Discovery of 4,5,6,7-Tetrahydrothieno [3,2-b] Pyridine as Novel Fungicide Lead Scaffold
Abstract
1. Introduction
2. Materials and Methods
2.1. General Information
2.2. Chemical Synthesis Procedures
2.2.1. General Method for the Synthesis of Intermediates 3a~3q
2.2.2. General Method for the Synthesis of Intermediates 4a~4q
2.2.3. General Method for the Synthesis of Intermediates 5a~5q
2.2.4. General Method for the Synthesis of Target Compounds I-1~I-17
2.2.5. General Method for the Synthesis of Target Compounds II-1~II-5
2.3. Fungicidal Activity
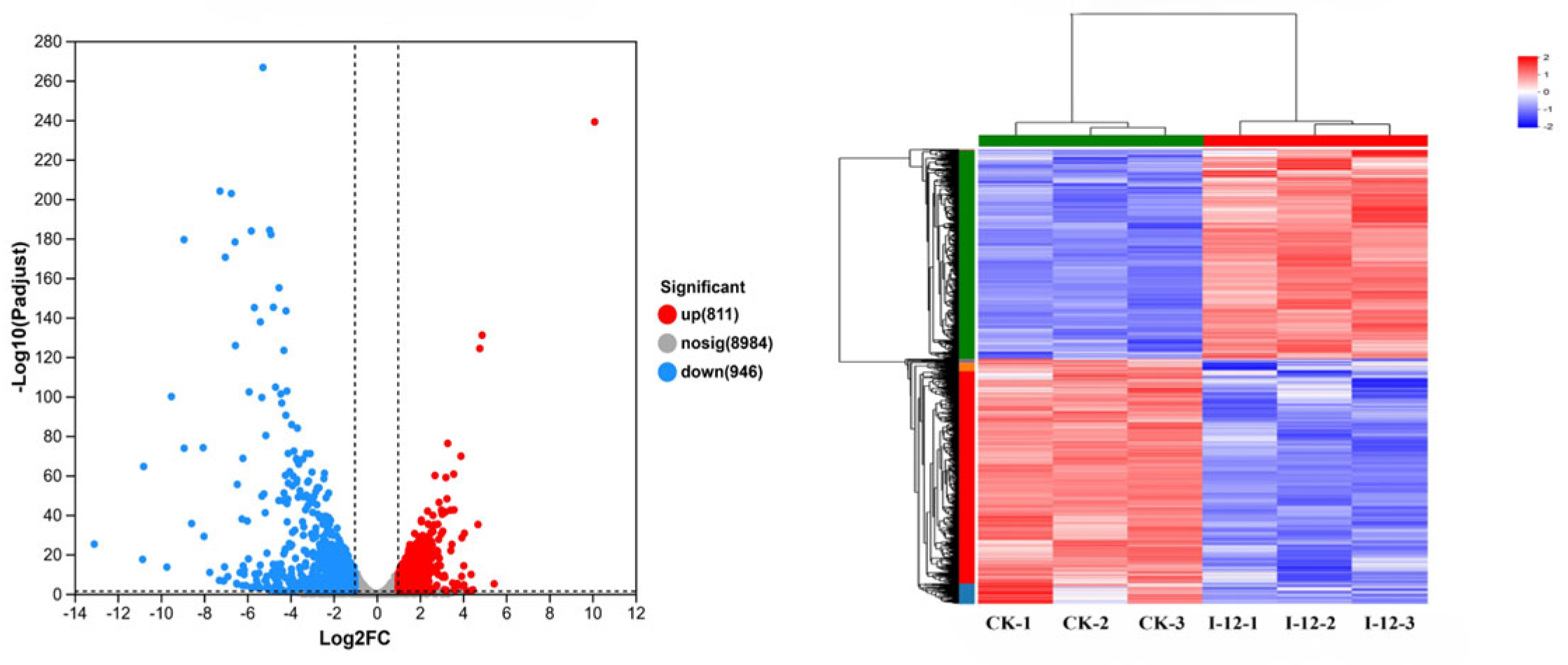
2.4. Transcriptome Analyses
2.5. Statistical Analysis
3. Results and Discussion
3.1. Chemistry
3.2. Fungicidal Activity and SAR
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jiang, X.; Yang, F.; Jia, W.; Jiang, Y.; Wu, X.; Song, S.; Shen, H.; Shen, J. Nanomaterials and Nano-technology in Agricultural Pesticide Delivery: A Review. Langmuir 2024, 40, 18806–18820. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Lv, F.; Yang, J. Pesticides Toxicity, Removal and Detoxification in Plants: A Review. Agronomy 2024, 14, 1260. [Google Scholar] [CrossRef]
- Giugliano, R.; Armenio, V.; Savio, V.; Vaccaro, E.; Ciccotelli, V.; Vivaldi, B. Monitoring of Non-Maximum-Residue-Level Pesticides in Animal Feed: A Study from 2019 to 2023. Toxics 2024, 12, 680. [Google Scholar] [CrossRef] [PubMed]
- Chio, E.H.; Li, Q.X. Pesticide Research and Development: General Discussion and Spinosad Case. J. Agric. Food Chem. 2022, 70, 8913–8919. [Google Scholar] [CrossRef]
- Clark, R.D. A Perspective on the Role of Quantitative Structure–Activity and Structure–Property Relationships in Herbicide Discovery. Pest Manag. Sci. 2012, 68, 513–518. [Google Scholar] [CrossRef]
- Barber, D.M. A Competitive Edge: Competitor Inspired Scaffold Hopping in Herbicide Lead Optimization. J. Agric. Food Chem. 2022, 70, 11075–11090. [Google Scholar] [CrossRef]
- Guan, A.; Liu, C.; Yang, X.; Dekeyser, M. Application of the Intermediate Derivatization Approach in Agrochemical Discovery. Chem. Rev. 2014, 114, 7079–7107. [Google Scholar] [CrossRef]
- Grossmann, K.; Christiansen, N.; Looser, R.; Tresch, S.; Hutzler, J.; Pollmann, S.; Ehrhardt, T. Physionomics and Metabolomics–Two Key Approaches in Herbicidal Mode of Action Discovery. Pest Manag. Sci. 2012, 68, 494–504. [Google Scholar] [CrossRef]
