
2.2. Soil Sampling
2.3. Measurement of Soil Properties
2.4. Soil DNA Extraction, Amplification, and Sequencing
2.5. Sequencing Data Analysis
2.6. Statistical Analysis
3. Results
3.1. Soil Physicochemical Properties
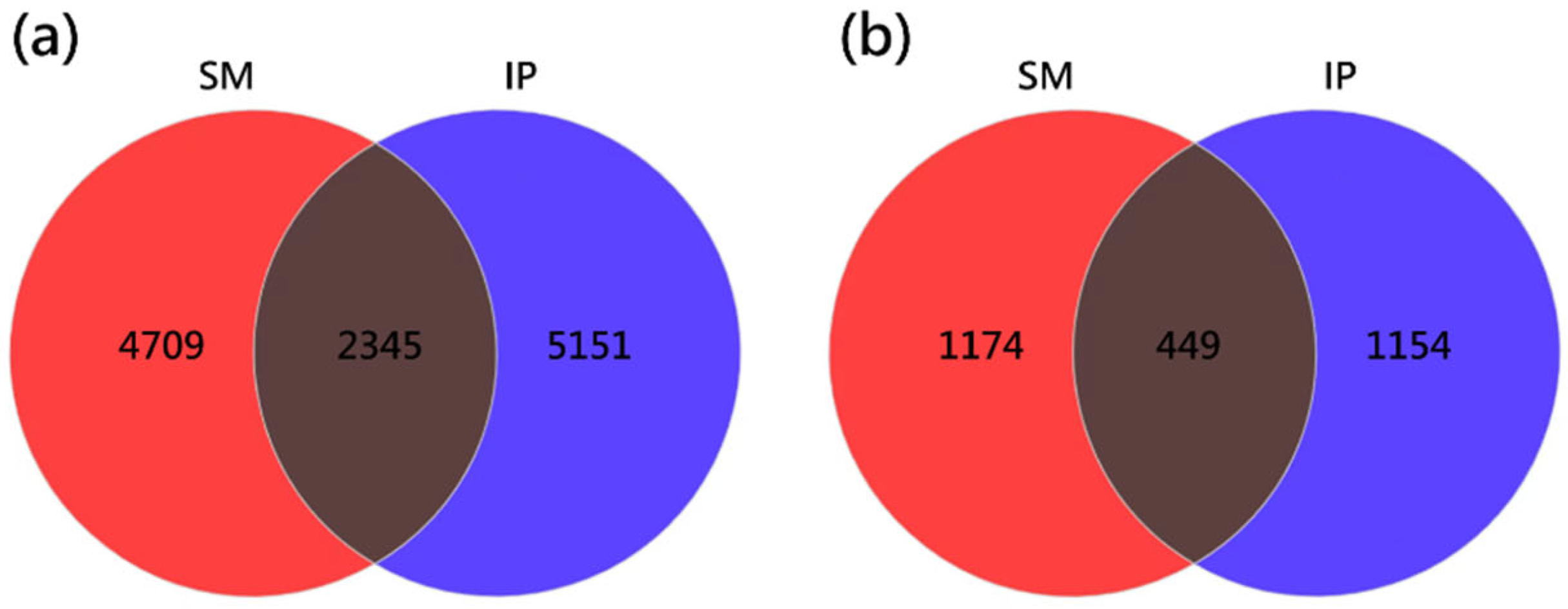
3.2. Amplicon Sequencing Data and Shared OTUs
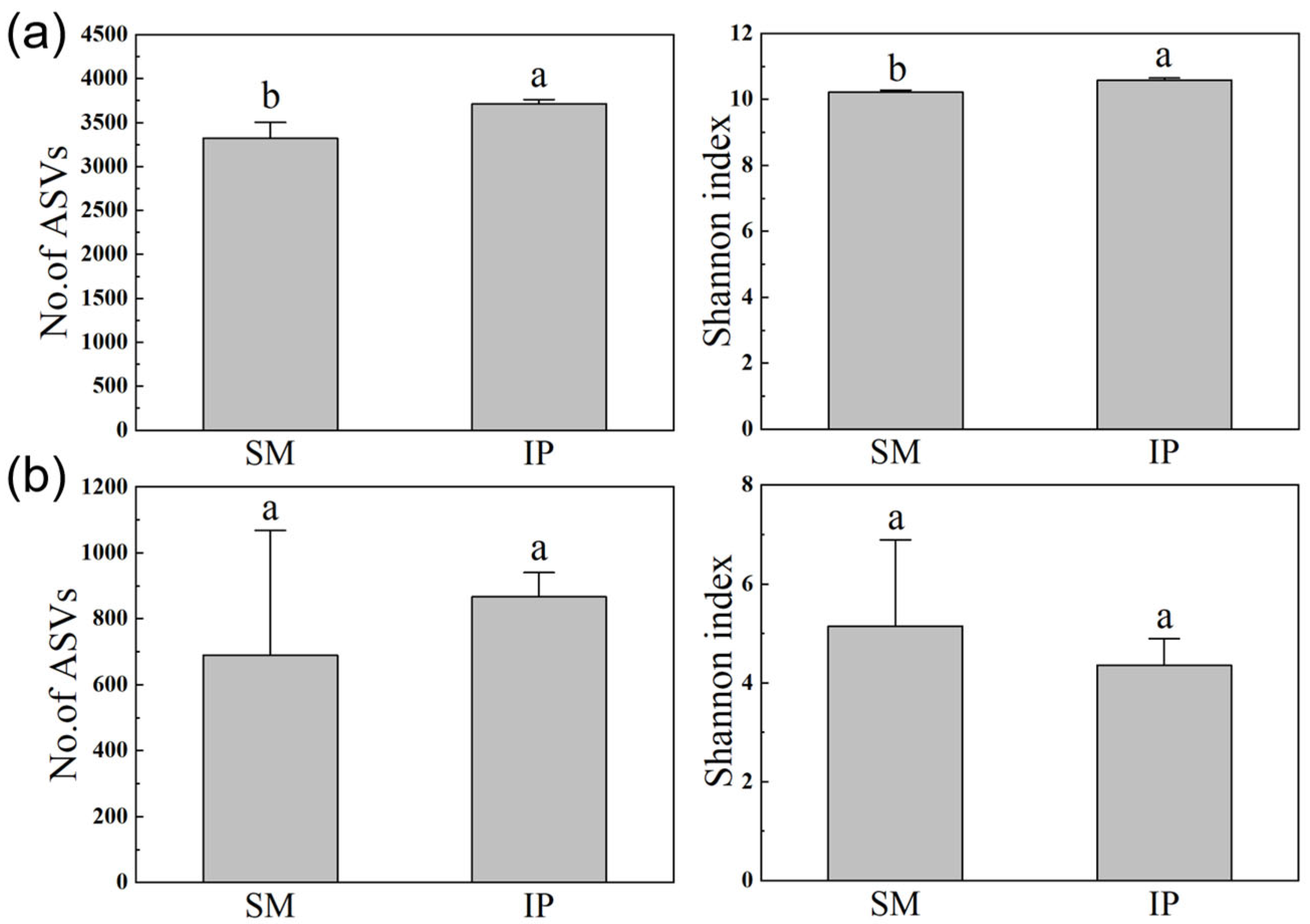
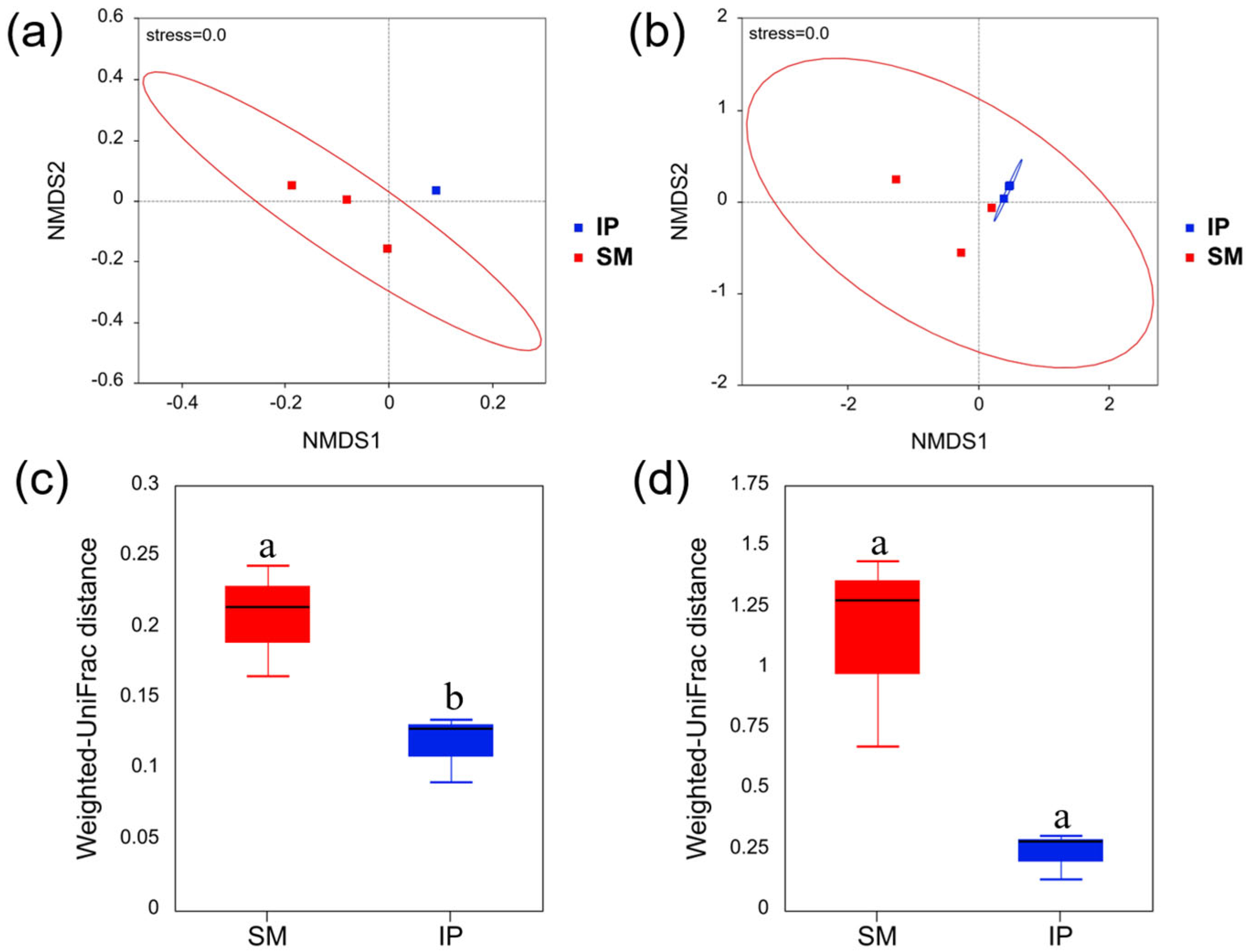
3.3. Soil Microbial Community Diversities and Structures
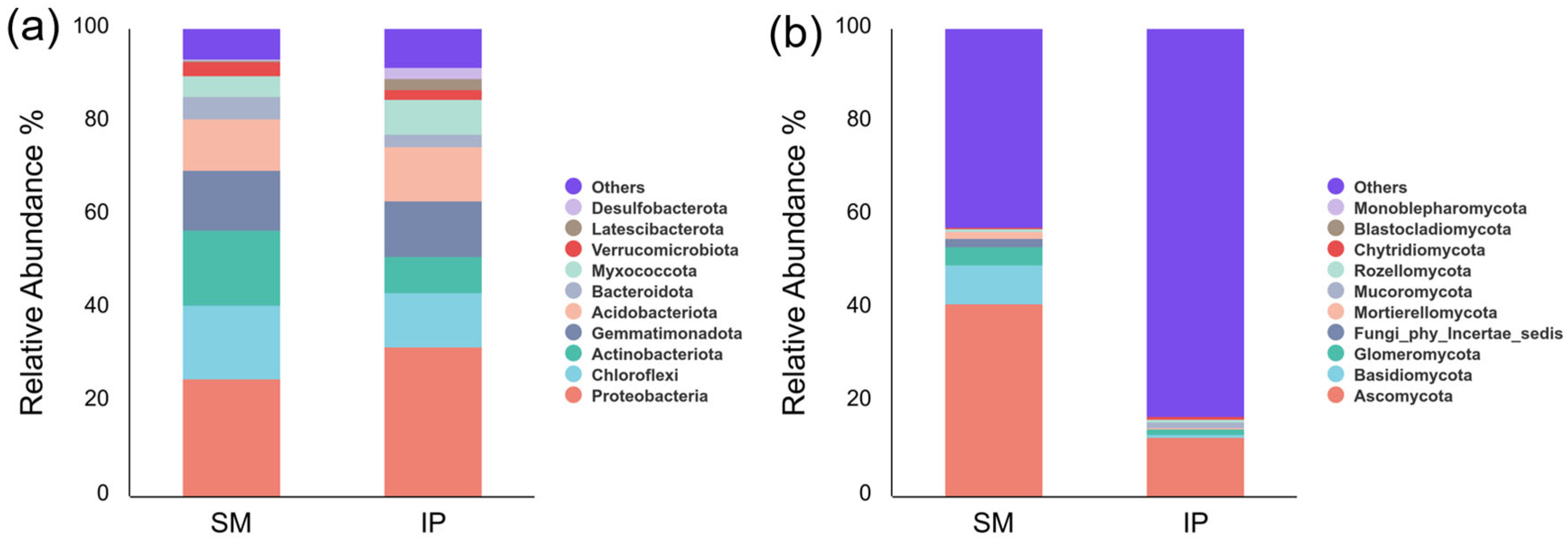
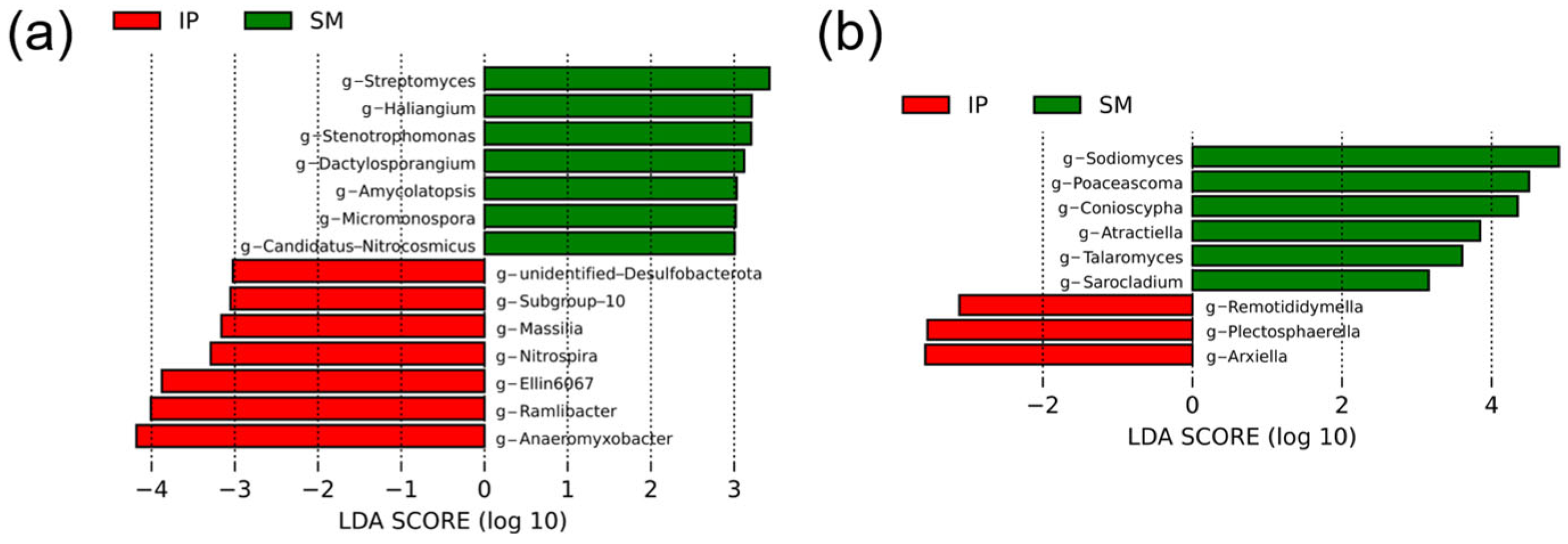
3.4. Soil Bacterial Community Composition
3.5. Soil Fungal Community Composition
3.6. Relationships Between Soil Microbial Communities and Soil Physiochemical Properties
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Reinprecht, Y.; Schram, L.; Smith, T.H.; Pauls, K.P. Enhancing In-Crop Diversity in Common Bean by Planting Cultivar Mixtures and Its Effect on Productivity. Front. Sustain. Food Syst. 2020, 4, 126. [Google Scholar] [CrossRef]
- Tang, X.; Zhang, Y.; Jiang, J.; Meng, X.; Huang, Z.; Wu, H.; He, L.; Xiong, F.; Liu, J.; Zhong, R.; et al. Sugarcane/Peanut Intercropping System Improves Physicochemical Properties by Changing N and P Cycling and Organic Matter Turnover in Root Zone Soil. PeerJ 2021, 9, e10880. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Gao, D.; Zhou, X.; Chen, S.; Li, C.; Wu, F. Intercropping with Potato-Onion Enhanced the Soil Microbial Diversity of Tomato. Microorganisms 2020, 8, 834. [Google Scholar] [CrossRef] [PubMed]
- Bybee-Finley, K.A.; Ryan, M.R. Advancing Intercropping Research and Practices in Industrialized Agricultural Landscapes. Agriculture 2018, 8, 80. [Google Scholar] [CrossRef]
- Apriasti, R.; Widyaningrum, S.; Hidayati, W.N.; Sawitri, W.D.; Darsono, N.; Hase, T.; Sugiharto, B. Full Sequence of the Coat Protein Gene Is Required for the Induction of Pathogen-Derived Resistance against Sugarcane Mosaic Virus in Transgenic Sugarcane. Mol. Biol. Rep. 2018, 45, 2749–2758. [Google Scholar] [CrossRef] [PubMed]
