Impact of Live Ligilactobacillus salivarius CCFM1332 and Its Postbiotics on Porphyromonas gingivalis Colonization, Alveolar Bone Resorption and Inflammation in a Rat Model of Periodontitis
Abstract
1. Introduction
2. Materials and Methods
2.1. Strain Preparation
2.2. Assessment of Antibacterial Activity
2.3. Assessment of Anti-Biofilm Activity
2.4. Animal Experimental Design
2.5. Determination of P. gingivalis Colonization
2.6. Determination of Gingival Index (GI) and Probing Depth (PD)
2.7. Measurement of Inflammatory Cytokines
2.8. Micro-Computed Tomography Imaging
2.9. Histopathological Analysis
2.10. TRAP Staining
2.11. Statistical Analysis
3. Results
3.1. Antibacterial Activity of Lactobacillus Against P. gingivalis
3.2. Anti-Biofilm Activity of Lactobacillus to P. gingivalis
3.3. The Effect of L. salivarius CCFM1332 on the Body Weight of Rats
3.4. The Effect of L. salivarius CCFM1332 on the GI and PD of Rats
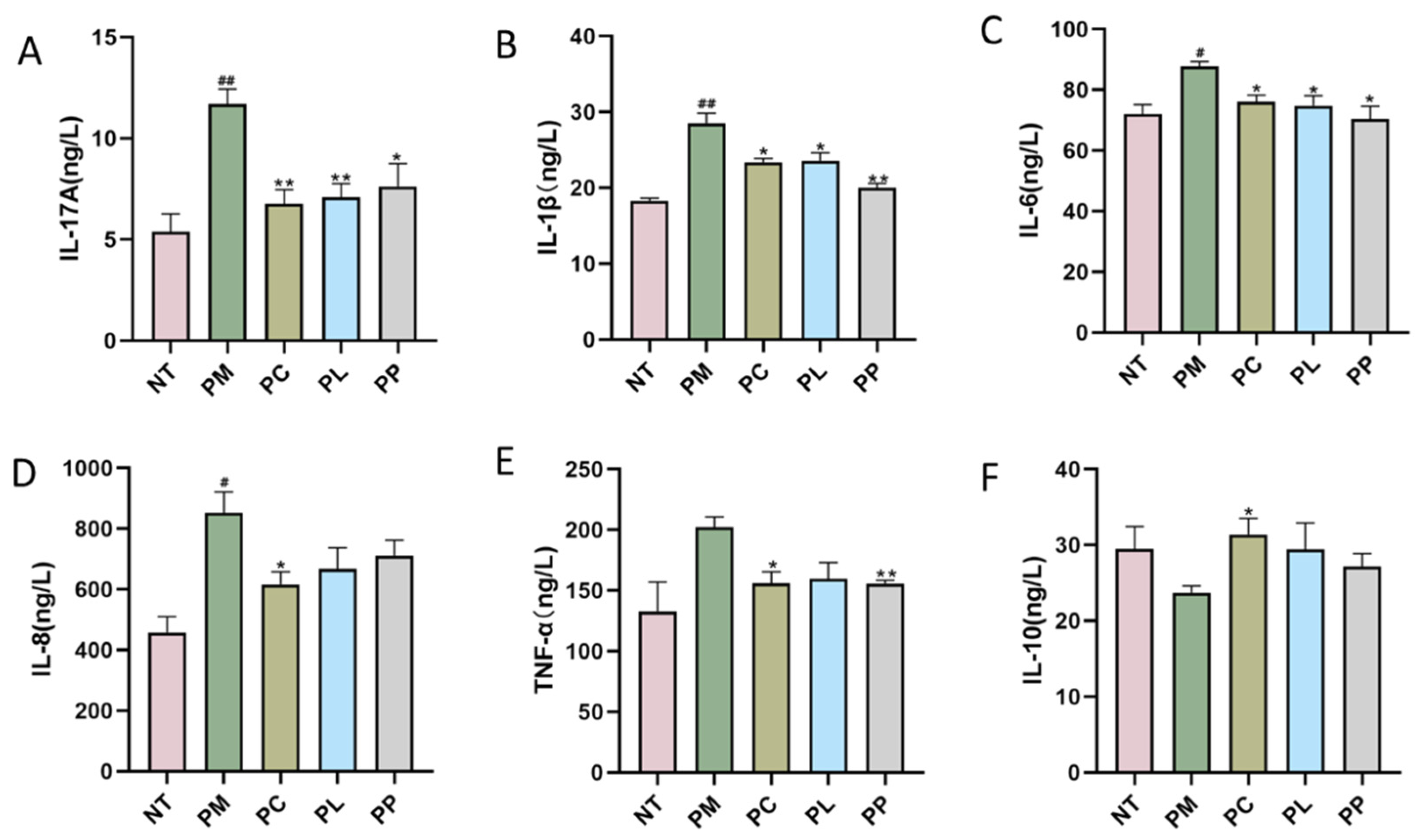
3.5. The Effect of L. salivarius CCFM1332 on Serum Inflammatory Cytokines of Rats
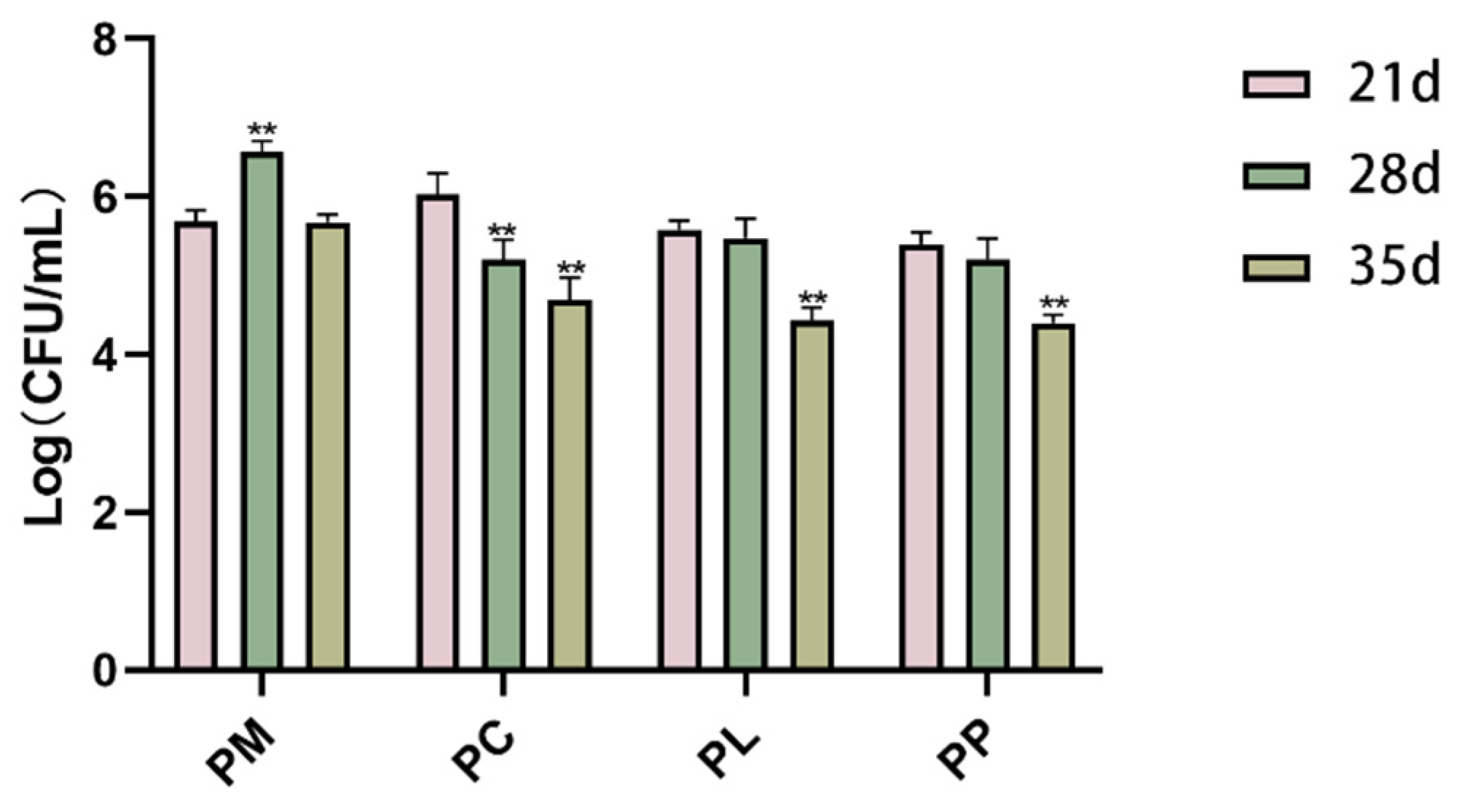
3.6. The Effect of L. salivarius CCFM1332 on the Load of P. gingivalis in the Oral Cavity of Rats
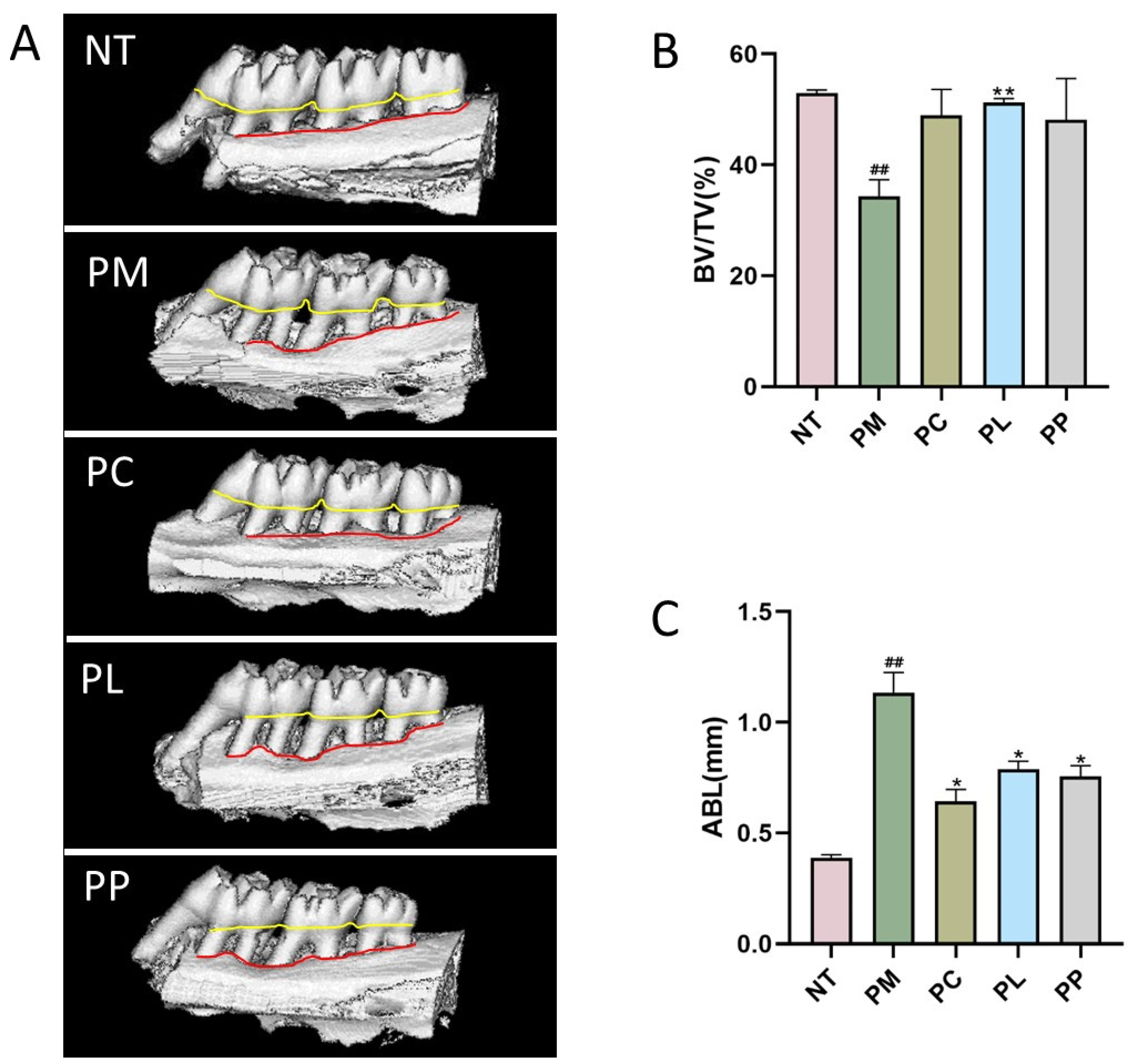
3.7. The Effect of L. salivarius CCFM1332 on Alveolar Bone Resorption of Rats
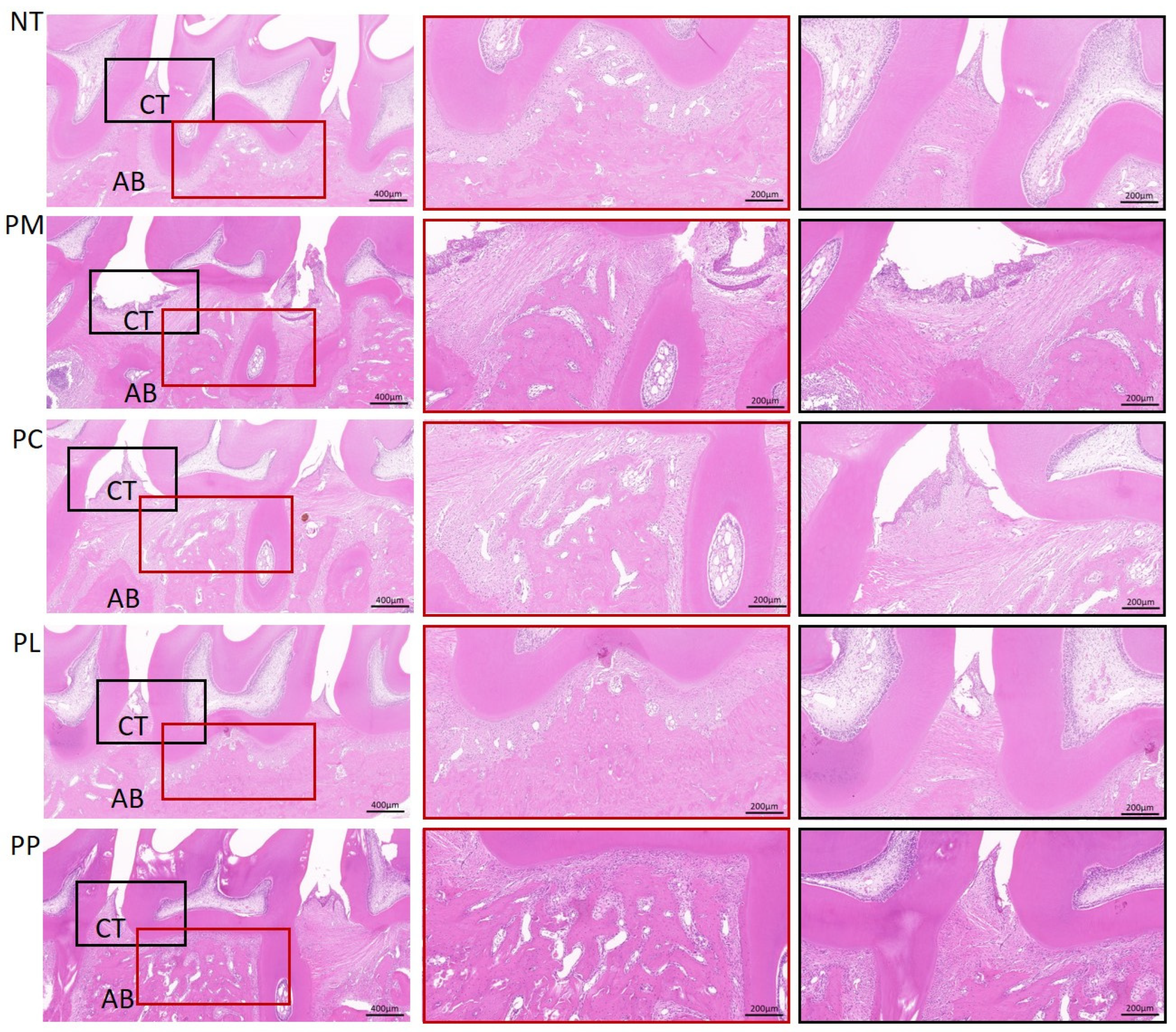
3.8. The Effect of L. salivarius CCFM1332 on the Morphological Structure of Alveolar Bone Tissue of Rats
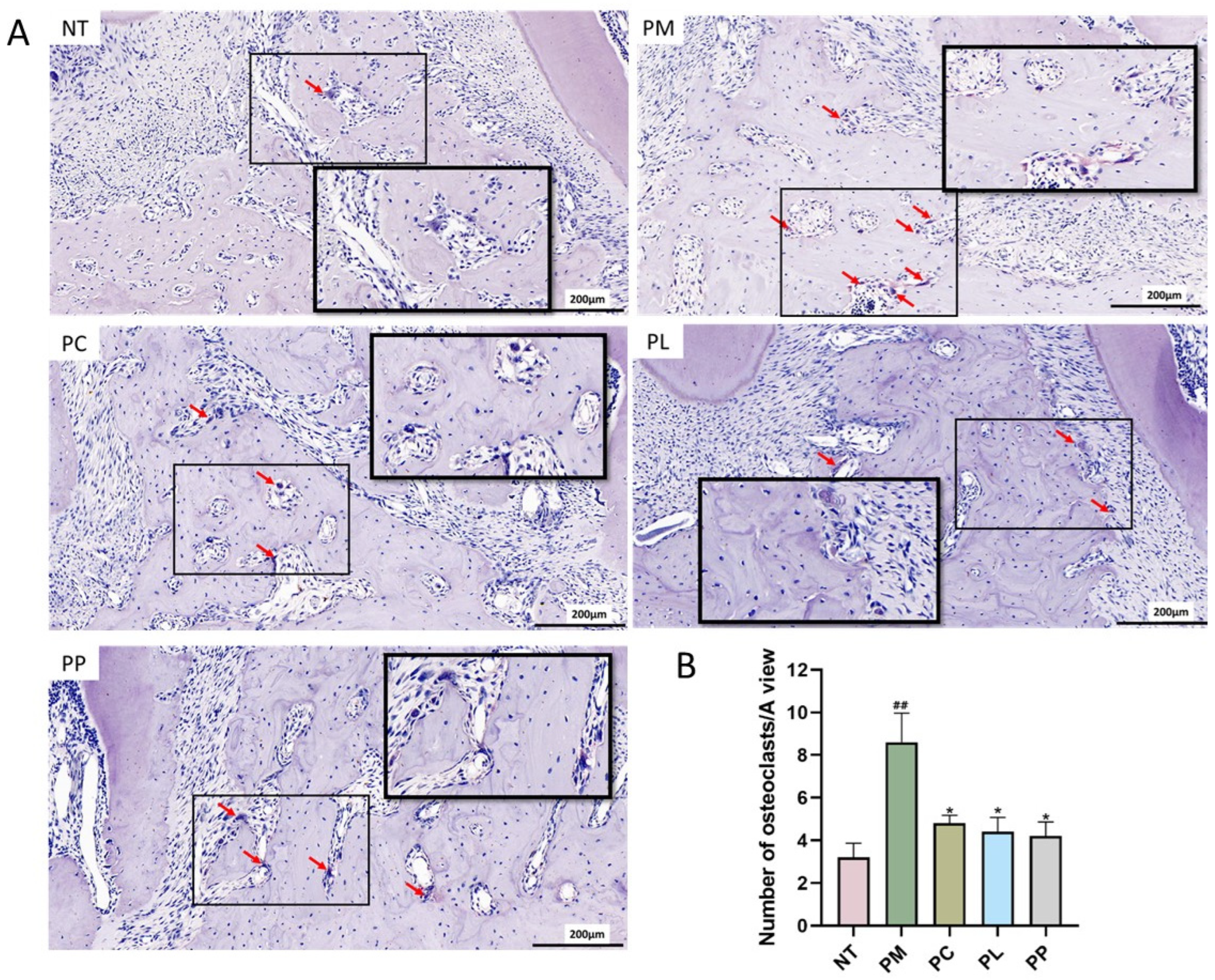
3.9. The Effect of L. salivarius CCFM1332 on Osteoclasts in the Alveolar Bone of Rats
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- de Molon, R.S.; Park, C.H.; Jin, Q.; Sugai, J.; Cirelli, J.A. Characterization of ligature-induced experimental periodontitis. Microsc. Res. Tech. 2018, 81, 1412–1421. [Google Scholar] [CrossRef] [PubMed]
- Kure, K.; Sato, H.; Suzuki, J.I.; Itai, A.; Aoyama, N.; Izumi, Y. A novel IkB kinase inhibitor attenuates ligature-induced periodontal disease in mice. J. Periodontal Res. 2019, 54, 164–173. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.X.; Zhong, Y.J.; Dong, Q.Q.; Wong, H.M.; Wen, Y.F. Global, regional, and national burden of severe periodontitis, 1990–2019: An analysis of the Global Burden of Disease Study 2019. J. Clin. Periodontol. 2021, 48, 1165–1188. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Liu, B.; Shen, J.; Qian, S.; Lai, H.; Yuan, C.; Tonetti, M.S. Low energy intake and nutritional maladaptation in terminal stage IV periodontitis. J. Clin. Periodontol. 2024, 51, 1147–1156. [Google Scholar] [CrossRef]
