Five-Year Analysis of Microbial Keratitis Incidence, Isolates, and In Vitro Antimicrobial Sensitivity in the South West of England: An Epidemiological Study
Abstract
1. Introduction
1.1. Aetiology and Epidemiology of Microbial Keratitis
1.2. Antimicrobial Susceptibility
2. Material and Methods
2.1. Data Collection
2.2. Hospital Scraping Protocols
2.3. Hospital Treatment Protocols
2.4. Ethical Approval
2.5. Statistical Analysis
3. Results
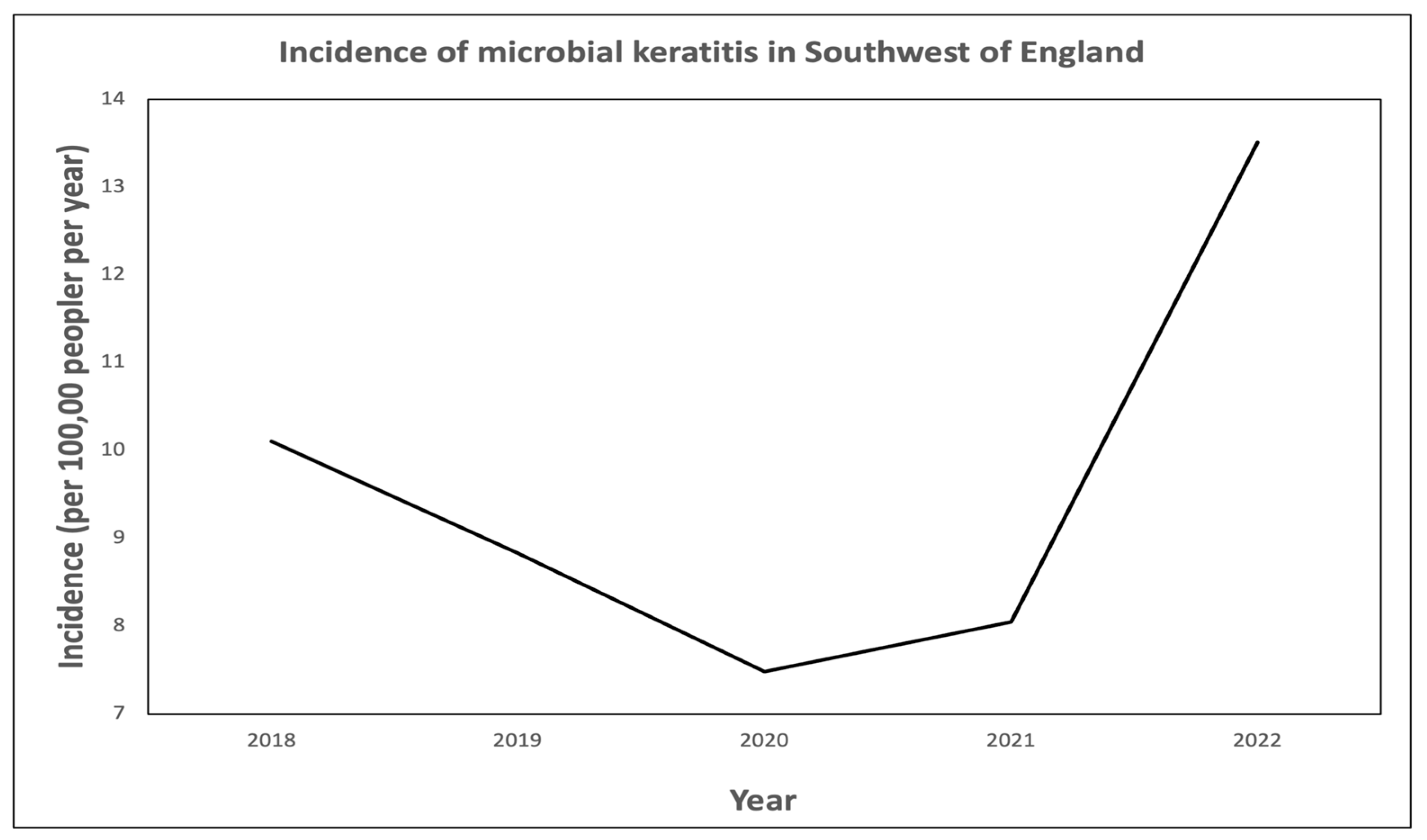
3.1. Corneal Scrape Outcomes and Incidence of Microbial Keratitis
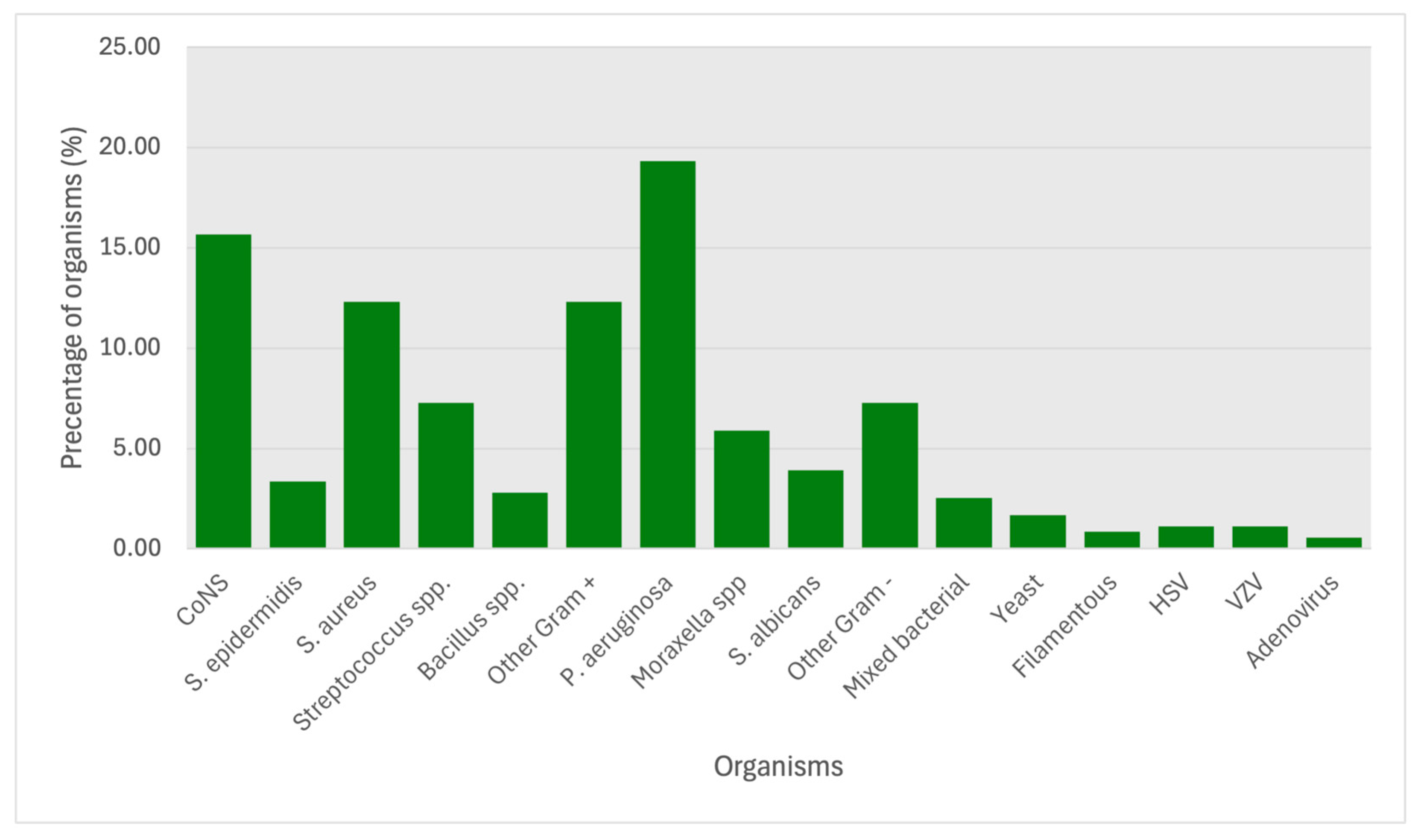
3.2. Analysis of Organisms Isolated
3.3. Antimicrobial Sensitivities
4. Discussion
4.1. Corneal Scrape Outcomes
4.2. Incidence of Microbial Keratitis
4.3. Isolate Analysis
4.4. Antimicrobial Sensitivities
4.5. Limitations
5. Conclusions
Key Points
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ung, L.; Bispo, P.J.M.; Shanbhag, S.S.; Gilmore, M.S.; Chodosh, J. The persistent dilemma of microbial keratitis: Global burden, diagnosis, and antimicrobial resistance. Surv. Ophthalmol. 2019, 64, 255–271. [Google Scholar] [CrossRef]
- Whitcher, J.P.; Srinivasan, M.; Upadhyay, M.P. Corneal blindness: A global perspective. Bull. World Health Organ. 2001, 79, 214–221. [Google Scholar] [PubMed] [PubMed Central]
- Moledina, M.; Roberts, H.W.; Mukherjee, A.; Spokes, D.; Pimenides, D.; Stephenson, C.; Bassily, R.; Rajan, M.S.; Myerscough, J. Analysis of microbial keratitis incidence, isolates and in-vitro antimicrobial susceptibility in the East of England: A 6-year study. Eye 2023, 37, 2716–2722. [Google Scholar] [CrossRef]
- Ting, D.S.J.; Ho, C.S.; Cairns, J.; Elsahn, A.; Al-Aqaba, M.; Boswell, T.; Said, D.G.; Dua, H.S. 12-year analysis of incidence, microbiological profiles and in vitro antimicrobial sensitivity of infectious keratitis: The Nottingham Infectious Keratitis Study. Br. J. Ophthalmol. 2021, 105, 328–333. [Google Scholar] [CrossRef]