- Waclawovsky, A.J.; Sato, P.M.; Lembke, C.G.; Moore, P.H.; Souza, G.M. Sugarcane for Bioenergy Production: An Assessment of Yield and Regulation of Sucrose Content. Plant Biotechnol. J. 2010, 8, 263–276. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.K.; Singh, P.; Li, H.-B.; Song, Q.-Q.; Guo, D.-J.; Solanki, M.K.; Verma, K.K.; Malviya, M.K.; Song, X.-P.; Lakshmanan, P.; et al. Diversity of Nitrogen-Fixing Rhizobacteria Associated with Sugarcane: A Comprehensive Study of Plant-Microbe Interactions for Growth Enhancement in Saccharum Spp. BMC Plant Biol. 2020, 20, 220. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.-F.; Zhao, Z.-H.; Chen, Y. Effects of Intercropping with Peanut and Silicon Application on Sugarcane Growth, Yield and Quality. Sugar Tech 2018, 21, 437–443. [Google Scholar] [CrossRef]
- Pevicharova, G.; Velkov, N. Sensory, Chemical and Morphological Characterization of Cucurbita Maxima and Cucurbita Moschata Genotypes from Different Geographical Origins. Genetika 2017, 49, 193–202. [Google Scholar] [CrossRef]
- Sun, Y.; Huang, Z.; Chen, S.; Yang, D.; Lin, X.; Liu, W.; Yang, S. Higher-Quality Pumpkin Cultivars Need to Recruit More Abundant Soil Microbes in Rhizospheres. Microorganisms 2022, 10, 2219. [Google Scholar] [CrossRef] [PubMed]
- Ells, J.E.; McSay, A.E.; Kruse, E.G.; Larson, G. Root Distribution and Proliferation of Field-Grown Acorn Squash as Influenced by Plastic Mulch and Water. HortTechnology 1994, 4, 248–252. [Google Scholar] [CrossRef]
- Smith, D.M.; Inman-Bamber, N.G.; Thorburn, P.J. Growth and Function of the Sugarcane Root System. Field Crops Res. 2005, 92, 169–183. [Google Scholar] [CrossRef]
- Pang, Z.; Dong, F.; Liu, Q.; Lin, W.; Hu, C.; Yuan, Z. Soil Metagenomics Reveals Effects of Continuous Sugarcane Cropping on the Structure and Functional Pathway of Rhizospheric Microbial Community. Front. Microbiol. 2021, 12, 627569. [Google Scholar] [CrossRef] [PubMed]
- Malviya, M.K.; Solanki, M.K.; Li, C.-N.; Wang, Z.; Zeng, Y.; Verma, K.K.; Singh, R.K.; Singh, P.; Huang, H.-R.; Yang, L.-T.; et al. Sugarcane-Legume Intercropping Can Enrich the Soil Microbiome and Plant Growth. Front. Sustain. Food Syst. 2021, 5, 606595. [Google Scholar] [CrossRef]
- Bao, S. Soil Analysis in Agricultural Chemistry, 3rd ed.; China Agricultural Press: Beijing, China, 2005. (In Chinese) [Google Scholar]