- Gao, W.; Li, J.; Zhang, Y.; Yuan, H.; Li, K.; Zhang, J.; Han, L.; Fan, Z.; Chen, L.; Tang, L. Pyruvate Kinase-Based Novel 2-Thiazol-2-yl-1,3,4-oxadiazoles Discovery as Fungicidal Highly Active Leads. J. Agric. Food Chem. 2025, 73, 1075–1085. [Google Scholar] [CrossRef]
- Zhao, B.; Liu, N.; Chen, L.; Geng, S.; Fan, Z.J.; Xing, J.H. Direct Label-Free Methods for Identification of Target Proteins in Agrochemicals. Int. J. Biol. Macromol. 2020, 164, 1475–1483. [Google Scholar] [CrossRef]
- Molina, D.M.; Jafari, R.; Ignatushchenko, M.; Seki, T.; Larsson, E.A.; Dan, C.; Sreekumar, L.; Cao, Y.H.; Nordlund, P. Monitoring drug target engagement in cells and tissues using the cellular thermal shift assay. Science 2013, 341, 84–87. [Google Scholar] [CrossRef] [PubMed]
- Martinez, M.D.; Nordlund, P. The cellular thermal shift assay: A novel biophysical assay for in situ drug target engagement and mechanistic biomarker studies. Annu. Rev. Pharmacol. 2016, 56, 141–161. [Google Scholar] [CrossRef]
- Savitski, M.M.; Reinhard, F.B.M.; Franken, H.; Werner, T.; Savitski, M.F.; Eberhard, D.; Molina, D.M.; Jafari, R.; Dovega, R.B.; Klaeger, S.; et al. Tracking cancer drugs in living cells by thermal profiling of the proteome. Science 2014, 346, 1255784. [Google Scholar] [CrossRef] [PubMed]
- Chin, R.M.; Fu, X.; Pai, M.Y.; Vergnes, L.; Hwang, H.; Deng, G.; Diep, S.; Lomenick, B.; Meli, V.S.; Monsalve, G.C.; et al. The metabolite α-ketoglutarate extends lifespan by inhibiting ATP synthase and TOR. Nature 2014, 510, 397–401. [Google Scholar] [CrossRef] [PubMed]
- West, G.M.; Tucker, C.L.; Xu, T.; Park, S.K.; Han, X.; Yates, J.R., III; Fitzgerald, M.C. Quantitative proteomics approach for identifying protein-drug interactions in complex mixtures using protein stability measurements. Proc. Natl. Acad. Sci. USA 2010, 107, 9078–9082. [Google Scholar] [CrossRef]
- Chan, J.N.Y.; Vuckovic, D.; Sleno, L.; Olsen, J.B.; Pogoutse, O.; Havugimana, P.; Hewel, J.A.; Bajaj, N.; Wang, Y.; Musteata, M.F.; et al. Target identification by chromatographic co-elution: Monitoring of drug-protein interactions without immobilization or chemical derivatization. Mol. Cell. Proteom. 2012, 11, M111.016642-1–M111.016642-14. [Google Scholar] [CrossRef]
- Škerlová, J.; Berndtsson, J.; Nolte, H.; Ott, M.; Stenmark, P. Structure of the native pyruvate dehydrogenase complex reveals the mechanism of substrate insertion. Nat. Commun. 2021, 12, 5277. [Google Scholar] [CrossRef]
- Kochetov, G.A.; Solovjeva, O.N. Structure and functioning mechanism of transketolase. BBA-Proteins Proteom. 2014, 1844, 1608–1618. [Google Scholar] [CrossRef]
