Dynamic Modeling of the Sulfur Cycle in Urban Sewage Pipelines Under High-Temperature and High-Salinity Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Design and Operating Conditions of Anaerobic Concrete Pipeline Reactors
2.2. The Inoculation of Sludge and Nutrition Medium
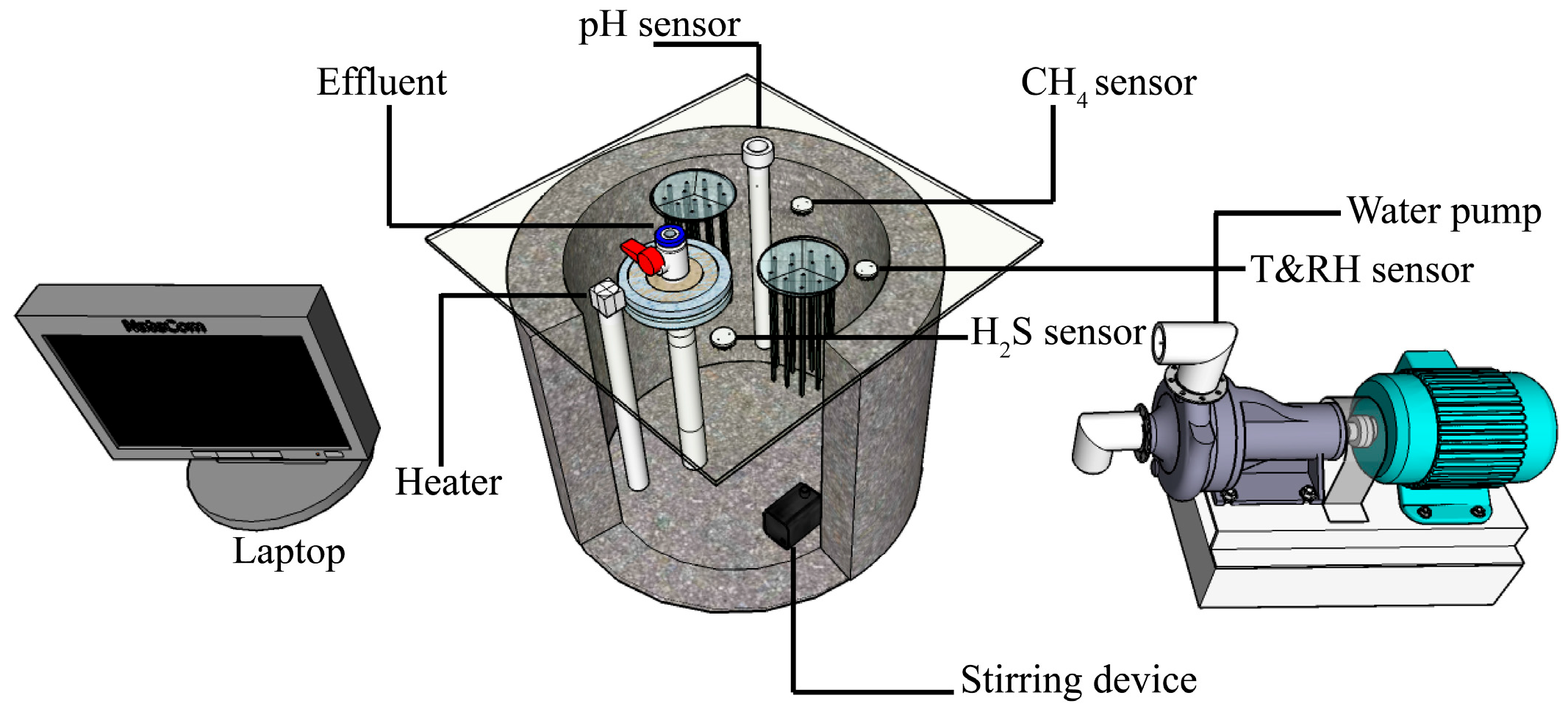
2.3. Real-Time Monitoring System
2.4. Collection of Basic Parameters
2.5. Mathematical Modeling
2.6. Correlation Analysis: Variable Group A
3. Results and Discussion
3.1. Basic Parameter Indicators
3.2. Correlation Analysis: Variable Group B
3.3. Biological Model
3.3.1. Sulfur Reduction Model
3.3.2. Sulfuric Acid Model in Sewage
3.3.3. Sulfuric Acid Model in in Gas Atmosphere
3.4. Model Validation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Grengg, C.; Mittermayr, F.; Baldermann, A.; Böttcher, M.E.; Leis, A.; Koraimann, G.; Grunert, P.; Dietzel, M. Microbiologically Induced Concrete Corrosion: A Case Study from a Combined Sewer Network. Cem. Concr. Res. 2015, 77, 16–25. [Google Scholar] [CrossRef]
- Li, X.; Johnson, I.; Mueller, K.; Wilkie, S.; Hanzic, L.; Bond, P.L.; O’Moore, L.; Yuan, Z.; Jiang, G. Corrosion Mitigation by Nitrite Spray on Corroded Concrete in a Real Sewer System. Sci. Total Environ. 2022, 806, 151328. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Liu, W.; Xu, D.; Hou, Y.; Ren, B.; Jin, X.; Wang, X.C.; Jin, P. Metagenomics Analysis of Ecosystem Integrating Methane and Sulfide Generation in Urban Sewer Systems. J. Clean. Prod. 2023, 382, 135372. [Google Scholar] [CrossRef]
- Knobel, A.N.; Lewis, A.E. A Mathematical Model of a High Sulphate Wastewater Anaerobic Treatment System. Water Res. 2002, 36, 257–265. [Google Scholar] [CrossRef]
- Liu, Y.; Tugtas, A.E.; Sharma, K.R.; Ni, B.-J.; Yuan, Z. Sulfide and Methane Production in Sewer Sediments: Field Survey and Model Evaluation. Water Res. 2016, 89, 142–150. [Google Scholar] [CrossRef]
- Liang, Z.-S.; Zhang, L.; Wu, D.; Chen, G.-H.; Jiang, F. Systematic Evaluation of a Dynamic Sewer Process Model for Prediction of Odor Formation and Mitigation in Large-Scale Pressurized Sewers in Hong Kong. Water Res. 2019, 154, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Khiewwijit, R.; Keesman, K.J.; Rijnaarts, H.; Temmink, H. Volatile Fatty Acids Production from Sewage Organic Matter by Combined Bioflocculation and Anaerobic Fermentation. Bioresour. Technol. 2015, 193, 150–155. [Google Scholar] [CrossRef]
- Batstone, D.J.; Puyol, D.; Flores-Alsina, X.; Rodríguez, J. Mathematical Modelling of Anaerobic Digestion Processes: Applications and Future Needs. Rev. Environ. Sci. Bio/Technol. 2015, 14, 595–613. [Google Scholar] [CrossRef]