- Nascimento, G.G.; Alves-Costa, S.; Romandini, M. Burden of severe periodontitis and edentulism in 2021, with projections up to 2050: The Global Burden of Disease 2021 study. J. Periodontal Res. 2024, 59, 823–867. [Google Scholar] [CrossRef]
- Gotsman, I.; Lotan, C.; Soskolne, W.A.; Rassovsky, S.; Pugatsch, T.; Lapidus, L.; Novikov, Y.; Masrawa, S.; Stabholz, A. Periodontal destruction is associated with coronary artery disease and periodontal infection with acute coronary syndrome. J. Periodontol. 2007, 78, 849–858. [Google Scholar] [CrossRef]
- Hickey, N.A.; Shalamanova, L.; Whitehead, K.A.; Dempsey-Hibbert, N.; van der Gast, C.; Taylor, R.L. Exploring the putative interactions between chronic kidney disease and chronic periodontitis. Crit. Rev. Microbiol. 2020, 46, 61–77. [Google Scholar] [CrossRef]
- Maekawa, T.; Krauss, J.L.; Abe, T.; Jotwani, R.; Triantafilou, M.; Triantafilou, K.; Hashim, A.; Hoch, S.; Curtis, M.A.; Nussbaum, G.; et al. Porphyromonas gingivalis manipulates complement and TLR signaling to uncouple bacterial clearance from inflammation and promote dysbiosis. Cell Host Microbe 2014, 15, 768–778. [Google Scholar] [CrossRef]
- Burns, E.; Eliyahu, T.; Uematsu, S.; Akira, S.; Nussbaum, G. TLR2-dependent inflammatory response to Porphyromonas gingivalis is MyD88 independent, whereas MyD88 is required to clear infection. J. Immunol. 2010, 184, 1455–1462. [Google Scholar] [CrossRef]
- Kinane, D.F.; Stathopoulou, P.G.; Papapanou, P.N. Periodontal diseases. Nat. Rev. Dis. Primers 2017, 3, 17038. [Google Scholar] [CrossRef]
- de Molon, R.S.; Hsu, C.; Bezouglaia, O.; Dry, S.M.; Pirih, F.Q.; Soundia, A.; Cunha, F.Q.; Cirelli, J.A.; Aghaloo, T.L.; Tetradis, S. Rheumatoid arthritis exacerbates the severity of osteonecrosis of the jaws (ONJ) in mice. A randomized, prospective, controlled animal study. J. Bone Miner. Res. 2016, 31, 1596–1607. [Google Scholar] [CrossRef] [PubMed]
- Cekici, A.; Kantarci, A.; Hasturk, H.; Van Dyke, T.E. Inflammatory and immune pathways in the pathogenesis of periodontal disease. Periodontol 2000 2014, 64, 57–80. [Google Scholar] [CrossRef] [PubMed]
- Herrera, D.; Matesanz, P.; Martín, C.; Oud, V.; Feres, M.; Teughels, W. Adjunctive effect of locally delivered antimicrobials in periodontitis therapy: A systematic review and meta-analysis. J. Clin. Periodontol. 2020, 47, 239–256. [Google Scholar] [CrossRef] [PubMed]
