Validation of a Real-Time PCR Assay for Fully Automated Detection of Bacillus cereus in Donor Human Milk
Abstract
1. Introduction
2. Materials and Methods
2.1. Donor Human Milk Samples
2.1.1. Donor Screening Criteria and Donor Human Milk Processing
2.1.2. Samples and Controls Preparation
2.2. Microorganisms
2.3. RT-PCR Detection of Bacillus cereus
2.3.1. Primers and Probes
2.3.2. Manual Procedure
2.3.3. Automated Procedure
2.4. BC Test Performance Assesment
2.5. Pre-Pasteurization Milk Culture
- Total aerobic microorganisms: ≤1 × 105 colony-forming units (CFU) per milliliter (mL);
- Staphylococcus aureus: ≤1 × 104 CFU/mL;
- Enterobacteriaceae: ≤1 × 104 CFU/mL;
- Bacillus cereus: <1 CFU/mL.
2.6. Quality System
2.7. Data Analysis
3. Results
3.1. Optimization of BC Test in cobas® 6800 System
- Raw milk sample (1.5 mL), extraction volume 850 µL;
- Centrifuge 1 mL of milk and remove 450 µL, leaving 550 µL for extraction volume of 350 µL;
- Centrifuge 1 mL of milk and remove 600 µL, leaving 400 µL, which is supplemented with 150 µL of sterile water to account for dead volume, for an extraction volume of 350 µL;
- Centrifuge 1 mL of milk and remove 600 µL, leaving 400 µL for an extraction volume of 150 µL;
- Centrifuge 1 mL of milk and remove 750 µL, leaving 250 µL, which is supplemented with 150 µL of sterile water to account for dead volume, for an extraction volume of 150 µL.
3.2. Analytical Sensitivity and Specificity
3.3. Cross-Contamination
3.4. Precision
3.5. Linearity, Limit of Detection and Correlation with Bacterial Concentration
3.6. Comparative Analysis of BC Test and BACARA® Plate to Detect Bacillus cereus in Donor Human Milk
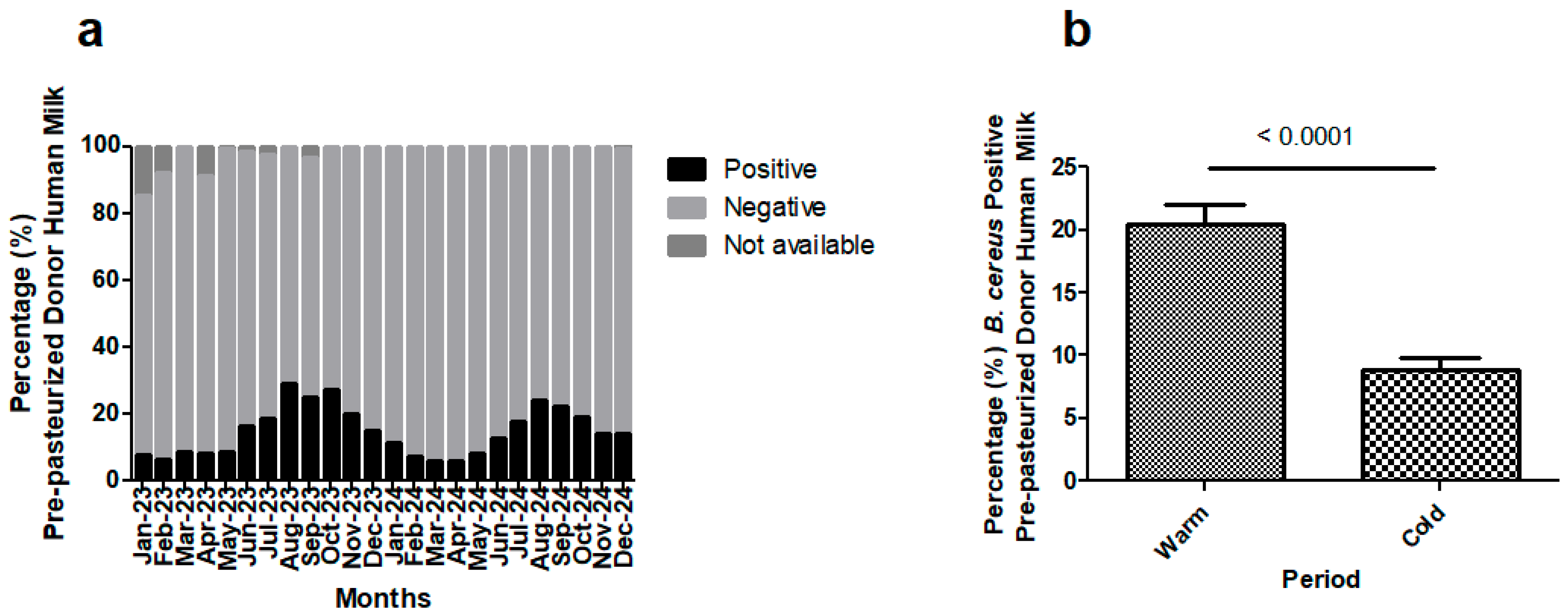
3.7. Routine Performance of BC Test: Detection of Bacillus cereus in Pre-Pasteurization Donor Human Milk
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| RT-PCR | real-time polymerase chain reaction |
| DHM | donor human milk |
| Ct | cycle threshold |
| HMB | human milk bank |
| HP | Holder pasteurization |
| BC test | RT-PCR specific for Bacillus cereus using the cobas® 6800 system |
References
- WHO Recommendations for Care of the Preterm or Low Birth Weight Infant; World Health Organization: Geneva, Switzerland, 2022; Licence: CC BY-NC-SA 3.0 IGO; Available online: https://www.who.int/publications/i/item/9789240058262 (accessed on 4 July 2025).
- Weaver, G.; Bertino, E.; Gebauer, C.; Grovslien, A.; Mileusnic-Milenovic, R.; Arslanoglu, S.; Barnett, D.; Boquien, C.Y.; Buffin, R.; Gaya, A.; et al. Recommendations for the Establishment and Operation of Human Milk Banks in Europe: A Consensus Statement From the European Milk Bank Association (EMBA). Front. Pediatr. 2019, 7, 53. [Google Scholar] [CrossRef]