- Shah, A.; Sachdev, A.; Coggon, D.; Hossain, P. Geographic variations in microbial keratitis: An analysis of the peer-reviewed liter-ature. Br. J. Ophthalmol. 2011, 95, 762–767. [Google Scholar] [CrossRef]
- Ibrahim, Y.W.; Boase, D.L.; Cree, I.A. Epidemiological characteristics, predisposing factors and microbiological profiles of infectious corneal ulcers: The Portsmouth corneal ulcer study. Br. J. Ophthalmol. 2009, 93, 1319–1324. [Google Scholar] [CrossRef]
- Bourcier, T.; Thomas, F.; Borderie, V.; Chaumeil, C.; Laroche, L. Bacterial keratitis: Predisposing factors, clinical and microbiological review of 300 cases. Br. J. Ophthalmol. 2003, 87, 834–838. [Google Scholar] [CrossRef]
- Lam, D.S.; Houang, E.; Fan, D.S.; Lyon, D.; Seal, D.; Wong, E.; Hong Kong Microbial Keratitis Study Group. Incidence and risk factors for microbial keratitis in Hong Kong: Comparison with Europe and North America. Eye 2002, 16, 608–618. [Google Scholar] [CrossRef]
- Orlans, H.O.; Hornby, S.J.; Bowler, I.C. In vitro antibiotic susceptibility patterns of bacterial keratitis isolates in Oxford, UK: A 10-year review. Eye 2011, 25, 489–493. [Google Scholar] [CrossRef]
- Goldstein, M.H.; Kowalski, R.P.; Gordon, Y.J. Emerging fluoroquinolone resistance in bacterial keratitis: A 5-year review. Ophthalmology 1999, 106, 1313–1318. [Google Scholar] [CrossRef]
- Afshari, N.A.; Ma, J.J.; Duncan, S.M.; Pineda, R.; Starr, C.E.; Decroos, F.C.; Johnson, C.S.; Adelman, R.A. Trends in resistance to ciprofloxacin, cefazolin, and gentamicin in the treatment of bacterial keratitis. J. Ocul. Pharmacol. Ther. 2008, 24, 217–223. [Google Scholar] [CrossRef]
- Stapleton, F. The epidemiology of infectious keratitis. Ocul. Surf. 2023, 28, 351–363. [Google Scholar] [CrossRef]
- Cabrera-Aguas, M.; Khoo, P.; Watson, S.L. Infectious keratitis: A review. Clin. Exp. Ophthalmol. 2022, 50, 543–562. [Google Scholar] [CrossRef]
- Suresh, L.; Hammoudeh, Y.; Ho, C.S.; Ong, Z.Z.; Cairns, J.; Gopal, B.P.; Krstic, L.; Elsahn, A.; Lister, M.M.; Said, D.G.; et al. Clinical features, risk factors and outcomes of contact lens-related bacterial keratitis in Nottingham, UK: A 7-year study. Eye 2024, 38, 3459–3466. [Google Scholar] [CrossRef]