- Jiang, G.; Zhou, M.; Chiu, T.H.; Sun, X.; Keller, J.; Bond, P.L. Wastewater-Enhanced Microbial Corrosion of Concrete Sewers. Environ. Sci. Technol. 2016, 50, 8084–8092. [Google Scholar] [CrossRef]
- Liu, H.; Fu, C.; Gu, T.; Zhang, G.; Lv, Y.; Wang, H.; Liu, H. Corrosion Behavior of Carbon Steel in the Presence of Sulfate Reducing Bacteria and Iron Oxidizing Bacteria Cultured in Oilfield Produced Water. Corros. Sci. 2015, 100, 484–495. [Google Scholar] [CrossRef]
- Kubota, K.; Hayashi, M.; Matsunaga, K.; Iguchi, A.; Ohashi, A.; Li, Y.-Y.; Yamaguchi, T.; Harada, H. Microbial Community Composition of a Down-Flow Hanging Sponge (DHS) Reactor Combined with an up-Flow Anaerobic Sludge Blanket (UASB) Reactor for the Treatment of Municipal Sewage. Bioresour. Technol. 2014, 151, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Cayford, B.I.; Dennis, P.G.; Keller, J.; Tyson, G.W.; Bond, P.L. High-Throughput Amplicon Sequencing Reveals Distinct Communities within a Corroding Concrete Sewer System. Appl. Environ. Microbiol. 2012, 78, 7160–7162. [Google Scholar] [CrossRef] [PubMed]
- Grengg, C.; Mittermayr, F.; Ukrainczyk, N.; Koraimann, G.; Kienesberger, S.; Dietzel, M. Advances in Concrete Materials for Sewer Systems Affected by Microbial Induced Concrete Corrosion: A Review. Water Res. 2018, 134, 341–352. [Google Scholar] [CrossRef]
- Sharma, K.R.; Yuan, Z.; de Haas, D.; Hamilton, G.; Corrie, S.; Keller, J. Dynamics and Dynamic Modelling of H2S Production in Sewer Systems. Water Res. 2008, 42, 2527–2538. [Google Scholar] [CrossRef] [PubMed]
- Mathews, E.R.; Wood, J.L.; Phillips, D.; Billington, N.; Barnett, D.; Franks, A.E. Town-Scale Microbial Sewer Community and H2S Emissions Response to Common Chemical and Biological Dosing Treatments. J. Environ. Sci. 2020, 87, 133–148. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, B.; Li, R.; Cui, Y.; Xie, F.; Zhou, A.; Li, J.; Yue, X. Study on the Feasibility of Carbon Source Recovery by Upflow Anaerobic Sludge Blanket in Simulated Municipal Wastewater. Sci. Total Environ. 2023, 878, 163157. [Google Scholar] [CrossRef]
- Van Den Brand, T.P.H.; Roest, K.; Chen, G.H.; Brdjanovic, D.; Van Loosdrecht, M.C.M. Adaptation of Sulfate-Reducing Bacteria to Sulfide Exposure. Environ. Eng. Sci. 2016, 33, 242–249. [Google Scholar] [CrossRef]
- Zhou, J.; Yin, S.; Fu, Q.; Wang, Q.; Huang, Q.; Wang, J. Microbial-Induced Concrete Corrosion under High-Salt Conditions: Microbial Community Composition and Environmental Multivariate Association Analysis. Int. Biodeterior. Biodegrad. 2021, 164, 105287. [Google Scholar] [CrossRef]