- Juffs, H.; Deeth, H. Scientific Evaluation of Pasteurization for Pathogen Reduction in Milk and Milk Products. Food Standards. 2007. Available online: https://www.foodstandards.gov.au/sites/default/files/food-standards-code/proposals/Documents/Scientific%20Evaluation.pdf (accessed on 4 July 2025).
- Liao, S.L.; Tsai, M.H. Bacillus cereus bacteremia in a preterm infant caused by consumption of contaminated breastmilk. Pediatr. Neonatol. 2021, 62, 337–338. [Google Scholar] [CrossRef]
- Berthold-Pluta, A.; Pluta, A.; Garbowska, M. The effect of selected factors on the survival of Bacillus cereus in the human gastrointestinal tract. Microb Pathog. 2015, 82, 7–14. [Google Scholar] [CrossRef]
- Jessberger, N.; Dietrich, R.; Granum, P.E.; Märtlbauer, E. The Bacillus cereus Food Infection as Multifactorial Process. Toxins 2020, 12, 701. [Google Scholar] [CrossRef]
- McDowell, R.H.; Sands, E.M.; Friedman, H. Bacillus Cereus. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK459121/ (accessed on 23 January 2023).
- Decousser, J.W.; Ramarao, N.; Duport, C.; Dorval, M.; Bourgeois-Nicolaos, N.; Guinebretière, M.H.; Razafimahefa, H.; Doucet-Populaire, F. Bacillus cereus and severe intestinal infections in preterm neonates: Putative role of pooled breast milk. Am. J. Infect. Control. 2013, 41, 918–921. [Google Scholar] [CrossRef]
- Blackshaw, K.; Valtchev, P.; Koolaji, N.; Berry, N.; Schindeler, A.; Dehghani, F.; Banati, R.B. The risk of infectious pathogens in breast-feeding, donated human milk and breast milk substitutes. Public Health Nutr. 2021, 24, 1725–1740. [Google Scholar] [CrossRef]
- Cormontagne, D.; Rigourd, V.; Vidic, J.; Rizzotto, F.; Bille, E.; Ramarao, N. Bacillus cereus Induces Severe Infections in Preterm Neonates: Implication at the Hospital and Human Milk Bank Level. Toxins 2021, 13, 123. [Google Scholar] [CrossRef]
- Moro, G.E.; Billeaud, C.; Rachel, B.; Calvo, J.; Cavallarin, L.; Christen, L.; Escuder-Vieco, D.; Gaya, A.; Lembo, D.; Wesolowska, A.; et al. Processing of Donor Human Milk: Update and Recommendations From the European Milk Bank Association (EMBA). Front. Pediatr. 2019, 7, 49. [Google Scholar] [CrossRef]
- HMBANA Standards for Donor Human Milk Banking: An Overview. Human Milk Banking Association of North America. 2024. Available online: https://www.hmbana.org/news/blog.html/article/2024/01/24/hmbana-unveils-updated-2024-public-standards-to-safeguard-donor-human-milk (accessed on 4 July 2025).