- Berg, J.; Brandt, K.K.; Al-Soud, W.A.; Holm, P.E.; Hansen, L.H.; Sørensen, S.J.; Nybroe, O. Selection for Cu-Tolerant Bacterial Communities with Altered Composition, but Unaltered Richness, via Long-Term Cu Exposure. Appl. Environ. Microbiol. 2012, 78, 7438–7446. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Liu, D.; Ling, N.; Chen, F.; Fang, W.; Shen, Q. Bio-Organic Fertilizer Application Significantly Reduces the Fusarium Oxysporum Population and Alters the Composition of Fungi Communities of Watermelon Fusarium Wilt Rhizosphere Soil. Biol. Fertil. Soils 2014, 50, 765–774. [Google Scholar] [CrossRef]
- Magoc, T.; Salzberg, S.L. FLASH: Fast Length Adjustment of Short Reads to Improve Genome Assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. Fastp: An Ultra-Fast All-In-One FASTQ Preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C.; Haas, B.J.; Clemente, J.C.; Quince, C.; Knight, R. UCHIME Improves Sensitivity and Speed of Chimera Detection. Bioinformatics 2011, 27, 2194–2200. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Shao, D.; Zhou, J.; Gu, J.; Qin, J.; Chen, W.; Wei, W. Signatures within esophageal microbiota with progression of esophageal squamous cell carcinoma. Chin. J. Cancer Res. = Chung-Kuo Yen Cheng Yen Chiu 2020, 32, 755–767. [Google Scholar] [CrossRef] [PubMed]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA Ribosomal RNA Gene Database Project: Improved Data Processing and Web-Based Tools. Nucleic Acids Res. 2012, 41, D590–D596. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, R.H. Molecular Identification of Fungi: Rationale, Philosophical Concerns, and the UNITE Database. Open Appl. Inform. J. 2011, 5, 81–86. [Google Scholar] [CrossRef]
- Daims, H.; Lebedeva, E.V.; Pjevac, P.; Han, P.; Herbold, C.; Albertsen, M.; Jehmlich, N.; Palatinszky, M.; Vierheilig, J.; Bulaev, A.; et al. Complete Nitrification by Nitrospira Bacteria. Nature 2015, 528, 504–509. [Google Scholar] [CrossRef] [PubMed]
- Sekaran, U.; Loya, J.R.; Abagandura, G.O.; Subramanian, S.; Owens, V.; Kumar, S. Intercropping of Kura Clover (Trifolium Ambiguum M. Bieb) with Prairie Cordgrass (Spartina Pectinata Link.) Enhanced Soil Biochemical Activities and Microbial Community Structure. Appl. Soil Ecol. 2019, 147, 103427. [Google Scholar] [CrossRef]