- Tavassoli, S.; Nayar, G.; Darcy, K.; Grzeda, M.; Luck, J.; Williams, O.M.; Tole, D. An 11-year analysis of microbial keratitis in the South West of England using brain-heart infusion broth. Eye 2019, 33, 1619–1625. [Google Scholar] [CrossRef]
- Ting, D.S.J.; Settle, C.; Morgan, S.J.; Baylis, O.; Ghosh, S. A 10-year analysis of microbiological profiles of microbial keratitis: The North East England Study. Eye 2018, 32, 1416–1417. [Google Scholar] [CrossRef]
- Tan, S.Z.; Walkden, A.; Au, L.; Fullwood, C.; Hamilton, A.; Qamruddin, A.; Armstrong, M.; Brahma, A.K.; Carley, F. Twelve-year analysis of microbial keratitis trends at a UK tertiary hospital. Eye 2017, 31, 1229–1236. [Google Scholar] [CrossRef]
- Royal College of Pathologists. Available online: https://www.rcpath.org/trainees/training/training-by-specialty.html (accessed on 1 June 2025).
- Maier, P.; Betancor, P.K.; Reinhard, T. Contact Lens-Associated Keratitis-an Often Underestimated Risk. Dtsch. Arztebl. Int. 2022, 119, 669–674. [Google Scholar] [CrossRef]
- Seal, D.V.; Kirkness, C.M.; Bennett, H.G.; Peterson, M.; Keratitis Study Group. Population-based cohort study of microbial keratitis in Scotland: Incidence and features. Cont. Lens Anterior Eye 1999, 22, 49–57. [Google Scholar] [CrossRef]
- Brown, L.; Leck, A.K.; Gichangi, M.; Burton, M.J.; Denning, D.W. The global incidence and diagnosis of fungal keratitis. Lancet Infect. Dis. 2021, 21, e49–e57. [Google Scholar] [CrossRef]
- Sidhu, S.K.; Malhotra, S.; Devi, P.; Tuli, A.K. Significance of coagulase negative Staphylococcus from blood cultures: Persisting problems and partial progress in resource constrained settings. Iran. J. Microbiol. 2016, 8, 366–371. [Google Scholar]
| 2018 | 2019 | 2020 | 2021 | 2022 | Total | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All Pathogens | 68 | 53 | 57 | 76 | 103 | 357 | ||||||
| Gram-positive | 40 | 58.8% | 27 | 50.9% | 28 | 49.1% | 41 | 53.9% | 56 | 54.4% | 192 | 53.8% |