- Escuder-Vieco, D.; Espinosa-Martos, I.; Rodríguez, J.M.; Corzo, N.; Montilla, A.; Siegfried, P.; Pallás-Alonso, C.R.; Fernández, L. High-Temperature Short-Time Pasteurization System for Donor Milk in a Human Milk Bank Setting. Front. Microbiol. 2018, 9, 926. [Google Scholar] [CrossRef]
- Herson, M.; Weaver, G. A comparative review of human milk banking and national tissue banking programs. Matern Child Nutr. 2024, 20 (Suppl. 4), e13584. [Google Scholar] [CrossRef]
- Kontopodi, E.; Arslanoglu, S.; Bernatowicz-Lojko, U.; Bertino, E.; Bettinelli, M.E.; Buffin, R.; Cassidy, T.; van Elburg, R.M.; Gebauer, C.; Grovslien, A.; et al. “Donor milk banking: Improving the future”. A survey on the operation of the European donor human milk banks. PLoS ONE 2021, 16, e0256435. [Google Scholar] [CrossRef] [PubMed]
- Regulation-2024/1938; Regulation (EU) 2024/1938 of the European Parliament and of the Council of 13 June 2024 on Standards of Quality and Safety for Substances of Human Origin Intended for Human Application and Repealing Directives 2002/98/EC and 2004/23/ECText with EEA Relevance. Office Journal of the European Union: Luxembourg, 2024. Available online: http://data.europa.eu/eli/reg/2024/1938/oj (accessed on 4 July 2025).
- European Directorate for the Quality of Medicines and Healthcare. Guide to the Quality and Safety of Tissues and Cells for Human Application, 5th ed.; European Directorate for the Quality of Medicines and Healthcare: Strasbourg, France, 2022; Available online: https://www.edqm.eu/en/guide-to-the-quality-and-safety-of-tissues-and-cells-for-human-application1 (accessed on 4 July 2025).
- Unger, S.L.; O’Connor, D.L. Review of current best practices for human milk banking. Matern. Child Nutr. 2024, 20, e13657. [Google Scholar] [CrossRef]
- Lewin, A.; Delage, G.; Bernier, F.; Germain, M. Banked Human Milk and Quantitative Risk Assessment of Bacillus cereus Infection in Premature Infants: A Simulation Study. Can. J. Infect. Dis. Med. Microbiol. 2019, 2019, 6348281. [Google Scholar] [CrossRef] [PubMed]
- Jandová, M.; Měřička, P.; Fišerová, M.; Landfeld, A.; Paterová, P.; Hobzová, L.; Jarkovská, E.; Kacerovský, M.; Houška, M. Bacillus cereus as a Major Cause of Discarded Pasteurized Human Banked Milk: A Single Human Milk Bank Experience. Foods 2021, 10, 2955. [Google Scholar] [CrossRef] [PubMed]
- Billeaud, C. High Hydrostatic Pressure Treatment Ensures the Microbiological Safety of Human Milk Including Bacillus cereus and Preservation of Bioactive Proteins Including Lipase and Immuno-Proteins: A Narrative Review. Foods 2021, 10, 1327. [Google Scholar] [CrossRef]
- Mullié, C.; Obin, O.; Outurquin, G.; Grognet, S.; Léké, A.; Adjidé, C. Breastmilk donations: Bacteriological assessment, analysis of causes of non-compliance and suggestions for improvement. Arch Pediatr. 2018, 25, 263–268. [Google Scholar] [CrossRef]
- Almutawif, Y.; Hartmann, B.; Lloyd, M.; Erber, W.; Geddes, D. A retrospective audit of bacterial culture results of donated human milk in Perth, Western Australia. Early Hum. Dev. 2017, 105, 1–6. [Google Scholar] [CrossRef]
- Fuchs, E.; Raab, C.; Brugger, K.; Ehling-Schulz, M.; Wagner, M.; Stessl, B. Performance Testing of Bacillus cereus Chromogenic Agar Media for Improved Detection in Milk and Other Food Samples. Foods 2022, 11, 288. [Google Scholar] [CrossRef]