- Shao, M.; Wang, C.; Zhou, L.; Peng, F.; Zhang, G.; Gao, J.; Chen, S.; Zhao, Q. Rhizosphere Soil Properties of Waxy Sorghum under Different Row Ratio Configurations in Waxy Sorghum-Soybean Intercropping Systems. PLoS ONE 2023, 18, e0288076. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Zhang, A.; Qin, X.; Yu, H.; Ji, X.; He, S.; Zong, Y.; Wang, J.; Tang, J. Effects of Intercropping Pandanus Amaryllifolius on Soil Properties and Microbial Community Composition in Areca Catechu Plantations. Forests 2022, 13, 1814. [Google Scholar] [CrossRef]
- Walia, S.S.; Dhaliwal, S.S.; Gill, R.S.; Kaur, T.; Kaur, K.; Randhawa, M.K.; Obročník, O.; Bárek, V.; Brestic, M.; Gaber, A.; et al. Improvement of Soil Health and Nutrient Transformations under Balanced Fertilization with Integrated Nutrient Management in a Rice-Wheat System in Indo-Gangetic Plains—A 34-Year Research Outcomes. Heliyon 2024, 10, e25113. [Google Scholar] [CrossRef] [PubMed]
- Lian, T.; Mu, Y.; Jin, J.; Ma, Q.; Cheng, Y.; Cai, Z.; Nian, H. Impact of Intercropping on the Coupling between Soil Microbial Community Structure, Activity, and Nutrient-Use Efficiencies. PeerJ 2019, 7, e6412. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Xiong, K.; Chi, Y.; Song, S. Effects of Crop and Grass Intercropping on the Soil Environment in the Karst Area. Sustainability 2021, 13, 5484. [Google Scholar] [CrossRef]
- Ntamwira, J.; Ocimati, W.; Blomme, G.; Lubobo, A.K.; Mwarabu Lolonga Pyame, D.; Dhed’a Djailo, B. Innovative Agroecological Practices Can Restore Degraded Farmlands and Revive Crop Yields. Front. Sustain. Food Syst. 2023, 7, 1017341. [Google Scholar] [CrossRef]
- He, C.; Zhou, B.; Wang, H.; Wei, Y.; Huang, J. A first-year maize/cassava relay intercropping system improves soil nutrients and changes the soil microbial community in the symbiotic period. Front. Microbiol. 2023, 14, 1087202. [Google Scholar] [CrossRef] [PubMed]
- Yitbarek, T. Carbon Stock of Luvisols as Influenced by Cropping System of Abela Lida, Southern Ethiopia. Agric. Res. Technol. Open Access J. 2019, 21, 556164. [Google Scholar] [CrossRef]