- Sanz, M.; Herrera, D.; Kebschull, M.; Chapple, I.; Jepsen, S.; Beglundh, T.; Sculean, A.; Tonetti, M.S.; EFP Workshop Participants and Methodological Consultants. Treatment of stage I-III periodontitis-The EFP S3 level clinical practice guideline. J. Clin. Periodontol. 2020, 47, 4–60. [Google Scholar] [CrossRef]
- Schaudinn, C.; Gorur, A.; Keller, D.; Sedghizadeh, P.P.; Costerton, J.W. Periodontitis: An archetypical biofilm disease. J. Am. Dent. Assoc. 2009, 140, 978–986. [Google Scholar] [CrossRef]
- Santi-Rocca, J. Advances in experimental research about periodontitis: Lessons from the past, ideas for the future. Adv. Exp. Med. Biol. 2022, 1373, 1–15. [Google Scholar] [CrossRef]
- Butera, A.; Folini, E.; Cosola, S.; Russo, G.; Scribante, A.; Gallo, S.; Stablum, G.; Menchini Fabris, G.B.; Covani, U.; Genovesi, A. Evaluation of the Efficacy of Probiotics Domiciliary Protocols for the Management of Periodontal Disease, in Adjunction of Non-Surgical Periodontal Therapy (NSPT): A Systematic Literature Review. Appl. Sci. 2023, 13, 663. [Google Scholar] [CrossRef]
- Shirbhate, U.; Bajaj, P.; Chandak, M.; Jaiswal, P.; Sarangi, S.; Suchak, D.; Bharti, L. Clinical implications of probiotics in oral and periodontal health: A comprehensive review. Cureus 2023, 15, e51177. [Google Scholar] [CrossRef]
- Homayouni Rad, A.; Pourjafar, H.; Mirzakhani, E. A comprehensive review of the application of probiotics and postbiotics in oral health. Front. Cell. Infect. Microbiol. 2023, 13, 1120995. [Google Scholar] [CrossRef]
- Kainulainen, V.; Tang, Y.; Spillmann, T.; Kilpinen, S.; Reunanen, J.; Saris, P.E.; Satokari, R. The canine isolate Lactobacillus acidophilus LAB20 adheres to intestinal epithelium and attenuates LPS-induced IL-8 secretion of enterocytes in vitro. BMC Microbiol. 2015, 15, 4. [Google Scholar] [CrossRef]
- Matsubara, V.H.; Bandara, H.M.; Ishikawa, K.H.; Mayer, M.P.; Samaranayake, L.P. The role of probiotic bacteria in managing periodontal disease: A systematic review. Expert Rev. Anti Infect. Ther. 2016, 14, 643–655. [Google Scholar] [CrossRef]
- Zhou, K.; Xie, J.; Su, Y.; Fang, J. Lactobacillus reuteri for chronic periodontitis: Focus on underlying mechanisms and future perspectives. Biotechnol. Genet. Eng. Rev. 2024, 40, 381–408. [Google Scholar] [CrossRef]
- Wang, L.; Wang, R.; He, Q.; Hu, Q.; Yang, J.; Tang, X. Assessment of the effectiveness of probiotics-assisted physical interventions in the management of chronic periodontitis: A randomized controlled clinical trial. Probiotics Antimicrob. Proteins, 2024; prepublish. [Google Scholar] [CrossRef]