- Muñoz, M.E.; Ponce, E. Pyruvate kinase: Current status of regulatory and functional properties. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2003, 135, 197–218. [Google Scholar] [CrossRef]
- Reis, R.A.G.; Calil, F.A.; Feliciano, P.R.; Pinheiro, M.P.; Nonato, M.C. The dihydroorotate dehydrogenases: Past and present. Arch. Biochem. Biophys. 2017, 632, 175–191. [Google Scholar] [CrossRef]
- Ridley, S.M.; Elliott, A.C.; Yeung, M.; Youle, D. High-throughput screening as a tool for agrochemical discovery: Automated synthesis, compound input, assay design and process management. Pestic. Sci. 1998, 54, 327–333. [Google Scholar] [CrossRef]
- Srivastava, B.K.; Solanki, M.; Mishra, B.; Soni, R.; Jayadev, S.; Valani, D.; Jain, M.; Patel, P.R. Synthesis and antibacterial activity of 4,5,6,7-tetrahydro-thieno[3,2-c]pyridine quinolones. Bioorg. Med. Chem. Lett. 2007, 17, 1924–1929. [Google Scholar] [CrossRef] [PubMed]
- Moloney, G.P. Methyl 3-Hydroxythieno[2,3-b]pyridine-2-carboxylate. Molecules 2001, 6, M203. [Google Scholar] [CrossRef]
- Pinheiro, L.C.; Borges, J.C.; Oliveira, C.D.; Ferreira, V.F.; Romeiro, G.A.; Marques, I.P.; Abreu, P.A.; Frugulhetti, I.; Rodrigues, C.R.; Albuquerque, M.G. Synthesis of new 4-(phenylamino)thieno[2,3-b]pyridines and derivatives of the novel benzo[b]thieno[3,2-h]-1,6-naphthyridine tetracyclic system. Arkivoc 2008, 14, 77–87. [Google Scholar] [CrossRef]
- Pérez-Areales, F.J.; Betari, N.; Viayna, A.; Pont, C.; Espargaró, A.; Bartolini, M.; De Simone, A.; Alvarenga, J.F.R.; Pérez, B.; Sabate, R. Design, synthesis and multitarget biological profiling of second-generation anti-Alzheimer rhein–huprine hybrids. Future Med. Chem. 2017, 9, 965–981. [Google Scholar] [CrossRef]
- Lodha, K.K.; Wavhal, D.S.; Bhalekar, S.B.; Meshram, R.J.; Shinde, V.S. Exploring new tetrahydrothienopyridine derivatives as platelet agglutination inhibitors: Synthesis, biological evaluation and in silico study. ChemistrySelect 2022, 7, e202103428. [Google Scholar] [CrossRef]
- Ionescu, A.; Cornut, D.; Soriano, S.; Guissart, C.; Van Antwerpen, P.; Jabin, I. Efficient ‘one-pot’ methodology for the synthesis of novel tetrahydro-β-carboline, tetrahydroisoquinoline and tetrahydrothienopyridine derivatives. Tetrahedron Lett. 2013, 54, 6087–6089. [Google Scholar] [CrossRef]