- Aye, N.S.; Butterly, C.R.; Sale, P.W.; Tang, C. Interactive Effects of Initial PH and Nitrogen Status on Soil Organic Carbon Priming by Glucose and Lignocellulose. Soil Biol. Biochem. 2018, 123, 33–44. [Google Scholar] [CrossRef]
- Chen, R.; Senbayram, M.; Blagodatsky, S.; Myachina, O.; Dittert, K.; Lin, X.; Blagodatskaya, E.; Kuzyakov, Y. Soil c and N Availability Determine the Priming Effect: Microbial N Mining and Stoichiometric Decomposition Theories. Glob. Change Biol. 2014, 20, 2356–2367. [Google Scholar] [CrossRef] [PubMed]
- Rayne, N.; Aula, L. Livestock Manure and the Impacts on Soil Health: A Review. Soil Syst. 2020, 4, 64. [Google Scholar] [CrossRef]
- Meng, C.; Tian, D.; Zeng, H.; Li, Z.; Yi, C.; Niu, S. Global Soil Acidification Impacts on Belowground Processes. Environ. Res. Lett. 2019, 14, 074003. [Google Scholar] [CrossRef]
- Wang, J.; Xie, J.; Li, L.; Luo, Z.; Zhang, R.; Wang, L.; Jiang, Y. The Impact of Fertilizer Amendments on Soil Autotrophic Bacteria and Carbon Emissions in Maize Field on the Semiarid Loess Plateau. Front. Microbiol. 2021, 12, 664120. [Google Scholar] [CrossRef] [PubMed]
- Barros-Rodríguez, A.; Rangseekaew, P.; Lasudee, K.; Pathom-Aree, W.; Manzanera, M. Impacts of Agriculture on the Environment and Soil Microbial Biodiversity. Plants 2021, 10, 2325. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Xie, H. Daylily Intercropping: Effects on Soil Nutrients, Enzyme Activities, and Microbial Community Structure. Front. Plant Sci. 2023, 14, 1107690. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Wang, Y.; Sun, L.; Qi, X.; Song, F.; Zhu, X. Impact of Maize–Mushroom Intercropping on the Soil Bacterial Community Composition in Northeast China. Agronomy 2020, 10, 1526. [Google Scholar] [CrossRef]
- Rousk, J.; Bååth, E. Growth of Saprotrophic Fungi and Bacteria in Soil. FEMS Microbiol. Ecol. 2011, 78, 17–30. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.M.; Zheng, Y.; Tang, L.; Long, G.Q. Crop Rhizospheric Microbial Community Structure and Functional Diversity as Affected by Maize and Potato Intercropping. J. Plant Nutr. 2017, 40, 2402–2412. [Google Scholar] [CrossRef]
- Vlassi, A.; Nesler, A.; Parich, A.; Puopolo, G.; Schuhmacher, R. Volatile-Mediated Inhibitory Activity of Rhizobacteria as a Result of Multiple Factors Interaction: The Case of Lysobacter Capsici AZ78. Microorganisms 2020, 8, 1761. [Google Scholar] [CrossRef] [PubMed]