- Lee, Y.; Jung, B.H.; Yoo, K.Y.; Lim, H.J.; Shin, K.J.; Lee, J.K. Lactobacillus fermentum attenuates the alveolar bone loss in ligature-induced periodontitis in mice. Oral Dis. 2024, 30, 3328–3335. [Google Scholar] [CrossRef]
- D’Agostino, S.; Valentini, G.; Iarussi, F.; Dolci, M. Effect of Probiotics Lactobacillus rhamnosus and Lactobacillus plantarum on caries and periodontal diseases: A systematic review. Dent. J. 2024, 12, 102. [Google Scholar] [CrossRef] [PubMed]
- Karaca, B.; Yilmaz, M.; Gursoy, U.K. Targeting Nrf2 with probiotics and postbiotics in the treatment of periodontitis. Biomolecules 2022, 12, 729. [Google Scholar] [CrossRef] [PubMed]
- Moraes, R.M.; Schlagenhauf, U.; Anbinder, A.L. Outside the limits of bacterial viability: Postbiotics in the management of periodontitis. Biochem. Pharmacol. 2022, 201, 115072. [Google Scholar] [CrossRef] [PubMed]
- Żółkiewicz, J.; Marzec, A.; Ruszczyński, M.; Feleszko, W. Postbiotics-a step beyond pre- and probiotics. Nutrients 2020, 12, 2189. [Google Scholar] [CrossRef]
- Aguilar-Toalá, J.E.; Garcia-Varela, R.; Garcia, H.S.; Mata-Haro, V.; González-Córdova, A.F.; Vallejo-Cordoba, B.; Hernández-Mendoza, A. Postbiotics: An evolving term within the functional foods field. Trends Food Sci. Technol. 2018, 75, 105–114. [Google Scholar] [CrossRef]
- Zhou, Q.; Gu, R.; Li, P.; Lu, Y.; Chen, L.; Gu, Q. Anti-Salmonella mode of action of natural L-phenyl lactic acid purified from Lactobacillus plantarum ZJ316. Appl. Microbiol. Biotechnol. 2020, 104, 5283–5292. [Google Scholar] [CrossRef]
- Ye, Y.; Xu, X.; Mao, B.; Tang, X.; Cui, S.; Zhao, J.; Zhang, Q. Evaluation of heat-inactivated Limosilactobacillus fermentum CCFM1139 and its supernatant for the relief of experimental periodontitis in rats. Food Funct. 2023, 14, 2847–2856. [Google Scholar] [CrossRef]
- Loo, C.Y.; Corliss, D.A.; Ganeshkumar, N. Streptococcus gordonii biofilm formation: Identification of genes that code for biofilm phenotypes. J. Bacteriol. 2000, 182, 1374–1382. [Google Scholar] [CrossRef]
- Zhang, Q.; Xu, W.; Xu, X.; Lu, W.; Zhao, J.; Zhang, H.; Chen, W. Effects of Limosilactobacillus fermentum CCFM1139 on experimental periodontitis in rats. Food Funct. 2021, 12, 4670–4678. [Google Scholar] [CrossRef] [PubMed]
- Löe, H. The Gingival Index, the Plaque Index and the Retention Index Systems. J. Periodontol. 1967, 38, 610–616. [Google Scholar] [CrossRef] [PubMed]