| CoNS | 17 | 42.5% | 7 | 25.9% | 7 | 25.0% | 13 | 31.7% | 12 | 21.4% | 56 | 29.2% |
| S. epidermidis | 0 | 0.0% | 1 | 3.7% | 1 | 3.6% | 5 | 12.2% | 5 | 8.9% | 12 | 6.3% |
| S. aureus | 6 | 15.0% | 6 | 22.2% | 10 | 35.7% | 8 | 19.5% | 14 | 25.0% | 44 | 22.9% |
| Streptoccus spp. | 6 | 15.0% | 6 | 22.2% | 3 | 10.7% | 3 | 7.3% | 8 | 14.3% | 26 | 13.5% |
| Bacillus spp. | 2 | 5.0% | 2 | 7.4% | 2 | 7.1% | 2 | 4.9% | 2 | 3.6% | 10 | 5.2% |
| Other | 9 | 22.5% | 5 | 18.5% | 5 | 17.9% | 10 | 24.4% | 15 | 26.8% | 44 | 22.9% |
| Gram-negative | 19 | 27.9% | 20 | 37.7% | 23 | 40.4% | 30 | 39.5% | 38 | 36.9% | 130 | 36.4% |
| P. aeruginosa | 9 | 47.4% | 10 | 50.0% | 10 | 43.5% | 17 | 56.7% | 23 | 60.5% | 69 | 19.3% |
| Moraxella spp. | 3 | 15.8% | 5 | 25.0% | 5 | 21.7% | 3 | 10.0% | 5 | 13.2% | 21 | 5.9% |
| S. albicans | 2 | 10.5% | 2 | 10.0% | 3 | 13.0% | 2 | 6.7% | 5 | 13.2% | 14 | 3.9% |
| Other | 5 | 26.3% | 3 | 15.0% | 5 | 21.7% | 8 | 26.7% | 5 | 13.2% | 26 | 7.3% |
| Mixed Growth | 1 | 1.5% | 4 | 7.5% | 3 | 5.3% | 1 | 1.3% | 0 | 0.0% | 9 | 2.5% |
| Fungi | 5 | 7.4% | 2 | 3.8% | 0 | 0.0% | 1 | 1.3% | 1 | 1.0% | 9 | 2.5% |
| Yeast | 4 | 80.0% | 1 | 50.0% | 0 | 0.0% | 0 | 0.0% | 1 | 100.0% | 6 | 1.7% |
| Filamentous | 1 | 20.0% | 1 | 50.0% | 0 | 0.0% | 1 | 100.0% | 0 | 0.0% | 3 | 0.8% |
| Acanthamoeba | 1 | 1.5% | 0 | 0.0% | 1 | 1.8% | 2 | 2.6% | 3 | 2.9% | 7 | 2.0% |
| Viruses | 2 | 2.9% | 0 | 0.0% | 2 | 3.5% | 1 | 1.3% | 5 | 4.9% | 10 | 2.8% |
| Herpes simplex Virus (HSV) | 2 | 100.0% | 0 | 0.0% | 1 | 50.0% | 1 | 0.0% | 0 | 0.0% | 4 | 1.1% |
| Varicella zoster Virus (VZV) | 0 | 0.0% | 0 | 0.0% | 1 | 50.0% | 0 | 0.0% | 3 | 60.0% | 4 | 1.1% |
| Adenovirus | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% | 2 | 40.0% | 2 | 0.6% |
| 2018–2020 | 2021–2022 | Total | p | ||||
|---|---|---|---|---|---|---|---|
| Sensitivity | % | Sensitivity | % | Sensitivity | % | ||
| Gram-positive | |||||||
| Chloramphenicol | 30/32 | 93.8% | 22/22 | 100.0% | 52/54 | 96.3% | 0.818 |
| Ciprofloxacin | 29/30 | 96.7% | 22/24 | 91.7% | 51/54 | 94.4% | 0.851 |