- De Filippis, F.; Laiola, M.; Blaiotta, G.; Ercolini, D. Different Amplicon Targets for Sequencing-Based Studies of Fungal Diversity. Appl. Environ. Microbiol. 2017, 83, e00905-17. [Google Scholar] [CrossRef] [PubMed]
- Gardes, M.; Bruns, T.D. ITS Primers with Enhanced Specificity for Basidiomycetes—Application to the Identification of Mycorrhizae and Rusts. Mol. Ecol. 1993, 2, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yang, Z.; Zhang, L.; Wan, H.; Deng, F.; Zhao, Z.; Wang, J. Characteristics of Bacterial Community Structure and Function in Artificial Soil Prepared Using Red Mud and Phosphogypsum. Microorganisms 2024, 12, 1886. [Google Scholar] [CrossRef] [PubMed]
- Landesman, W.J.; Freedman, Z.B.; Nelson, D.M. Seasonal, Sub-Seasonal and Diurnal Variation of Soil Bacterial Community Composition in a Temperate Deciduous Forest. FEMS Microbiol. Ecol. 2019, 95, fiz002. [Google Scholar] [CrossRef] [PubMed]
- Kaspar, F.; Neubauer, P.; Gimpel, M. Bioactive Secondary Metabolites from Bacillus subtilis: A Comprehensive Review. J. Nat. Prod. 2019, 82, 2038–2053. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Yang, D.; Zhu, Y.; Qin, Y.; Liang, T.; Yang, S.; Tan, H. Changes in Root Metabolites and Soil Microbial Community Structures in Rhizospheres of Sugarcanes under Different Propagation Methods. Microb. Biotechnol. 2023, 17, e14372. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Lin, H.; Han, M.; Yang, L. Soil Metagenomics Reveals the Effect of Nitrogen on Soil Microbial Communities and Nitrogen-Cycle Functional Genes in the Rhizosphere of Panax Ginseng. Front. Plant Sci. 2024, 15, 1411073. [Google Scholar] [CrossRef] [PubMed]
- Fontaine, S.; Hénault, C.; Aamor, A.; Bdioui, N.; Bloor, J.M.; Maire, V.; Mary, B.; Revaillot, S.; Maron, P.A. Fungi Mediate Long Term Sequestration of Carbon and Nitrogen in Soil through Their Priming Effect. Soil Biol. Biochem. 2011, 43, 86–96. [Google Scholar] [CrossRef]
- Pranamuda, H.; Tokiwa, Y.; Tanaka, H. Polylactide Degradation by an Amycolatopsis Sp. Appl. Environ. Microbiol. 1997, 63, 1637–1640. [Google Scholar] [CrossRef] [PubMed]
