Taxon-Dependent Community Assembly of Bacteria and Protists in River Ecosystems: A Case Study from the Yujiang River
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area and Sample Collection
2.2. Measurements of Environmental Factors
2.3. DNA Extraction and High-Throughput Sequencing
2.4. Data Processing
2.5. Statistical Analysis
3. Results
3.1. Differences in the Alpha Diversity and Niche Breadth Between Bacteria and Protists
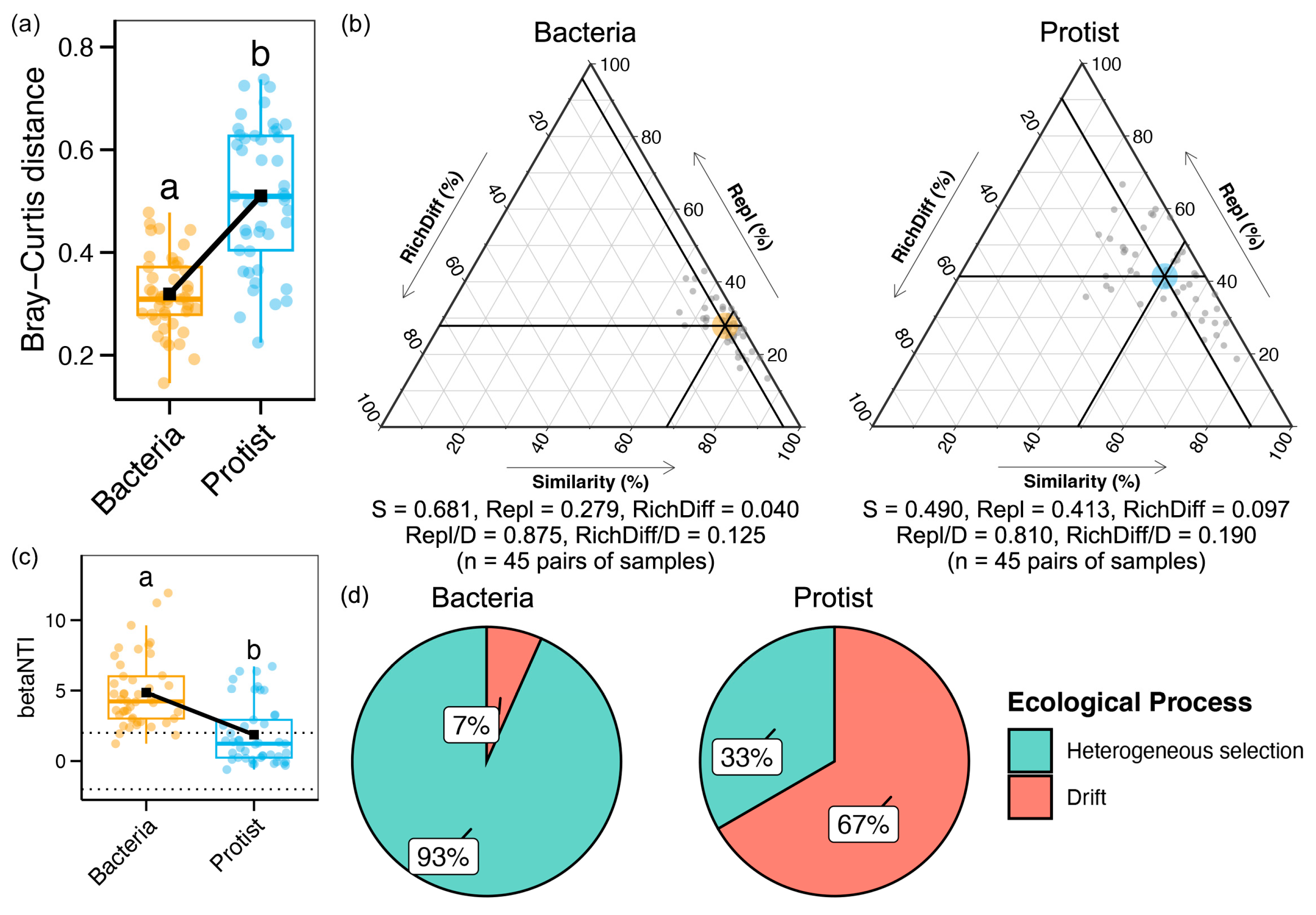
3.2. Assembly Mechanisms of Bacterial and Protistan Communities
3.3. Driving Factors for Variations in Bacterial and Protistan Communities
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Besemer, K. Biodiversity, Community Structure and Function of Biofilms in Stream Ecosystems. Res. Microbiol. 2015, 166, 774–781. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yan, B.; Wu, Y.; Yin, M.; Wang, M.; Fu, C. Microbial Community Diversity and Potential Functionality in Response to Dam Construction along the Three Gorge Reservoir, China. Front. Microbiol. 2023, 14, 1218806. [Google Scholar] [CrossRef] [PubMed]
- Karlicki, M.; Bednarska, A.; Hałakuc, P.; Maciszewski, K.; Karnkowska, A. Spatio-Temporal Changes of Small Protist and Free-Living Bacterial Communities in a Temperate Dimictic Lake: Insights from Metabarcoding and Machine Learning. FEMS Microbiol. Ecol. 2024, 100, fiae104. [Google Scholar] [CrossRef] [PubMed]
- Ruhl, I.A.; Sheremet, A.; Smirnova, A.V.; Sharp, C.E.; Grasby, S.E.; Strous, M.; Dunfield, P.F. Microbial Functional Diversity Correlates with Species Diversity along a Temperature Gradient. mSystems 2022, 7, e00991-21. [Google Scholar] [CrossRef]
- Wagg, C.; Hautier, Y.; Pellkofer, S.; Banerjee, S.; Schmid, B.; van der Heijden, M.G. Diversity and Asynchrony in Soil Microbial Communities Stabilizes Ecosystem Functioning. eLife 2021, 10, e62813. [Google Scholar] [CrossRef]
- Teachey, M.E.; McDonald, J.M.; Ottesen, E.A. Rapid and Stable Microbial Community Assembly in the Headwaters of a Third-Order Stream. Appl. Environ. Microbiol. 2019, 85, e00188-19. [Google Scholar] [CrossRef]
- Yuan, H.; Zhang, W.; Yin, H.; Zhang, R.; Wang, J. Taxonomic Dependency of Beta Diversity for Bacteria, Archaea, and Fungi in a Semi-Arid Lake. Front. Microbiol. 2022, 13, 998496. [Google Scholar] [CrossRef]