- Hosadurga, R.R.; Rao, S.N.; Jose, J.; Rompicharla, N.C.; Shakil, M.; Shashidhara, R. Evaluation of the efficacy of 2% curcumin gel in the treatment of experimental periodontitis. Pharmacogn. Res. 2014, 6, 326–333. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.H.; Tsai, T.Y.; Pan, T.M. Effects of an ethanol extract from Lactobacillus paracasei subsp. paracasei NTU 101 fermented skimmed milk on lipopolysaccharide-induced periodontal inflammation in rats. Food Funct. 2018, 9, 4916–4925. [Google Scholar] [CrossRef]
- Cai, X.; Li, C.; Du, G.; Cao, Z. Protective effects of baicalin on ligature-induced periodontitis in rats. J. Periodontal Res. 2008, 43, 14–21. [Google Scholar] [CrossRef]
- Korah, L.; Amri, N.; Bugueno, I.M.; Hotton, D.; Tenenbaum, H.; Huck, O.; Berdal, A.; Davideau, J.L. Experimental periodontitis in Msx2 mutant mice induces alveolar bone necrosis. J. Periodontol. 2020, 91, 693–704. [Google Scholar] [CrossRef]
- Jiang, L.; Ma, Y.; Xiong, Y.; Tan, Y.; Duan, X.; Liao, X.; Wang, J. Ruthenium polypyridine complexes with triphenylamine groups as antibacterial agents against Staphylococcus aureus with membrane-disruptive mechanism. Front. Chem. 2022, 10, 1035741. [Google Scholar] [CrossRef]
- Wang, J.; Liu, Y.; Wang, W.; Ma, J.; Zhang, M.; Lu, X.; Liu, J.; Kou, Y. The rationale and potential for using Lactobacillus in the management of periodontitis. J. Microbiol. 2022, 60, 355–363. [Google Scholar] [CrossRef]
- Wang, H.Y.; Lin, L.; Fu, W.; Yu, H.Y.; Yu, N.; Tan, L.S.; Cheng, J.W.; Pan, Y.P. Preventive effects of the novel antimicrobial peptide Nal-P-113 in a rat periodontitis model by limiting the growth of Porphyromonas gingivalis and modulating IL-1β and TNF-α production. BMC Complement. Altern. Med. 2017, 17, 426. [Google Scholar] [CrossRef]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef]
- Kobayashi, R.; Kobayashi, T.; Sakai, F.; Hosoya, T.; Yamamoto, M.; Kurita-Ochiai, T. Oral administration of Lactobacillus gasseri SBT2055 is effective in preventing Porphyromonas gingivalis-accelerated periodontal disease. Sci. Rep. 2017, 7, 545. [Google Scholar] [CrossRef]
- Ishikawa, K.H.; Bueno, M.R.; Kawamoto, D.; Simionato, M.R.L.; Mayer, M.P.A. Lactobacilli postbiotics reduce biofilm formation and alter transcription of virulence genes of Aggregatibacter actinomycetemcomitans. Mol. Oral Microbiol. 2021, 36, 92–102. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.I.; Baek, S.M.; Nguyen, T.H.; Kim, J.W.; Kang, C.H.; Kim, S.; Imm, J.Y. Effects of probiotic culture supernatant on cariogenic biofilm formation and RANKL-induced osteoclastogenesis in RAW 264.7 macrophages. Molecules 2021, 26, 733. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.M.; Kim, J.S.; Kim, H.S.; Kim, Y.Y.; Oh, J.K.; Jung, H.W.; Park, D.S.; Bae, K.H. Lactobacillus reuteri AN417 cell-free culture supernatant as a novel antibacterial agent targeting oral pathogenic bacteria. Sci. Rep. 2021, 11, 1631. [Google Scholar] [CrossRef] [PubMed]
- Graves, D. Cytokines that promote periodontal tissue destruction. J. Periodontol. 2008, 79, 1585–1591. [Google Scholar] [CrossRef]
- Park, S.Y.; Park, D.J.; Kim, Y.H.; Kim, Y.; Kim, S.G.; Shon, K.J.; Choi, Y.W.; Lee, S.J. Upregulation of heme oxygenase-1 via PI3K/Akt and Nrf-2 signaling pathways mediates the anti-inflammatory activity of Schisandrin in Porphyromonas gingivalis LPS-stimulated macrophages. Immunol. Lett. 2011, 139, 93–101. [Google Scholar] [CrossRef]
- Jiang, H.; Zhang, Y.; Xiong, X.; Harville, E.W.; O, K.; Qian, X. Salivary and serum inflammatory mediators among pre-conception women with periodontal disease. BMC Oral Health 2016, 16, 131. [Google Scholar] [CrossRef]
- Bagavad Gita, J.; George, A.V.; Pavithra, N.; Chandrasekaran, S.C.; Latchumanadhas, K.; Gnanamani, A. Dysregulation of miR-146a by periodontal pathogens: A risk for acute coronary syndrome. J. Periodontol. 2019, 90, 756–765. [Google Scholar] [CrossRef]
- Zhu, H.; Lin, X.; Zheng, P.; Chen, H. Inflammatory cytokine levels in patients with periodontitis and/or coronary heart disease. Int. J. Clin. Exp. Pathol. 2015, 8, 2214–2220. [Google Scholar]
- Acharya, A.B.; Thakur, S.; Muddapur, M.V.; Kulkarni, R.D. Tumor necrosis factor-α, interleukin-4 and -6 in the serum of health, chronic periodontitis, and type 2 diabetes mellitus. J. Indian Soc. Periodontol. 2016, 20, 509–513. [Google Scholar] [CrossRef]
- Chauhan, A.; Yadav, S.S.; Dwivedi, P.; Lal, N.; Usman, K.; Khattri, S. Correlation of serum and salivary cytokines level with clinical parameters in metabolic syndrome with periodontitis. J. Clin. Lab. Anal. 2016, 30, 649–655. [Google Scholar] [CrossRef]
- Awang, R.A.; Lappin, D.F.; MacPherson, A.; Riggio, M.; Robertson, D.; Hodge, P.; Ramage, G.; Culshaw, S.; Preshaw, P.M.; Taylor, J.; et al. Clinical associations between IL-17 family cytokines and periodontitis and potential differential roles for IL-17A and IL-17E in periodontal immunity. Inflamm. Res. 2014, 63, 1001–1012. [Google Scholar] [CrossRef] [PubMed]