- Trujillo, M.E.; Riesco, R.; Benito, P.; Carro, L. Endophytic Actinobacteria and the Interaction of Micromonospora and Nitrogen Fixing Plants. Front. Microbiol. 2015, 6, 1341. [Google Scholar] [CrossRef] [PubMed]
- Özbolat, O.; Sánchez-Navarro, V.; Zornoza, R.; Egea-Cortines, M.; Cuartero, J.; Ros, M.; Pascual, J.A.; Boix-Fayos, C.; Almagro, M.; de Vente, J.; et al. Long-Term Adoption of Reduced Tillage and Green Manure Improves Soil Physicochemical Properties and Increases the Abundance of Beneficial Bacteria in a Mediterranean Rainfed Almond Orchard. Geoderma 2023, 429, 116218. [Google Scholar] [CrossRef]
- Masuda, Y.; Yamanaka, H.; Xu, Z.-X.; Shiratori, Y.; Aono, T.; Amachi, S.; Senoo, K.; Itoh, H. Diazotrophic Anaeromyxobacter Isolates from Soils. Appl. Environ. Microbiol. 2020, 86, e00956-20. [Google Scholar] [CrossRef] [PubMed]
- Koch, H.; Lücker, S.; Albertsen, M.; Kitzinger, K.; Herbold, C.; Spieck, E.; Nielsen, P.H.; Wagner, M.; Daims, H. Expanded Metabolic Versatility of Ubiquitous Nitrite-Oxidizing Bacteria from the GenusNitrospira. Proc. Natl. Acad. Sci. USA 2015, 112, 11371–11376. [Google Scholar] [CrossRef] [PubMed]
- Xu, A.; Liu, C.; Zhao, S.; Song, Z.; Sun, H. Dynamic Distribution of Massilia Spp. In Sewage, Substrate, Plant Rhizosphere/Phyllosphere and Air of Constructed Wetland Ecosystem. Front. Microbiol. 2023, 14, 1211649. [Google Scholar] [CrossRef] [PubMed]
- Ofek, M.; Hadar, Y.; Minz, D. Ecology of Root Colonizing Massilia (Oxalobacteraceae). PLoS ONE 2012, 7, e40117. [Google Scholar] [CrossRef] [PubMed]
- Ling, N.; Deng, K.; Song, Y.; Wu, Y.; Zhao, J.; Raza, W.; Huang, Q.; Shen, Q. Variation of Rhizosphere Bacterial Community in Watermelon Continuous Mono-Cropping Soil by Long-Term Application of a Novel Bioorganic Fertilizer. Microbiol. Res. 2014, 169, 570–578. [Google Scholar] [CrossRef] [PubMed]
- Hei, J.; Li, Y.; Wang, Q.; Wang, S.; He, X. Effects of Exogenous Organic Acids on the Soil Metabolites and Microbial Communities of Panax Notoginseng from the Forest Understory. Agronomy 2024, 14, 601. [Google Scholar] [CrossRef]