- Härer, A.; Dominguez, J.; Shurin, J.B.; Rennison, D.J. Contrasting Alpha, Beta, and Gamma Diversity in the Littoral Zones of Mountain Lakes: Effects of Habitat Size and within-Lake Community Structuring on Bacterial Biogeography. FEMS Microbiol. Ecol. 2025, 101, fiaf026. [Google Scholar] [CrossRef]
- Cai, S.; Zhao, J.; Sheng, E.; Fan, L.; Shen, Z.; Li, Y. Similar but Different Assembly Processes of Bacterial and Micro-Eukaryotic Communities in an Urban River. Sci. Rep. 2025, 15, 6974. [Google Scholar] [CrossRef]
- Nemergut, D.R.; Schmidt, S.K.; Fukami, T.; O’Neill, S.P.; Bilinski, T.M.; Stanish, L.F.; Knelman, J.E.; Darcy, J.L.; Lynch, R.C.; Wickey, P.; et al. Patterns and Processes of Microbial Community Assembly. Microbiol. Mol. Biol. Rev. 2013, 77, 342–356. [Google Scholar] [CrossRef]
- Wang, J.; Pan, Z.; Yu, J.; Zhang, Z.; Li, Y. Global Assembly of Microbial Communities. mSystems 2023, 8, e01289-22. [Google Scholar] [CrossRef] [PubMed]
- Ning, D.; Wang, Y.; Fan, Y.; Wang, J.; Van Nostrand, J.D.; Wu, L.; Zhang, P.; Curtis, D.J.; Tian, R.; Lui, L.; et al. Environmental Stress Mediates Groundwater Microbial Community Assembly. Nat. Microbiol. 2024, 9, 490–501. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Xiang, X.; Yang, Y.; Huang, G.; Fu, K.; Che, R.; Chen, L. Seasonal Effects of River Flow on Microbial Community Coalescence and Diversity in a Riverine Network. FEMS Microbiol. Ecol. 2020, 96, fiaa132. [Google Scholar] [CrossRef] [PubMed]
- Cruaud, P.; Vigneron, A.; Dorea, C.C.; Rodriguez, M.J.; Charette, S.J. Rapid Changes in Microbial Community Structures along a Meandering River. Microorganisms 2020, 8, 1631. [Google Scholar] [CrossRef]
- Sun, Y.; Li, H.; Zhang, J.; Wang, H.; Cui, X.; Gao, X.; Qiao, W.; Yang, Y. Assembly Mechanisms of Microbial Communities in Plastisphere Related to Species Taxonomic Types and Habitat Niches. Mar. Pollut. Bull. 2024, 198, 115894. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, X.; Wang, X.; Wang, H.; Sun, Y.; Zhang, J.; Li, H. Contrasting Benthic Bacterial and Fungal Communities in Two Temperate Coastal Areas Affected by Different Levels of Anthropogenic Pressure. Mar. Environ. Res. 2024, 198, 106501. [Google Scholar] [CrossRef]
- Zhao, Z.; Li, H.; Sun, Y.; Shao, K.; Wang, X.; Ma, X.; Hu, A.; Zhang, H.; Fan, J. How Habitat Heterogeneity Shapes Bacterial and Protistan Communities in Temperate Coastal Areas near Estuaries. Environ. Microbiol. 2022, 24, 1775–1789. [Google Scholar] [CrossRef]