- Song, Y.; Tian, Y.; Li, X.; Wei, J.; Zhang, H.; Bond, P.L.; Yuan, Z.; Jiang, G. Distinct Microbially Induced Concrete Corrosion at the Tidal Region of Reinforced Concrete Sewers. Water Res. 2019, 150, 392–402. [Google Scholar] [CrossRef]
- D’ Bastiani, C.; Kennedy, D.; Reynolds, A. CFD Simulation of Anaerobic Granular Sludge Reactors: A Review. Water Res. 2023, 242, 120220. [Google Scholar] [CrossRef]
- Zuo, Z.; Ren, D.; Qiao, L.; Li, H.; Huang, X.; Liu, Y. Rapid Dynamic Quantification of Sulfide Generation Flux in Spatially Heterogeneous Sediments of Gravity Sewers. Water Res. 2021, 203, 117494. [Google Scholar] [CrossRef]
- Or, D.; Smets, B.F.; Wraith, J.M.; Dechesne, A.; Friedman, S.P. Physical Constraints Affecting Bacterial Habitats and Activity in Unsaturated Porous Media—A Review. Adv. Water Resour. 2007, 30, 1505–1527. [Google Scholar] [CrossRef]
- Rooyackers, F.A.M.; Bosco, E.; Suiker, A.S.J.; Clemens, F.H.L.R. A Chemo-Mechanical Model for Biogenic Sulphide Corrosion of Concrete. Cem. Concr. Res. 2022, 160, 106809. [Google Scholar] [CrossRef]
- Sharifi, S.; Murthy, S.; Takács, I.; Massoudieh, A. Probabilistic Parameter Estimation of Activated Sludge Processes Using Markov Chain Monte Carlo. Water Res. 2014, 50, 254–266. [Google Scholar] [CrossRef]
- Scheller, S.; Yu, H.; Chadwick, G.L.; McGlynn, S.E.; Orphan, V.J. Artificial Electron Acceptors Decouple Archaeal Methane Oxidation from Sulfate Reduction. Science 2016, 351, 703–707. [Google Scholar] [CrossRef] [PubMed]
- Ge, R.; Zhou, M.; Luo, Y.; Meng, Q.; Mai, G.; Ma, D.; Wang, G.; Zhou, F. McTwo: A Two-Step Feature Selection Algorithm Based on Maximal Information Coefficient. BMC Bioinform. 2016, 17, 142. [Google Scholar] [CrossRef]
- Liu, C.; Liu, J.; Li, J.; He, H.; Peng, S.; Li, C.; Chen, Y. Removal of H2S by Co-Immobilized Bacteria and Fungi Biocatalysts in a Bio-Trickling Filter. Process Saf. Environ. Prot. 2013, 91, 145–152. [Google Scholar] [CrossRef]
- Cao, J.; Zhang, G.; Mao, Z.-S.; Li, Y.; Fang, Z.; Yang, C. Influence of Electron Donors on the Growth and Activity of Sulfate-Reducing Bacteria. Int. J. Miner. Process. 2012, 106–109, 58–64. [Google Scholar] [CrossRef]
- Cardoso, R.B.; Sierra-Alvarez, R.; Rowlette, P.; Flores, E.R.; Gómez, J.; Field, J.A. Sulfide Oxidation under Chemolithoautotrophic Denitrifying Conditions. Biotechnol. Bioeng. 2006, 95, 1148–1157. [Google Scholar] [CrossRef]
- You, H.S.; Marshall, J.A.; Delgado, C. Assessing Students’ Disciplinary and Interdisciplinary Understanding of Global Carbon Cycling. J. Res. Sci. Teach. 2017, 55, 377–398. [Google Scholar] [CrossRef]