- Martínez-Blanch, J.F.; Sánchez, G.; Garay, E.; Aznar, R. Development of a real-time PCR assay for detection and quantification of enterotoxigenic members of Bacillus cereus group in food samples. Int. J. Food Microbiol. 2009, 135, 15–21. [Google Scholar] [CrossRef]
- Cattani, F.; Barth, V.C.; Nasário, J.S.R.; Ferreira, C.A.S.; Oliveira, S.D. Detection and quantification of viable Bacillus cereus group species in milk by propidium monoazide quantitative real-time PCR. J. Dairy Sci. 2016, 99, 2617–2624. [Google Scholar] [CrossRef]
- Liang, L.; Wang, P.; Qu, T.; Zhao, X.; Ge, Y.; Chen, Y. Detection and quantification of Bacillus cereus and its spores in raw milk by qPCR, and distinguish Bacillus cereus from other bacteria of the genus Bacillus. Food Qual. Saf. 2022, 6, fyab035. [Google Scholar] [CrossRef]
- Ramarao, N.; Tran, S.L.; Marin, M.; Vidic, J. Advanced Methods for Detection of Bacillus cereus and Its Pathogenic Factors. Sensors 2020, 20, 2667. [Google Scholar] [CrossRef]
- COBAS 6800 System 2024. Available online: https://diagnostics.roche.com/es/es/products/instruments/cobas-6800-ins-2693.html (accessed on 4 July 2025).
- International Council for Harmonisation of Technical Requirements of Pharmaceuticals for Human Use. ICH Q14 Guideline on Analytical Procedure Development—Step 5. EMA/CHMP/ICH/195040/2022. 2024. Available online: https://www.ema.europa.eu/en/ich-q14-analytical-procedure-development-scientific-guideline (accessed on 4 July 2025).
- International Council for Harmonisation of Technical Requirements of Pharmaceuticals for Human Use. ICH Q2(R2) Guideline on Validation of Analytical Procedures—Step 5—Revision 1. EMA/CHMP/ICH/82072/2006. 2023. Available online: https://www.ema.europa.eu/en/ich-q2r2-validation-analytical-procedures-scientific-guideline (accessed on 4 July 2025).
- Park, Y.; Roh, J.; Kim, S. Performance Evaluation of the Aptima Assays in Comparison with the cobas 6800 Assays for the Detection of HIV-1, HBV, and HCV in Clinical Samples. Ann. Lab. Med. 2022, 42, 447–456. [Google Scholar] [CrossRef] [PubMed]
- Alternative Methods for Control of Microbiological Quality, 11th ed.; Council of Europe: Strasbourg, France, 2025; Chapter 5.1.6.
- Landis, J.R.; Koch, G.G. The measurement of observer agreement for categorical data. Biometrics 1977, 33, 159–174. [Google Scholar] [CrossRef]
- Song, J.; Kim, S.; Kwak, E.; Park, Y. Routine breast milk monitoring using automated molecular assay system reduced postnatal CMV infection in preterm infants. Front. Microbiol. 2023, 14, 1257124. [Google Scholar] [CrossRef] [PubMed]
- Schraft, H.; Griffiths, M.W. Specific Oligonucleotide Primers for Detection of Lecithinase-Positive Bacillus spp. by PCR. Appl. Environ. Microbiol. 1995, 61, 98–102. [Google Scholar] [CrossRef]
- Fritzsche, A.; Berneking, L.; Nörz, D.; Reucher, S.; Fischer, N.; Roggenkamp, H.; Aepfelbacher, M.; Rohde, H.; Pfefferle, S.; Lütgehetmann, M. Clinical evaluation of a laboratory-developed quantitative BK virus-PCR assay using the cobas® omni Utility Channel. J. Virol. Methods 2021, 290, 114093. [Google Scholar] [CrossRef]