- Mauro, M.; Villanova, V.; Valvo, M.L.; Alduina, R.; Radovic, S.; Vizzini, A.; Orecchio, G.; Longo, F.; Badalamenti, R.; Ferrantelli, V.; et al. Environmental DNA: Preliminary Characterization of Microbiota in Three Sicilian Lakes. Ecol. Evol. 2025, 15, e71276. [Google Scholar] [CrossRef]
- GB 11893-89; Water Quality—Determination of Total Phosphorus—Ammonium Molybdate Spectrophotometric Method. State Bureau of Technical Supervision: Beijing, China, 1989.
- Stoeck, T.; Bass, D.; Nebel, M.; Christen, R.; Jones, M.D.M.; Breiner, H.-W.; Richards, T.A. Multiple Marker Parallel Tag Environmental DNA Sequencing Reveals a Highly Complex Eukaryotic Community in Marine Anoxic Water. Mol. Ecol. 2010, 19, 21–31. [Google Scholar] [CrossRef]
- Sampson, T.R.; Debelius, J.W.; Thron, T.; Janssen, S.; Shastri, G.G.; Ilhan, Z.E.; Challis, C.; Schretter, C.E.; Rocha, S.; Gradinaru, V.; et al. Gut Microbiota Regulate Motor Deficits and Neuroinflammation in a Model of Parkinson’s Disease. Cell 2016, 167, 1469–1480.e12. [Google Scholar] [CrossRef]
- Chen, W.; Sang, S.; Shao, L.; Li, Y.; Li, T.; Gan, L.; Liu, L.; Wang, D.; Zhou, L. Biogeographic Patterns and Community Assembly Processes of Bacterioplankton and Potential Pathogens in Subtropical Estuaries in China. Microbiol. Spectr. 2023, 11, e0368322. [Google Scholar] [CrossRef] [PubMed]
- Zou, K.; Wang, R.; Xu, S.; Li, Z.; Liu, L.; Li, M.; Zhou, L. Changes in Protist Communities in Drainages across the Pearl River Delta under Anthropogenic Influence. Water Res. 2021, 200, 117294. [Google Scholar] [CrossRef] [PubMed]
- Rognes, T.; Flouri, T.; Nichols, B.; Quince, C.; Mahé, F. VSEARCH: A Versatile Open Source Tool for Metagenomics. PeerJ 2016, 4, e2584. [Google Scholar] [CrossRef]
- Edgar, R.C. Search and Clustering Orders of Magnitude Faster than BLAST. Bioinformatics 2010, 26, 2460–2461. [Google Scholar] [CrossRef]
- Guillou, L.; Bachar, D.; Audic, S.; Bass, D.; Berney, C.; Bittner, L.; Boutte, C.; Burgaud, G.; de Vargas, C.; Decelle, J.; et al. The Protist Ribosomal Reference Database (PR2): A Catalog of Unicellular Eukaryote Small Sub-Unit rRNA Sequences with Curated Taxonomy. Nucleic Acids Res. 2013, 41, D597–D604. [Google Scholar] [CrossRef]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA Ribosomal RNA Gene Database Project: Improved Data Processing and Web-Based Tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing, Version 4.3.0; R Foundation for Statistical Computing: Vienna, Austria, 2023; Available online: https://www.r-project.org/ (accessed on 10 May 2025).