- Rossetti, A.; Bono, N.; Candiani, G.; Meneghetti, F.; Roda, G.; Sacchetti, A. Synthesis and antimicrobial evaluation of novel chiral 2-amino-4,5,6,7-tetrahydrothieno[2,3-c]pyridine derivatives. Chem. Biodivers. 2019, 16, e1900097. [Google Scholar] [CrossRef]
- Sangshetti, J.N.; Zambare, A.S.; Khan, F.A.K.; Gonjari, I.; Zaheer, Z. Synthesis and biological activity of substituted-4,5,6,7-tetrahydrothieno pyridines: A review. Mini-Rev. Med. Chem. 2014, 14, 988–1020. [Google Scholar] [CrossRef]
- Wang, S.; Liu, H.; Wang, X.; Lei, K.; Li, G.; Li, X.; Wei, L.; Quan, Z. Synthesis and evaluation of anticonvulsant activities of 7-phenyl-4,5,6,7-tetrahydrothieno[3,2-b]pyridine derivatives. Arch. Pharm. Chem. Life Sci. 2019, 352, 1900106. [Google Scholar] [CrossRef]
- Wang, S.; Liu, H.; Wang, X.; Lei, K.; Li, G.; Quan, Z. Synthesis and evaluation of antidepressant activities of 5-aryl-4,5-dihydrotetrazolo[1,5-a]thieno[2,3-e]pyridine derivatives. Molecules 2019, 24, 1857. [Google Scholar] [CrossRef]
- Price, C.L.; Parker, J.E.; Warrilow, A.G.S.; Kelly, D.E.; Kelly, S.L. Azole fungicides—Understanding resistance mechanisms in agricultural fungal pathogens. Pest Manag. Sci. 2015, 71, 1054–1058. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-S.; Chun, S.-J.; Jeon, J.-J.; Lee, S.-W.; Joe, G.-H. Synthesis and fungicidal activity of ethaboxam against Oomycetes. Pest Manag. Sci. 2004, 60, 1007–1012. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.; Wang, Y.; Hou, Y.; Zhou, M. Activity of the succinate dehydrogenase inhibitor fungicide penthiopyrad against Sclerotinia sclerotiorum. Plant Dis. 2020, 104, 2696–2703. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Gao, B.; He, Z.; Li, L.; Zhang, Q.; Kaziem, A.E.; Wang, M. Stereoselective bioactivity of the chiral triazole fungicide prothioconazole and its metabolite. Pestic. Biochem. Physiol. 2019, 160, 112–118. [Google Scholar] [CrossRef]
- Ghosh, S.; Gupta, S.K.; Jha, G. Identification and functional analysis of AG1-IA specific genes of Rhizoctonia solani. Curr. Genet. 2014, 60, 327–341. [Google Scholar] [CrossRef]
- Lv, Y.; Liu, H.L.; Wang, L.F.; Li, K.; Gao, W.; Liu, X.Y.; Tang, L.F.; Kalinina, T.A.; Glukhareva, T.V.; Fan, Z.J. Discovery of novel 3,4-dichloroisothiazole-containing coumarins as fungicidal leads. J. Agric. Food Chem. 2021, 69, 4253–4262. [Google Scholar] [CrossRef]
- Gao, W.; Zhang, J.; Zhang, Y.; Huang, Y.T.; Wang, C.L.; Liang, Q.M.; Yu, Z.C.; Fan, R.H.; Tang, L.F.; Fan, Z.J. CoMFA directed molecular design for significantly improving fungicidal activity of novel [1,2,4]-triazolo-[3,4-b][1,3,4]-thiadiazoles. J. Agric. Food Chem. 2023, 71, 14125–14136. [Google Scholar] [CrossRef]