- Corrêa, J.D.; Madeira, M.F.; Resende, R.G.; Correia-Silva, J.d.F.; Gomez, R.S.; de Souza, D.d.G.; Teixeira, M.M.; Queiroz-Junior, C.M.; da Silva, T.A. Association between polymorphisms in interleukin-17A and -17F genes and chronic periodontal disease. Mediat. Inflamm. 2012, 2012, 846052. [Google Scholar] [CrossRef] [PubMed]
- Robati, M.; Ranjbari, A.; Ghafourian Boroujerdnia, M.; Chinipardaz, Z. Detection of IL-4, IL-6 and IL-12 serum levels in generalized aggressive periodontitis. Iran. J. Immunol. 2011, 8, 170–175. [Google Scholar] [PubMed]
- Miranda, T.S.; Heluy, S.L.; Cruz, D.F.; da Silva, H.D.P.; Feres, M.; Figueiredo, L.C.; Duarte, P.M. The ratios of pro-inflammatory to anti-inflammatory cytokines in the serum of chronic periodontitis patients with and without type 2 diabetes and/or smoking habit. Clin. Oral Investig. 2019, 23, 641–650. [Google Scholar] [CrossRef]
- Gümüş, P.; Nizam, N.; Lappin, D.F.; Buduneli, N. Saliva and serum levels of B-cell activating factors and tumor necrosis factor-α in patients with periodontitis. J. Periodontol. 2014, 85, 270–280. [Google Scholar] [CrossRef]
- Nie, Q.; Wan, X.; Tao, H.; Yang, Q.; Zhao, X.; Liu, H.; Hu, J.; Luo, Y.; Shu, T.; Geng, R.; et al. Multi-function screening of probiotics to improve oral health and evaluating their efficacy in a rat periodontitis model. Front. Cell. Infect. Microbiol. 2023, 13, 1261189. [Google Scholar] [CrossRef]
- Finoti, L.S.; Nepomuceno, R.; Pigossi, S.C.; Corbi, S.C.; Secolin, R.; Scarel-Caminaga, R.M. Association between interleukin-8 levels and chronic periodontal disease: A PRISMA-compliant systematic review and meta-analysis. Medicine 2017, 96, e6932. [Google Scholar] [CrossRef]
- Shi, T.; Jin, Y.; Miao, Y.; Wang, Y.; Zhou, Y.; Lin, X. IL-10 secreting B cells regulate periodontal immune response during periodontitis. Odontology 2020, 108, 350–357. [Google Scholar] [CrossRef]
- Mejía, K.; Rodríguez-Hernández, A.P.; Martínez-Hernández, M. Insights Into the Mechanism of Action of Chlorhexidine on Porphyromonas gingivalis. Int. J. Dent. 2025, 2025, 1492069. [Google Scholar] [CrossRef]
- Ranney, R.R. Immunologic mechanisms of pathogenesis in periodontal diseases: An assessment. J. Periodontal Res. 1991, 26, 243–254. [Google Scholar] [CrossRef]
- Sukumaran, A.; Lo Russo, L.; Peeran, S.W.; Das, N.; Neeta, P.; Nazir, M.; Wilson, S.; Rastogi, P.; Tope, O.; Gandhi, K. Overview of periodontal disease: Causes, pathogenesis, and characteristics. In Periodontal Disease and Overall Health: A Clinician′s Guide, 2nd ed.; Robert, J.G., Ray, C.W., Eds.; Professional Audience Communications, Inc.: Yardley, PA, USA.