| Levofloxacin | 7/7 | 100.0% | 4/4 | 100.0% | 11/11 | 100.0% | 1 |
| Ofloxacin | 17/18 | 94.4% | 4/4 | 100.0% | 21/22 | 95.5% | 1 |
| All fluoroquinolones | 29/30 | 96.7% | 22/24 | 91.7% | 51/54 | 94.4% | 0.851 |
| Fusidic acid | 21/29 | 72.4% | 15/22 | 68.2% | 36/51 | 70.6% | 0.859 |
| Gentamicin | 32/35 | 91.4% | 23/25 | 92.0% | 55/60 | 91.7% | 0.982 |
| Neomycin | 0/0 | 0.0% | 4/4 | 100.0% | 4/4 | 100.0% | N/A |
| Tobramycin | 4/4 | 100.0% | 0/0 | 0.0% | 4/4 | 100.0% | N/A |
| All aminoglycosides | 32/35 | 91.4% | 24/25 | 96.0% | 56/60 | 93.3% | 0.857 |
| Vancomycin | 5/5 | 100.0% | 18/18 | 100.0% | 23/23 | 100.0% | 1 |
| Teicoplanin | 2/2 | 100.0% | 2/2 | 100.0% | 4/4 | 100.0% | 1 |
| Penicillins | 31/35 | 88.6% | 33/34 | 97.1% | 64/69 | 92.8% | 0.714 |
| Co-Amoxiclav | 1/2 | 50.0% | 2/2 | 100.0% | 3/4 | 75.0% | 1 |
| Tazocin | 4/4 | 100.0% | 1/1 | 100.0% | 5/5 | 100.0% | 1 |
| Cephalosporins | 20/26 | 76.9% | 6/7 | 85.7% | 26/33 | 78.8% | 0.816 |
| Meropenem | 6/6 | 100.0% | 1/1 | 100.0% | 7/7 | 100.0% | 1 |
| Clindamycin | 20/25 | 80.0% | 5/7 | 71.4% | 25/32 | 78.1% | 0.821 |
| Macrolides | 22/32 | 68.8% | 28/28 | 100.0% | 50/60 | 83.3% | 0.186 |
| Tetracyclines | 23/26 | 88.5% | 28/28 | 100.0% | 51/54 | 94.4% | 0.663 |
| Rifampicin | 21/21 | 100.0% | 3/4 | 75.0% | 24/25 | 96.0% | 0.16 |
| Linezolid | 16/16 | 100.0% | 15/16 | 93.8% | 31/32 | 96.9% | 0.857 |
| Mupirocin | 10/14 | 71.4% | 18/18 | 100.0% | 28/32 | 87.5% | 0.391 |
| Co-Trimoxazole | 2/2 | 100.0% | 6/8 | 75.0% | 8/10 | 80.0% | 1 |
| Gram-negative | |||||||
| Chloramphenicol | 16/17 | 94.1% | 12/12 | 100.0% | 28/29 | 96.6% | 0.874 |
| Ciprofloxacin | 37/38 | 97.4% | 31/31 | 100.0% | 68/69 | 98.6% | 0.913 |
| Levofloxacin | 3/3 | 100.0% | 2/2 | 100.0% | 5/5 | 100.0% | 1 |
| Ofloxacin | 2/2 | 100.0% | 0/0 | 0.0% | 2/2 | 100.0% | N/A |
| Fluoroquinolones | 37/38 | 97.4% | 31/31 | 100.0% | 68/69 | 98.6% | 0.913 |
| Fusidic Acid | 2/2 | 100.0% | 0/0 | 0.0% | 2/4 | 50.0% | N/A |
| Gentamicin | 25/25 | 100.0% | 29/29 | 100.0% | 54/54 | 100.0% | 1 |