- Zhang, L.; Jin, Q.; Guan, Y.; Liu, Z.; Pan, X.; Zhang, Y.; Zhang, Y.; Wang, Q. Trichoderma spp. promotes ginseng biomass by influencing the soil microbial community. Front. Microbiol. 2024, 15, 1283492. [Google Scholar] [CrossRef] [PubMed]
- Amtmann, A.; Troufflard, S.; Armengaud, P. The Effect of Potassium Nutrition on Pest and Disease Resistance in Plants. Physiol. Plant. 2008, 133, 682–691. [Google Scholar] [CrossRef] [PubMed]
- Wan, P.; Peng, H.; Ji, X.; Chen, X.; Zhou, H. Effect of Stand Age on Soil Microbial Communities of a Plantation Ormosia Hosiei Forest in Southern China. Ecol. Inform. 2021, 62, 101282. [Google Scholar] [CrossRef]
- Jiang, Y.; Yang, L.; Wu, S.; Zhou, T. Warming Changes the Composition and Diversity of Fungal Communities in Permafrost. Ann. Microbiol. 2023, 73, 7. [Google Scholar] [CrossRef]

| Treatments | PH | AN (mg kg−1) | AP (mg kg−1) | AK (mg kg−1) | TK (g kg−1) | SOM (g kg−1) |
|---|---|---|---|---|---|---|
| SM | 6.69 ± 0.16 b | 66.89 ± 6.84 a | 24.74 ± 6.95 a | 58.29 ± 1.51 a | 9.82 ± 0.19 b | 17.37 ± 0.27 a |
| IP | 7.28 ± 0.08 a | 58.64 ± 5.69 a | 22.68 ± 2.48 a | 59.56 ± 2.49 a | 11.58 ± 0.84 a | 12.93 ± 1.64 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Cheng, Z.; Su, L.; Huang, X.; Deng, Y.; Bai, W.; Chen, Z.; Chen, B.; Wang, P.; Pang, H.; et al. Impact of Sugarcane–Pumpkin Intercropping on Soil Microbial Diversity. Microorganisms 2025, 13, 1703. https://doi.org/10.3390/microorganisms13071703
Chen X, Cheng Z, Su L, Huang X, Deng Y, Bai W, Chen Z, Chen B, Wang P, Pang H, et al. Impact of Sugarcane–Pumpkin Intercropping on Soil Microbial Diversity. Microorganisms. 2025; 13(7):1703. https://doi.org/10.3390/microorganisms13071703
Chicago/Turabian StyleChen, Xianglei, Zhikui Cheng, Liwen Su, Xialei Huang, Yan Deng, Wenhui Bai, Zhihao Chen, Baoshan Chen, Peng Wang, Hongguang Pang, and et al. 2025. "Impact of Sugarcane–Pumpkin Intercropping on Soil Microbial Diversity" Microorganisms 13, no. 7: 1703. https://doi.org/10.3390/microorganisms13071703
APA StyleChen, X., Cheng, Z., Su, L., Huang, X., Deng, Y., Bai, W., Chen, Z., Chen, B., Wang, P., Pang, H., & Liu, Z. (2025). Impact of Sugarcane–Pumpkin Intercropping on Soil Microbial Diversity. Microorganisms, 13(7), 1703. https://doi.org/10.3390/microorganisms13071703