- Dixon, P. VEGAN, a Package of R Functions for Community Ecology. J. Veg. Sci. 2003, 14, 927–930. [Google Scholar] [CrossRef]
- Finn, D.R.; Yu, J.; Ilhan, Z.E.; Fernandes, V.M.C.; Penton, C.R.; Krajmalnik-Brown, R.; Garcia-Pichel, F.; Vogel, T.M. MicroNiche: An R Package for Assessing Microbial Niche Breadth and Overlap from Amplicon Sequencing Data. FEMS Microbiol. Ecol. 2020, 96, fiaa131. [Google Scholar] [CrossRef]
- Granot, I.; Shenkar, N.; Belmaker, J. Habitat Niche Breadth Predicts Invasiveness in Solitary Ascidians. Ecol. Evol. 2017, 7, 7838–7847. [Google Scholar] [CrossRef]
- Xiong, J.; Li, X.; Yan, M.; Lu, J.; Qiu, Q.; Chen, J. Comparable Ecological Processes Govern the Temporal Succession of Gut Bacteria and Microeukaryotes as Shrimp Aged. Microb. Ecol. 2020, 80, 935–945. [Google Scholar] [CrossRef]
- Wan, W.; Grossart, H.; He, D.; Liu, W.; Wang, S.; Yang, Y. Differentiation Strategies for Planktonic Bacteria and Eukaryotes in Response to Aggravated Algal Blooms in Urban Lakes. Imeta 2023, 2, e84. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Ning, D. Stochastic Community Assembly: Does It Matter in Microbial Ecology? Microbiol. Mol. Biol. Rev. 2017, 81, e00002-17. [Google Scholar] [CrossRef] [PubMed]
- Huang, H. linkET: Everything Is Linkable. R Package Version 0.0.3. 2021. Available online: https://github.com/Hy4m/linkET (accessed on 10 May 2025).
- Hijmans, R.J. Geosphere: Spherical Trigonometry 2010, 1.5-20. Available online: https://uribo.github.io/rpkg_showcase/spatial/geosphere.html (accessed on 10 May 2025).
- Goslee, S.C.; Urban, D.L. The Ecodist Package for Dissimilarity-Based Analysis of Ecological Data. J. Stat. Softw. 2007, 22, 1–19. [Google Scholar] [CrossRef]
- Xiao, Z.; Li, G.; Zhao, Y.; Xiao, K.; Chen, Q.; Bao, P.; Tang, J.; Ruan, T.; Zama, E.F.; Xu, Y. Bacterioplankton Richness and Composition in a Seasonal Urban River. Front. Environ. Sci. 2021, 9, 731227. [Google Scholar] [CrossRef]
- Kim, M.K.; Lim, B.-S.; Lee, C.S.; Srinivasan, S. Bacterial Diversity in the Different Ecological Niches Related to the Yonghwasil Pond (Republic of Korea). Microorganisms 2024, 12, 2547. [Google Scholar] [CrossRef]
- Garner, R.E.; Kraemer, S.A.; Onana, V.E.; Huot, Y.; Gregory-Eaves, I.; Walsh, D.A. Protist Diversity and Metabolic Strategy in Freshwater Lakes Are Shaped by Trophic State and Watershed Land Use on a Continental Scale. mSystems 2022, 7, e00316-22. [Google Scholar] [CrossRef]
- Davison, J.; Vasar, M.; Sepp, S.-K.; Oja, J.; Al-Quraishy, S.; Bueno, C.G.; Cantero, J.J.; Chimbioputo Fabiano, E.; Decocq, G.; Fraser, L.; et al. Dominance, Diversity, and Niche Breadth in Arbuscular Mycorrhizal Fungal Communities. Ecology 2022, 103, e3761. [Google Scholar] [CrossRef]
- Carscadden, K.A.; Emery, N.C.; Arnillas, C.A.; Cadotte, M.W.; Afkhami, M.E.; Gravel, D.; Livingstone, S.W.; Wiens, J.J. Niche Breadth: Causes and Consequences for Ecology, Evolution, and Conservation. Q. Rev. Biol. 2020, 95, 179–214. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, Y.; Yang, X.; Li, K.; Mai, B.; He, Z.; Wu, R. Deterministic Processes Shape Bacterial Community Assembly in a Karst River across Dry and Wet Seasons. Front. Microbiol. 2022, 13, 938490. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, Y.; Fang, H.; Wang, C.; Wang, Z.; Zhang, W.; Mai, B.; He, Z.; Wu, R.; Li, K. The Assembly, Biogeography and Co-Occurrence of Abundant and Rare Microbial Communities in a Karst River. Front. Mar. Sci. 2023, 10, 1228813. [Google Scholar] [CrossRef]