| Amikacin | 4/4 | 100.0% | 7/7 | 100.0% | 11/11 | 100.0% | 1 |
| Tobramycin | 5/5 | 100.0% | 2/2 | 100.0% | 7/7 | 100.0% | 1 |
| All aminoglycosides | 25/25 | 100.0% | 29/29 | 100.0% | 54/54 | 100.0% | 1 |
| Vancomycin | 1/2 | 50.0% | 0/0 | 0.0% | 1/1 | 100.0% | N/A |
| Teicoplanin | 0/0 | 0.0% | 1/1 | 100.0% | 1/0 | 100.0% | N/A |
| Penicillins | 6/7 | 85.7% | 4/4 | 100.0% | 10/11 | 90.9% | 1 |
| Co-Amoxiclav | 7/9 | 77.8% | 0/0 | 0.0% | 7/9 | 77.8% | N/A |
| Tazocin | 15/15 | 107.1% | 15/18 | 83.3% | 30/33 | 90.9% | 0.617 |
| Colistin | 0/0 | 0.0% | 5/5 | 100.0% | 5/5 | 100.0% | N/A |
| Meropenem | 20/20 | 100.0% | 22/22 | 100.0% | 42/42 | 100.0% | 1 |
| Clindamycin | 1/3 | 33.3% | 12/13 | 92.3% | 13/16 | 81.3% | 0.071 |
| Macrolides | 2/3 | 66.7% | 5/5 | 100.0% | 7/8 | 87.5% | 0.375 |
| Tetracyclines | 4/5 | 80.0% | 9/9 | 100.0% | 13/14 | 92.9% | 0.357 |
| Rifampicin | 2/2 | 100.0% | 1/1 | 100.0% | 3/3 | 100.0% | 1 |
| Daptomycin | 0/0 | 0.0% | 4/6 | 66.7% | 4/6 | 66.7% | N/A |
| Linezolid | 2/2 | 100.0% | 0/0 | 0.0% | 2/2 | 100.0% | N/A |
| Mupirocin | 2/2 | 100.0% | 0/0 | 0.0% | 2/2 | 100.0% | N/A |
| Co-Trimoxazole | 0/1 | 0.0% | 0/0 | 0.0% | 0/1 | 0.0% | N/A |
| Aztreonam | 2/2 | 100.0% | 0/0 | 0.0% | 2/0 | 100.0% | N/A |
| Trimethoprim | 1/1 | 100.0% | 1/1 | 100.0% | 2/2 | 100.0% | 1 |
| Ticarcillin/Clavulan | 1/2 | 50.0% | 2/2 | 100.0% | 3/4 | 75.0% | 1 |
| Fungal | |||||||
| Voriconazole | 0/0 | 0.0% | 1/2 | 50.0% | 1/2 | 50.0% | N/A |
| Caspofungin | 0/0 | 0.0% | 1/1 | 100.0% | 1/1 | 100.0% | N/A |
| Fluconazole | 0/0 | 0.0% | 1/1 | 100.0% | 1/1 | 100.0% | N/A |
| Amphotericin B | 0/0 | 0.0% | 1/1 | 100.0% | 1/1 | 100.0% | N/A |
| Pathogen | Chloramphenicol | Fusidic Acid | Fluoroquinolones | Cephalosporins | Gentamicin | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sensitivity | % | Sensitivity | % | Sensitivity | % | Sensitivity | % | Sensitivity | % | |
| Gram-positive | 103/109 | 94.5 | 57/89 | 64.0 | 174/182 | 95.6 | 15/15 | 100.0 | 81/89 | 91.0 |
| CoNS | 38/42 | 90.5 | 29/54 | 53.7 | 103/107 | 96.3 | 5/5 | 100.0 | 49/54 | 90.7 |