- Yossa, N.; Huang, S.; Canida, T.; Binet, R.; Macarisin, D.; Bell, R.; Tallent, S.; Brown, E.; Hammack, T. qPCR detection of viable Bacillus cereus group cells in cosmetic products. Sci. Rep. 2023, 13, 4477. [Google Scholar] [CrossRef]
- Zhou, P.; Xie, G.; Liang, T.; Yu, B.; Aguilar, Z.; Xu, H. Rapid and quantitative detection of viable emetic Bacillus cereus by PMA-qPCR assay in milk. Mol. Cell Probes 2019, 47, 101437. [Google Scholar] [CrossRef]
- Yu, S.; Yan, L.; Wu, X.; Li, F.; Wang, D.; Xu, H. Multiplex PCR coupled with propidium monoazide for the detection of viable Cronobacter sakazakii, Bacillus cereus, and Salmonella spp. in milk and milk products. J. Dairy Sci. 2017, 100, 7874–7882. [Google Scholar] [CrossRef]
- Zhang, Z.; Feng, L.; Xu, H.; Liu, C.; Shah, N.P.; Wei, H. Detection of viable enterotoxin-producing Bacillus cereus and analysis of toxigenicity from ready-to-eat foods and infant formula milk powder by multiplex PCR. J. Dairy Sci. 2016, 99, 1047–1055. [Google Scholar] [CrossRef] [PubMed]
- Yang, I.C.; Shih, D.Y.C.; Wang, J.Y.; Pan, T.M. Development of Rapid Real-Time PCR and Most-Probable-Number Real-Time PCR Assays to Quantify Enterotoxigenic Strains of the Species in the Bacillus cereus Group. J. Food Prot. 2007, 70, 2774–2781. [Google Scholar] [CrossRef]
- Tallent, S.M.; Kotewicz, K.M.; Strain, E.A.; Bennett, R.W. Efficient Isolation and Identification of Bacillus cereus Group. J. AOAC Int. 2012, 95, 446–451. [Google Scholar] [CrossRef]
- Kabir, M.S.; Hsieh, Y.H.; Simpson, S.; Kerdahi, K.; Sulaiman, I.M. Evaluation of Two Standard and Two Chromogenic Selective Media for Optimal Growth and Enumeration of Isolates of 16 Unique Bacillus Species. J. Food Prot. 2017, 80, 952–962. [Google Scholar] [CrossRef]
- Cobb, B.; Simon, C.O.; Stramer, S.L.; Body, B.; Mitchell, P.S.; Reisch, N.; Stevens, W.; Carmona, S.; Katz, L.; Will, S.; et al. The cobas® 6800/8800 System: A new era of automation in molecular diagnostics. Expert. Rev. Mol. Diagn. 2017, 17, 167–180. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, M.; Arcenas, R.; Couto-Parada, X.; Lewinski, M.; Njoya, M.; Perinpanathan, D.; Sheriff, R.; Hansra, A.; Singh, S. PivNG primers and probes set used in the cobas omni Utility Channel is a reliable supplemental test for detection of Neisseria gonorrhoeae in oropharyngeal, urogenital and rectal specimens collected in cobas PCR Media. Sex. Transm. Infect. 2023, 99, 416–419. [Google Scholar] [CrossRef] [PubMed]
- Miura, K.; Tanaka, M.; Date, M.; Ito, M.; Mizuno, N.; Mizuno, K. Comparison of bacterial profiles in human milk from mothers of term and preterm infants. Int. Breastfeed J. 2023, 18, 29. [Google Scholar] [CrossRef]
- Chang, F.Y.; Cheng, S.W.; Wu, T.Z.; Fang, L.J. Characteristics of the First Human Milk Bank in Taiwan. Pediatr. Neonatol. 2013, 54, 28–33. [Google Scholar] [CrossRef]
- Adjidé, C.C.; Léké, A.; Mullié, C. Bacillus cereus contamination of pasteurized human milk donations: Frequency, origin, seasonal distribution, molecular typing of strains and proposed corrective/preventive actions. J. Matern. Fetal. Neonatal. Med. 2022, 35, 1554–1561. [Google Scholar] [CrossRef]
- Tran, H.T.; Nguyen, T.T.; Nguyen, O.T.X.; Huynh, L.T.; Nguyen, L.T.; Nguyen, T.T.; Le, H.T.T.; Barnett, D.; Weaver, G.; Mathisen, R. Differences in the Microbiological Profile of Raw and Pasteurized Breastmilk from Hospital and Community-Based Donors at the First Human Milk Bank in Vietnam. Nutrients 2023, 15, 412. [Google Scholar] [CrossRef]