- Menéndez-Serra, M.; Ontiveros, V.J.; Cáliz, J.; Alonso, D.; Casamayor, E.O. Understanding Stochastic and Deterministic Assembly Processes in Microbial Communities along Temporal, Spatial and Environmental Scales. Mol. Ecol. 2023, 32, 1629–1638. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Ren, K.; Isabwe, A.; Chen, H.; Liu, M.; Yang, J. Stochastic Processes Shape Microeukaryotic Community Assembly in a Subtropical River across Wet and Dry Seasons. Microbiome 2019, 7, 138. [Google Scholar] [CrossRef]
- Wu, W.; Lu, H.-P.; Sastri, A.; Yeh, Y.-C.; Gong, G.-C.; Chou, W.-C.; Hsieh, C.-H. Contrasting the Relative Importance of Species Sorting and Dispersal Limitation in Shaping Marine Bacterial versus Protist Communities. ISME J. 2018, 12, 485–494. [Google Scholar] [CrossRef] [PubMed]
- Aguilar, P.; Sommaruga, R. The Balance between Deterministic and Stochastic Processes in Structuring Lake Bacterioplankton Community over Time. Mol. Ecol. 2020, 29, 3117–3130. [Google Scholar] [CrossRef]
- Hou, D.; Huang, Z.; Zeng, S.; Liu, J.; Wei, D.; Deng, X.; Weng, S.; He, Z.; He, J. Environmental Factors Shape Water Microbial Community Structure and Function in Shrimp Cultural Enclosure Ecosystems. Front. Microbiol. 2017, 8, 2359. [Google Scholar] [CrossRef]
- Liu, Y.; Ding, C.; Li, X.; Su, D.; He, J. Biotic Interactions Contribute More than Environmental Factors and Geographic Distance to Biogeographic Patterns of Soil Prokaryotic and Fungal Communities. Front. Microbiol. 2023, 14, 1134440. [Google Scholar] [CrossRef]
- Marshall, I.P.G.; Ren, G.; Jaussi, M.; Lomstein, B.A.; Jørgensen, B.B.; Røy, H.; Kjeldsen, K.U. Environmental Filtering Determines Family-Level Structure of Sulfate-Reducing Microbial Communities in Subsurface Marine Sediments. ISME J. 2019, 13, 1920–1932. [Google Scholar] [CrossRef]
- Peng, J.; Wang, D.; He, P.; Wei, P.; Zhang, L.; Lan, W.; Li, Y.; Chen, W.; Zhao, Z.; Jiang, L.; et al. Exploring the Environmental Influences and Community Assembly Processes of Bacterioplankton in a Subtropical Coastal System: Insights from the Beibu Gulf in China. Environ. Res. 2024, 259, 119561. [Google Scholar] [CrossRef]
- David, A.S.; Hernandez, D.J.; Menges, E.S.; Sclater, V.L.; Afkhami, M.E.; Searcy, C.A. Heterogeneous Landscape Promotes Distinct Microbial Communities in an Imperiled Scrub Ecosystem. Mycologia 2023, 115, 739–748. [Google Scholar] [CrossRef]