| S. epidermidis | 2/2 | 100.0 | 2/4 | 50.0 | 7/7 | 100.0 | N/A | 4/4 | 100.0 | |
| S. aureus | 31/31 | 100.0 | 26/29 | 89.7 | 53/54 | 98.1 | 4/4 | 100.0 | 31/33 | 93.4 |
| Streptococcus spp. | 18/18 | 100.0 | 1/4 | 25.0 | 18/20 | 90.0 | 2/2 | 100.0 | 3/5 | 60.0 |
| Bacillus spp. | 7/8 | 87.5 | 2/8 | 25.0 | 16/16 | 100.0 | 2/2 | 100.0 | 8/8 | 100.0 |
| Other | 14/15 | 93.3 | 9/10 | 90.0 | 17/17 | 100.0 | 2/2 | 100.0 | 8/8 | 100.0 |
| Gram-negative | 41/52 | 78.8 | 3/19 | 15.8 | 77/79 | 97.5 | 35/38 | 92.1 | 32/33 | 96.9 |
| P. aeruginosa | 1/12 | 8.3 | 0/11 | 0.0 | 25/25 | 100.0 | 10/11 | 90.0 | 12/12 | 100.0 |
| Moraxella spp. | 17/17 | 100.0 | 2/2 | 100.0 | 22/22 | 100.0 | 6/6 | 100.0 | 5/5 | 100.0 |
| S. albicans | 7/7 | 100.0 | 7/7 | 100.0 | 10/10 | 100.0 | 5/8 | 62.5 | 6/6 | 100.0 |
| Other | 16/16 | 100.0 | 1/5 | 20.0 | 20/22 | 90.9 | 13/13 | 100.0 | 9/10 | 90.0 |
| 2018–2020 | 2021–2022 | Total | p | ||||
|---|---|---|---|---|---|---|---|
| All Pathogens | 178 | 179 | 357 | ||||
| Gram-positive | 95/178 | 53.4% | 97/179 | 54.2% | 192/357 | 53.8% | 0.916 |
| CoNS | 31/95 | 32.6% | 25/97 | 25.8% | 56/192 | 29.2% | 0.411 |
| S. epidermidis | 2/95 | 2.1% | 10/97 | 10.3% | 12/192 | 6.3% | 0.021 |
| S. aureus | 22/95 | 23.2% | 22/97 | 22.7% | 44/192 | 22.9% | 0.985 |
| Streptococcus spp. | 15/95 | 15.8% | 11/97 | 11.3% | 26/192 | 13.5% | 0.424 |
| Bacillus spp. | 6/95 | 6.3% | 4/97 | 4.1% | 10/192 | 5.2% | 0.521 |
| Other | 19/95 | 20.0% | 25/97 | 25.8% | 44/192 | 22.9% | 0.376 |
| Gram-negative | 62/178 | 34.8% | 68/179 | 38.0% | 130/357 | 36.4% | 0.621 |
| P. aeruginosa | 29/62 | 46.8% | 40/68 | 58.8% | 69/130 | 53.1% | 0.193 |
| Moraxella spp. | 13/62 | 21.0% | 8/68 | 11.8% | 21/130 | 16.2% | 0.27 |
| S. albicans | 7/62 | 11.3% | 7/68 | 10.3% | 14/130 | 10.8% | 0.992 |
| Other | 13/62 | 21.0% | 13/68 | 19.1% | 26/130 | 20.0% | 0.989 |
| Mixed Growth | 8/178 | 4.5% | 1/179 | 0.6% | 9/357 | 2.5% | 0.02 |
| Fungi | 7/178 | 3.9% | 2/179 | 1.1% | 9/357 | 2.5% | 0.104 |
| Yeast | 5/7 | 71.4% | 1/2 | 50.0% | 6/9 | 66.7% | 0.101 |