| Comp. | A. s 2 | B. c 2 | C. a 2 | F. g 2 | P. p 2 | R. s 2 | S. s 2 |
|---|---|---|---|---|---|---|---|
| I-1 | 56 ± 1e | 34 ± 1h | 89 ± 1b | 43 ± 1f | 62 ± 1c | 54 ± 1g | 90 ± 1b |
| I-2 | 44 ± 1g | 58 ± 1d | 27 ± 1k | 45 ± 1d | 42 ± 1f | 17 ± 4l | 46 ± 1h |
| I-3 | 47 ± 4g | 39 ± 1g | 13 ± 1n | 17 ± 1k | 55 ± 0d | 42 ± 2i | 10 ± 1o |
| I-4 | 52 ± 1f | 24 ± 1j | 30 ± 1j | 15 ± 1k | 28 ± 1h | 65 ± 1d | 45 ± 1i |
| I-5 | 58 ± 2e | 24 ± 1j | 75 ± 1d | 24 ± 1j | 29 ± 1h | 38 ± 1i | 78 ± 1d |
| I-6 | 76 ± 1c | 64 ± 0c | 58 ± 1g | 44 ± 1e | 51 ± 0e | 76 ± 1c | 49 ± 1g |
| I-7 | 59 ± 4e | 61 ± 0c | 89 ± 1b | 61 ± 0b | 26 ± 0i | 80 ± 1c | 91 ± 1b |
| I-8 | 35 ± 1i | 47 ± 2f | 39 ± 1i | 46 ± 0d | 39 ± 1g | 51 ± 1h | 58 ± 1f |
| I-9 | 57 ± 1e | 61 ± 1c | 20 ± 1m | 45 ± 0e | 30 ± 0h | 68 ± 0d | 20 ± 0m |
| I-10 | 52 ± 1f | 36 ± 1g | 66 ± 1e | 45 ± 0e | 29 ± 1h | 54 ± 1g | 67 ± 1e |
| I-11 | 22 ± 1k | 57 ± 1e | 63 ± 1f | 63 ± 1b | 18 ± 1k | 37 ± 1i | 58 ± 1f |
| I-12 | 84 ± 1b | 76 ± 1b | 50 ± 1h | 49 ± 0c | 55 ± 0d | 79 ± 3c | 48 ± 1g |
| I-13 | 30 ± 1j | 25 ± 1j | 36 ± 1i | 36 ± 1g | 7 ± 1m | 59 ± 3f | 39 ± 1j |
| I-14 | 65 ± 1d | 48 ± 1f | 81 ± 1c | 35 ± 1h | 74 ± 1b | 41 ± 4i | 83 ± 1c |
| I-15 | 64 ± 1d | 62 ± 0c | 53 ± 1h | 33 ± 2i | 52 ± 2d | 62 ± 1e | 58 ± 0f |
| I-16 | 25 ± 1k | 34 ± 1h | 10 ± 1o | 1 ± 2l | 20 ± 2j | 59 ± 2f | 35 ± 1k |
| I-17 | 41 ± 1h | 38 ± 5g | 26 ± 1k | 35 ± 1h | 22 ± 1j | 22 ± 0k | 47 ± 2g |
| II-1 | 35 ± 1i | 47 ± 2f | 23 ± 1k | 16 ± 1k | 39 ± 1g | 26 ± 3k | 27 ± 2l |
| II-2 | 19 ± 4l | 15 ± 4k | 0q | 0l | 10 ± 1l | 31 ± 0j | 20 ± 1m |
| II-3 | 12 ± 1m | 10 ± 1l | 11 ± 2n | 0l | 21 ± 2j | 37 ± 3i | 16 ± 1n |
| II-4 | 24 ± 2k | 55 ± 1e | 22 ± 4l | 47 ± 1c | 29 ± 2h | 32 ± 4j | 40 ± 2j |
| II-5 | 30 ± 1j | 55 ± 1e | 25 ± 1k | 37 ± 1g | 23 ± 2j | 10 ± 1m | 39 ± 1j |
| Flutriafol | 100a | 97 ± 1a | 97 ± 2a | 100a | 99 ± 1a | 95 ± 1a | 95 ± 1a |
| Thifluzamide | 67 ± 2d | 32 ± 2i | 100a | 44 ± 1e | 22 ± 2j | 90 ± 1b | 15 ± 1n |
| Comp. | Fungi 1 | Regression Equation | R2 | EC50 (μg/mL) | 95% Confidence Interval (μg/mL) |
|---|---|---|---|---|---|
| I-1 | C. a | y = 3.0551 + 1.9245x | 0.9514 | 10.23 | 8.00–13.12 |
| S. s | y = 4.3421 + 0.9909x | 0.9580 | 4.61 | 3.31–6.42 | |
| I-5 | S. s | y = 4.1252 + 1.1353x | 0.9779 | 5.89 | 3.73–9.32 |