- Hauner, A.; Onwuchekwa, C.; Ariën, K.K. Sample-to-result molecular diagnostic platforms and their suitability for infectious disease testing in low- and middle-income countries. Expert. Rev. Mol. Diagn. 2024, 24, 423–438. [Google Scholar] [CrossRef] [PubMed]

| N | Extraction Volume | Matrix | Sample | BC Ct | IC Ct | Interpretation |
|---|---|---|---|---|---|---|
| 1 | 850 µL | Unprocessed milk | P | 35.20 | 39.09 | Valid |
| N | ND | ND | Invalid | |||
| 2 | 350 µL | Centrifuged milk (550 µL) | P | 34.58 | ND | Invalid |
| N | ND | 37.23 | Valid | |||
| 3 | 350 µL | Centrifuged milk (400 µL + 150 µL H2O) | P | 31.98 | 37.69 | Valid |
| N | ND | 37.19 | Valid | |||
| 4 | 150 µL | Centrifuged milk (400 µL) | P | 28.28 | 35.75 | Valid |
| N | ND | 35.19 | Valid | |||
| 5 | 150 µL | Centrifuged milk (250 µL + 150 µL H2O) | P | 27.45 | 36.38 | Valid |
| N | ND | 35.39 | Valid |
| Variability | Sample | Replicate | BC Ct | Mean Ct | SD | CV |
|---|---|---|---|---|---|---|
| Intra-run | High positive | 1 | 18.92 | 18.93 | 0.29 | 1.53 |
| 2 | 18.64 | |||||
| 3 | 19.22 | |||||
| Intra-run | Low positive | 1 | 29.21 | 29.45 | 0.25 | 0.85 |
| 2 | 29.43 | |||||
| 3 | 29.71 | |||||
| Inter-run | Positive | 1 | 27.30 | 26.58 | 0.61 | 2.31 |
| 2 | 26.30 | |||||
| 3 | 27.73 | |||||
| 4 | 27.90 | |||||
| 5 | 26.10 | |||||
| 6 | 26.80 | |||||
| 7 | 26.01 | |||||
| 8 | 26,00 | |||||
| 9 | 26.43 | |||||
| 10 | 26.30 | |||||
| 11 | 26.11 | |||||
| 12 | 26.25 | |||||
| 13 | 26.30 | |||||
| 14 | 26.32 | |||||
| 15 | 26.92 |
| BACARA® Positive | BACARA® Negative | Total | |
|---|---|---|---|
| RT-PCR Positive | 210 | 138 | 408 |
| RT-PCR Negative | 39 | 1987 | 2026 |
| Total | 309 | 2125 | 2434 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aran, G.; Pleguezuelos, V.; Blanco, M.; Garcia, C.; Jallow, M.; López, M.; Monge, S.; Casamitjana, N.; Alonso-Nogués, E.; Soria, G. Validation of a Real-Time PCR Assay for Fully Automated Detection of Bacillus cereus in Donor Human Milk. Microorganisms 2025, 13, 1640. https://doi.org/10.3390/microorganisms13071640
Aran G, Pleguezuelos V, Blanco M, Garcia C, Jallow M, López M, Monge S, Casamitjana N, Alonso-Nogués E, Soria G. Validation of a Real-Time PCR Assay for Fully Automated Detection of Bacillus cereus in Donor Human Milk. Microorganisms. 2025; 13(7):1640. https://doi.org/10.3390/microorganisms13071640
Chicago/Turabian StyleAran, Gemma, Vanessa Pleguezuelos, Margarita Blanco, Cristina Garcia, Mariama Jallow, Mar López, Sara Monge, Natalia Casamitjana, Eva Alonso-Nogués, and Gloria Soria. 2025. "Validation of a Real-Time PCR Assay for Fully Automated Detection of Bacillus cereus in Donor Human Milk" Microorganisms 13, no. 7: 1640. https://doi.org/10.3390/microorganisms13071640
APA StyleAran, G., Pleguezuelos, V., Blanco, M., Garcia, C., Jallow, M., López, M., Monge, S., Casamitjana, N., Alonso-Nogués, E., & Soria, G. (2025). Validation of a Real-Time PCR Assay for Fully Automated Detection of Bacillus cereus in Donor Human Milk. Microorganisms, 13(7), 1640. https://doi.org/10.3390/microorganisms13071640