- Erdirencelebi, D.; Ozturk, I. A Comparative Study of Sulfidogenic and Methanogenic Activities during the Treatment of Landfill Leachate: Part I. J. Environ. Sci. Health A Toxic/Hazard. Subst. Environ. Eng. 2006, 41, 687–701. [Google Scholar] [CrossRef]
- Subrahmanyam, G.; Kumar, K.; Shah, A.P.; Maurya, D.M.; Sharma, A.; Chamyal, L.S.; Archana, G. Geochemical Characteristics Control Potential Microbial Activity in Exposed Late Quaternary Alluvial Deposits. Pedobiologia 2021, 87–88, 150747. [Google Scholar] [CrossRef]
- Jiang, G.; Keller, J.; Bond, P.L. Determining the Long-Term Effects of H2S Concentration, Relative Humidity and Air Temperature on Concrete Sewer Corrosion. Water Res. 2014, 65, 157–169. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.; Feng, J.; Xiang, X.; Wang, J.; Salojärvi, J.; Liu, C.; Wu, Z.; Zhang, J.; Liang, X.; Jiang, Z.; et al. Two Divergent Haplotypes from a Highly Heterozygous Lychee Genome Suggest Independent Domestication Events for Early and Late-Maturing Cultivars. Nat. Genet. 2022, 54, 73–83. [Google Scholar] [CrossRef]
- Mohanakrishnan, J.; Sharma, K.R.; Meyer, R.L.; Hamilton, G.; Keller, J.; Yuan, Z. Variation in Biofilm Structure and Activity along the Length of a Rising Main Sewer. Water Environ. Res. 2009, 81, 800–808. [Google Scholar] [CrossRef] [PubMed]
- Yoda, M.; Kitagawa, M.; Miyaji, Y. Long Term Competition between Sulfate-Reducing and Methane-Producing Bacteria for Acetate in Anaerobic Biofilm. Water Res. 1987, 21, 1547–1556. [Google Scholar] [CrossRef]
- Jolliffe, I.T.; Cadima, J. Principal Component Analysis: A Review and Recent Developments. Philos. Trans. A Math. Phys. Eng. Sci. 2016, 374, 20150202. [Google Scholar] [CrossRef] [PubMed]
- Davidson, B.R. The Economic Dimensions of Pollution in Australia. Econ. Pap. 1971, 1, 1–15. [Google Scholar] [CrossRef]
- Cai, J.; Zheng, P.; Mahmood, Q. Effect of Sulfide to Nitrate Ratios on the Simultaneous Anaerobic Sulfide and Nitrate Removal. Bioresour. Technol. 2008, 99, 5520–5527. [Google Scholar] [CrossRef]
- Ling, A.L.; Robertson, C.E.; Harris, J.K.; Frank, D.N.; Kotter, C.V.; Stevens, M.J.; Pace, N.R.; Hernandez, M.T. Carbon Dioxide and Hydrogen Sulfide Associations with Regional Bacterial Diversity Patterns in Microbially Induced Concrete Corrosion. Environ. Sci. Technol. 2014, 48, 7357–7364. [Google Scholar] [CrossRef]
- Sun, X.; Jiang, G.; Chiu, T.H.; Zhou, M.; Keller, J.; Bond, P.L. Effects of Surface Washing on the Mitigation of Concrete Corrosion under Sewer Conditions. Cem. Concr. Compos. 2016, 68, 88–95. [Google Scholar] [CrossRef]