- Matsuoka, T.; Sugano, N.; Takigawa, S.; Takane, M.; Yoshinuma, N.; Ito, K.; Koga, Y. Effect of oral Lactobacillus salivarius TI2711 (LS1) administration on periodontopathic bacteria in subgingival plaque. J. Jpn. Soc. Periodontal Dis. 2006, 48, 315–324. [Google Scholar] [CrossRef]
- Assuma, R.; Oates, T.; Cochran, D.; Amar, S.; Graves, D.T. IL-1 and TNF antagonists inhibit the inflammatory response and bone loss in experimental periodontitis. J. Immunol. 1998, 160, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Redlich, K.; Smolen, J.S. Inflammatory bone loss: Pathogenesis and therapeutic intervention. Nat. Rev. Drug. Discov. 2012, 11, 234–250. [Google Scholar] [CrossRef]
- Lapérine, O.; Cloitre, A.; Caillon, J.; Huck, O.; Bugueno, I.M.; Pilet, P.; Sourice, S.; Le Tilly, E.; Palmer, G.; Davideau, J.L.; et al. Interleukin-33 and RANK-L interplay in the alveolar bone loss associated to periodontitis. PLoS ONE 2016, 11, e0168080. [Google Scholar] [CrossRef]
- Hienz, S.A.; Paliwal, S.; Ivanovski, S. Mechanisms of bone resorption in periodontitis. J. Immunol. Res. 2015, 2015, 615486. [Google Scholar] [CrossRef]
| Lactobacillus strains | DIZ (mm) |
|---|---|
| Positive control (0.02% CHX) | 12.46 ± 0.43 |
| Negative control | - |
| L. salivarius FHNXY73M9 | - |
| L. salivarius FGSYC47M10 | - |
| L. salivarius CCFM1332 | 20.15 ± 0.80 |
| L. salivarius FXJWS6M4 | - |
| L. salivarius FJSWX10-2 | - |
| L. plantarum QS6-12 | 14.18 ± 0.63 |
| L. plantarum CCFM242 | 11.86 ± 0.50 |
| L. plantarum DL2-1 | 11.28 ± 0.46 |
| L. plantarum CCFM10 | 10.82 ± 0.40 |
| Lactobacillus | OD600 | Biofilm Reduction Rate (%) |
|---|---|---|
| Positive control (0.02% CHX) | 0.18 ± 0.04 | 89.47 |
| Negative control | 1.71 ± 0.44 | - |
| L. salivarius FHNXY73M9 | 0.15 ± 0.01 | 91.23 |
| L. salivarius FGSYC47M10 | 0.18 ± 0.03 | 89.47 |
| L. salivarius CCFM1332 | 0.12 ± 0.04 | 92.98 |
| L. salivarius FXJWS6M4 | 0.25 ± 0.17 | 85.38 |
| L. salivarius FJSWX10-2 | 0.17 ± 0.02 | 90.06 |
| L. plantarum QS6-12 | 0.23 ± 0.02 | 86.48 |
| L. plantarum CCFM242 | 0.27 ± 0.01 | 84.03 |
| L. plantarum DL2-1 | 0.27 ± 0.03 | 83.98 |
| L. plantarum CCFM10 | 0.23 ± 0.02 | 86.74 |
| Groups | Body Weight (g) | Weight Gain (g) | ||||
|---|---|---|---|---|---|---|
| 7 d | 14 d | 21 d | 28 d | 35 d | ||
| NT | 253.3 ± 2.2 | 328.5 ± 6.0 | 370.4 ± 4.6 | 422.4 ± 1.8 | 457.6 ± 6.9 | 204.2 ± 5.2 |
| PM | 240.8 ± 9.7 | 304.5 ± 23.0 | 358.3 ± 17.0 | 387.9 ± 8.9 | 414.3 ± 5.4 | 173.5 ± 15.1 |
| PC | 251.9 ± 14.9 | 290.4 ± 16.4 | 374.4 ± 3.9 | 394.9 ± 19.9 | 417.6 ± 14.1 | 165.7 ± 23.0 |
| PL | 243.9 ± 8.2 | 313.3 ± 4.4 | 355.6 ± 4.7 | 391.3 ± 6.4 | 428.5 ± 6.4 | 183.3 ± 12.9 |
| PP | 254.9 ± 5.2 | 322.0 ± 3.6 | 362.3 ± 5.9 | 399.9 ± 7.8 | 423.3 ± 8.3 | 168.4 ± 6.5 |
| Groups | PD | GI |
|---|---|---|
| NT | 0.24 ± 0.05 | 0.00 ± 0.00 |
| PM | 1.24 ± 0.11 ## | 1.50 ± 0.29 ## |
| PC | 0.58 ± 0.09 ** | 0.50 ± 0.29 * |
| PL | 0.85 ± 0.06 * | 0.50 ± 0.29 * |
| PP | 0.92 ± 0.09 | 0.60 ± 0.24 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hong, Q.; Ren, Y.; Tang, X.; Mao, B.; Zhang, Q.; Zhao, J.; Cui, S.; Liu, Z. Impact of Live Ligilactobacillus salivarius CCFM1332 and Its Postbiotics on Porphyromonas gingivalis Colonization, Alveolar Bone Resorption and Inflammation in a Rat Model of Periodontitis. Microorganisms 2025, 13, 1701. https://doi.org/10.3390/microorganisms13071701
Hong Q, Ren Y, Tang X, Mao B, Zhang Q, Zhao J, Cui S, Liu Z. Impact of Live Ligilactobacillus salivarius CCFM1332 and Its Postbiotics on Porphyromonas gingivalis Colonization, Alveolar Bone Resorption and Inflammation in a Rat Model of Periodontitis. Microorganisms. 2025; 13(7):1701. https://doi.org/10.3390/microorganisms13071701
Chicago/Turabian StyleHong, Qing, Yu Ren, Xin Tang, Bingyong Mao, Qiuxiang Zhang, Jianxin Zhao, Shumao Cui, and Zhenmin Liu. 2025. "Impact of Live Ligilactobacillus salivarius CCFM1332 and Its Postbiotics on Porphyromonas gingivalis Colonization, Alveolar Bone Resorption and Inflammation in a Rat Model of Periodontitis" Microorganisms 13, no. 7: 1701. https://doi.org/10.3390/microorganisms13071701
APA StyleHong, Q., Ren, Y., Tang, X., Mao, B., Zhang, Q., Zhao, J., Cui, S., & Liu, Z. (2025). Impact of Live Ligilactobacillus salivarius CCFM1332 and Its Postbiotics on Porphyromonas gingivalis Colonization, Alveolar Bone Resorption and Inflammation in a Rat Model of Periodontitis. Microorganisms, 13(7), 1701. https://doi.org/10.3390/microorganisms13071701