- Feng, M.; Tripathi, B.M.; Shi, Y.; Adams, J.M.; Zhu, Y.-G.; Chu, H. Interpreting Distance-Decay Pattern of Soil Bacteria via Quantifying the Assembly Processes at Multiple Spatial Scales. MicrobiologyOpen 2019, 8, e00851. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, S.; Wang, J.; Huang, Y.; Freedman, Z.; Fu, S.; Liu, K.; Wang, H.; Li, X.; Yao, M.; et al. Local Community Assembly Mechanisms Shape Soil Bacterial β Diversity Patterns along a Latitudinal Gradient. Nat. Commun. 2020, 11, 5428. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Yang, Y.; Feng, J.; Tian, R.; Guo, X.; Ning, D.; Hale, L.; Wang, M.; Cheng, J.; Wu, L.; et al. The Spatial Scale Dependence of Diazotrophic and Bacterial Community Assembly in Paddy Soil. Glob. Ecol. Biogeogr. 2019, 28, 1093–1105. [Google Scholar] [CrossRef]
- Pearman, W.S.; Duffy, G.A.; Liu, X.P.; Gemmell, N.J.; Morales, S.E.; Fraser, C.I. Macroalgal Microbiome Biogeography Is Shaped by Environmental Drivers Rather than Geographical Distance. Ann. Bot. 2024, 133, 169–182. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Liu, Z.; Li, J.; Meng, D.; Yuan, H.; Zhang, M.; Zhang, H.; Yin, H.; Cong, J.; Xiao, N. Body Size as Key Trait Determining Aquatic Metacommunity Assemblies in Benthonic and Planktonic Habitats of Dongting Lake, China. Ecol. Indic. 2022, 143, 109355. [Google Scholar] [CrossRef]
- Li, Z.; Zhu, H.; García-Girón, J.; Gu, S.; Heino, J.; Xiong, X.; Yang, J.; Zhao, X.; Jia, Y.; Xie, Z.; et al. Historical and Dispersal Processes Drive Community Assembly of Multiple Aquatic Taxa in Glacierized Catchments in the Qinghai-Tibet Plateau. Environ. Res. 2024, 251, 118746. [Google Scholar] [CrossRef]




| Bacteria | Protist | |||
|---|---|---|---|---|
| r2 | p-Value | r2 | p-Value | |
| Temperature | 0.876 | 0.001 | 0.064 | 0.756 |
| DO | 0.546 | 0.048 | 0.499 | 0.091 |
| SPC | 0.903 | 0.002 | 0.219 | 0.418 |
| TDS | 0.903 | 0.002 | 0.219 | 0.418 |
| pH | 0.943 | 0.001 | 0.363 | 0.188 |
| ORP | 0.030 | 0.894 | 0.347 | 0.231 |
| Ammonia | 0.260 | 0.352 | 0.243 | 0.405 |
| Nitrate | 0.423 | 0.156 | 0.069 | 0.718 |
| TP | 0.239 | 0.391 | 0.574 | 0.055 |
| Transparent | 0.273 | 0.315 | 0.413 | 0.167 |
| Longitude | 0.877 | 0.002 | 0.748 | 0.027 |
| Latitude | 0.549 | 0.066 | 0.287 | 0.280 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Chen, W.; Han, Y.; Lei, J.; Huang, B.; Qin, Y.; Lin, F.; Li, C.; Wang, D.; Zhou, L. Taxon-Dependent Community Assembly of Bacteria and Protists in River Ecosystems: A Case Study from the Yujiang River. Microorganisms 2025, 13, 1650. https://doi.org/10.3390/microorganisms13071650
Li Y, Chen W, Han Y, Lei J, Huang B, Qin Y, Lin F, Li C, Wang D, Zhou L. Taxon-Dependent Community Assembly of Bacteria and Protists in River Ecosystems: A Case Study from the Yujiang River. Microorganisms. 2025; 13(7):1650. https://doi.org/10.3390/microorganisms13071650
Chicago/Turabian StyleLi, Yusen, Wenjian Chen, Yaoquan Han, Jianjun Lei, Bo Huang, Youjie Qin, Feng Lin, Caijin Li, Dapeng Wang, and Lei Zhou. 2025. "Taxon-Dependent Community Assembly of Bacteria and Protists in River Ecosystems: A Case Study from the Yujiang River" Microorganisms 13, no. 7: 1650. https://doi.org/10.3390/microorganisms13071650
APA StyleLi, Y., Chen, W., Han, Y., Lei, J., Huang, B., Qin, Y., Lin, F., Li, C., Wang, D., & Zhou, L. (2025). Taxon-Dependent Community Assembly of Bacteria and Protists in River Ecosystems: A Case Study from the Yujiang River. Microorganisms, 13(7), 1650. https://doi.org/10.3390/microorganisms13071650