| Filamentous | 2/7 | 28.6% | 1/2 | 50.0% | 3/9 | 33.3% | 0.56 |
| Acanthamoeba | 2/178 | 1.1% | 5/179 | 2.8% | 7/357 | 2.0% | 0.448 |
| Viruses | 4/178 | 2.2% | 6/179 | 3.4% | 10/357 | 2.8% | 0.75 |
| HSV | 3/4 | 75.0% | 1/6 | 16.7% | 4/10 | 40.0% | 0.315 |
| VZV | 1/4 | 25.0% | 3/6 | 50.0% | 4/10 | 40.0% | 0.32 |
| Adenovirus | 0/4 | 0.0% | 2/6 | 33.3% | 2/10 | 20.0% | 0.158 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharma, P.; Chipeta, C.; O’Kane, K.; Whiteman, A.; Francis, B.; Thornton, R.; Sian, I.; Buscombe, C.; Court, J.; Knox-Cartwright, N.; et al. Five-Year Analysis of Microbial Keratitis Incidence, Isolates, and In Vitro Antimicrobial Sensitivity in the South West of England: An Epidemiological Study. Microorganisms 2025, 13, 1578. https://doi.org/10.3390/microorganisms13071578
Sharma P, Chipeta C, O’Kane K, Whiteman A, Francis B, Thornton R, Sian I, Buscombe C, Court J, Knox-Cartwright N, et al. Five-Year Analysis of Microbial Keratitis Incidence, Isolates, and In Vitro Antimicrobial Sensitivity in the South West of England: An Epidemiological Study. Microorganisms. 2025; 13(7):1578. https://doi.org/10.3390/microorganisms13071578
Chicago/Turabian StyleSharma, Poonam, Chimwemwe Chipeta, Kieran O’Kane, Alexander Whiteman, Bryher Francis, Richard Thornton, Indy Sian, Charlotte Buscombe, Jennifer Court, Nathaniel Knox-Cartwright, and et al. 2025. "Five-Year Analysis of Microbial Keratitis Incidence, Isolates, and In Vitro Antimicrobial Sensitivity in the South West of England: An Epidemiological Study" Microorganisms 13, no. 7: 1578. https://doi.org/10.3390/microorganisms13071578
APA StyleSharma, P., Chipeta, C., O’Kane, K., Whiteman, A., Francis, B., Thornton, R., Sian, I., Buscombe, C., Court, J., Knox-Cartwright, N., & Roberts, H. (2025). Five-Year Analysis of Microbial Keratitis Incidence, Isolates, and In Vitro Antimicrobial Sensitivity in the South West of England: An Epidemiological Study. Microorganisms, 13(7), 1578. https://doi.org/10.3390/microorganisms13071578