| C. a | y = 3.7874 + 1.2820x | 0.9962 | 8.83 | 8.15–9.56 | |
| I-6 | S. s | y = 4.0716 + 1.0393x | 0.9628 | 7.82 | 5.76–10.63 |
| A. s | y = 2.2428 + 1.9286x | 0.9726 | 26.89 | 21.90–33.02 | |
| I-7 | R. s | y = 2.9379 + 1.6176x | 0.9918 | 18.83 | 16.89–20.99 |
| C. a | y = 4.0862 + 1.1276x | 0.9998 | 6.46 | 6.18–6.75 | |
| S. s | y = 2.9350 + 1.6853x | 0.9984 | 16.80 | 16.02–17.62 | |
| I-12 | A. s | y = 4.2756 + 0.7412x | 0.9789 | 9.49 | 7.75–11.62 |
| B. c | y = 3.6982 + 1.1403x | 0.9696 | 13.85 | 11.37–16.88 | |
| R. s | y = 4.4158 + 0.7095x | 0.9974 | 6.66 | 6.22–7.13 | |
| I-14 | C. a | y = 3.8396 + 1.6293x | 0.9769 | 5.15 | 4.30–6.17 |
| S. s | y = 2.5793 + 2.8281x | 0.9543 | 7.18 | 5.59–9.21 | |
| P. p | y = 3.0468 + 1.5726x | 0.9843 | 17.46 | 15.06–20.24 | |
| Flutriafol | A. s | y = 5.6465 + 1.2352x | 0.9938 | 0.30 | 0.25–0.35 |
| B. c | y = 4.6342 + 0.8530x | 0.9946 | 2.68 | 2.47–2.91 | |
| C. a | y = 5.5458 + 1.2204x | 0.9845 | 0.36 | 0.29–0.44 | |
| F. g | y = 5.0119 + 1.4412x | 0.9859 | 0.98 | 0.82–1.17 | |
| P. p | y = 5.6715 + 1.2163x | 0.9880 | 0.28 | 0.23–0.34 | |
| R. s | y = 5.4127 + 0.5878x | 0.9817 | 0.20 | 0.15–0.27 | |
| S. s | y = 5.5140 + 1.1679x | 0.9868 | 0.36 | 0.31–0.43 | |
| Thifluzamide | A. s | y = 4.5223 + 0.6386x | 0.9984 | 5.60 | 5.35–5.86 |
| C. a | y = 5.0057 + 0.3451x | 0.9900 | 0.96 | 0.78–1.20 | |
| R. s | y = 5.2730 + 0.1924x | 0.9855 | 0.04 | 0.03–0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, K.; Deng, D.; Yin, Y.; Xi, D.; Park, P.; Gao, W.; Liu, R.; Lei, K. Discovery of 4,5,6,7-Tetrahydrothieno [3,2-b] Pyridine as Novel Fungicide Lead Scaffold. Microorganisms 2025, 13, 1588. https://doi.org/10.3390/microorganisms13071588
Chen K, Deng D, Yin Y, Xi D, Park P, Gao W, Liu R, Lei K. Discovery of 4,5,6,7-Tetrahydrothieno [3,2-b] Pyridine as Novel Fungicide Lead Scaffold. Microorganisms. 2025; 13(7):1588. https://doi.org/10.3390/microorganisms13071588
Chicago/Turabian StyleChen, Ke, Difan Deng, Yupeng Yin, Dongmei Xi, Phumbum Park, Wei Gao, Rui Liu, and Kang Lei. 2025. "Discovery of 4,5,6,7-Tetrahydrothieno [3,2-b] Pyridine as Novel Fungicide Lead Scaffold" Microorganisms 13, no. 7: 1588. https://doi.org/10.3390/microorganisms13071588
APA StyleChen, K., Deng, D., Yin, Y., Xi, D., Park, P., Gao, W., Liu, R., & Lei, K. (2025). Discovery of 4,5,6,7-Tetrahydrothieno [3,2-b] Pyridine as Novel Fungicide Lead Scaffold. Microorganisms, 13(7), 1588. https://doi.org/10.3390/microorganisms13071588