- Nielsen, A.H.; Vollertsen, J.; Hvitved-Jacobsen, T. Determination of Kinetics and Stoichiometry of Chemical Sulfide Oxidation in Wastewater of Sewer Networks. Environ. Sci. Technol. 2003, 37, 3853–3858. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Ye, L.; Ni, B.-J.; Yuan, Z. Effect of pH on N2O Reduction and Accumulation during Denitrification by Methanol Utilizing Denitrifiers. Water Res. 2012, 46, 4832–4840. [Google Scholar] [CrossRef]
- Visser, J.M.; Robertson, L.A.; Van Verseveld, H.W.; Kuenen, J.G. Sulfur Production by Obligately Chemolithoautotrophic Thiobacillus Species. Appl. Environ. Microbiol. 1997, 63, 2300–2305. [Google Scholar] [CrossRef] [PubMed]
- Brunet, R.C.; Garcia-Gil, L.J. Sulfide-Induced Dissimilatory Nitrate Reduction to Ammonia in Anaerobic Freshwater Sediments. FEMS Microbiol. Ecol. 1996, 21, 131–138. [Google Scholar] [CrossRef]
- Sørensen, J.; Tiedje, J.M.; Firestone, R.B. Inhibition by Sulfide of Nitric and Nitrous Oxide Reduction by Denitrifying Pseudomonas Fluorescens. Appl. Environ. Microbiol. 1980, 39, 105–108. [Google Scholar] [CrossRef]
- Leeder, A.C.; Palma-Guerrero, J.; Glass, N.L. The Social Network: Deciphering Fungal Language. Nat. Rev. Microbiol. 2011, 9, 440–451. [Google Scholar] [CrossRef]
- Jou, F.-Y.; Mather, A.E. Solubility of Hydrogen Sulfide in [Bmim][PF6]. Int. J. Thermophys. 2007, 28, 490–495. [Google Scholar] [CrossRef]
- Yi, S.; Lai, Z.; He, Z.; Cheung, Y.; Liu, Y. Joint Sparse Principal Component Analysis. Pattern Recognit. 2017, 61, 524–536. [Google Scholar] [CrossRef]

| SN | Process Reaction | Reference Component | Reaction Rate |
|---|---|---|---|
| Fermentation | FCOD → VFA | SF | |
| Sulfide generation using fermentable substrate | 2FCOD + SO4 → H2S | ||
| Sulfide oxidation | 2O2 + H2S → SO4 | ||
| Concrete corrosion layer | 2O2 + H2S → SO4 |
| Symbol | Definition (Unit) | Typical Value |
|---|---|---|
| SF | Fermentable, readily biodegradable substrate (g COD m−3) | |
| SA | Fermentation products (g COD m−3) | |
| a | Rate constant for sulfide formation (g0.5 m−0.5 h−1) | 0.03 |
| XS1 | ||
| KO | Saturation constant for DO (g O2 m−3) | 0.01–0.5 |
| SO | Dissolved oxygen (g O2 m−3) | |
| KNO3 | Saturation constant for nitrate (g NO3− N m−3) | |
| SNO3 | Nitrate concentration in the bulk water phase (g NO3− N m−3) | |
| αw | Temperature coefficient for heterotrophic, aerobic water phase processes (–) | 1.07 |
| αf | Temperature coefficient for aerobic biofilm processes (–) | 1.05 |
| A | Biofilm surface area (m2) | |
| V | Water volume (m3) | |
| rs(–II) | Rate of sulfide oxidation (g S m−3 day−1) | |
| kS(–II) | Rate constant (unit depends on the values of n1 and n2) | |
| CS | Concentration of dissolved sulfide (g m−3) | |
| CO | Concentration of DO (g m−3) | |
| n = n1 + n2 | Reaction order (unit dependent on n1 and n2) | |
| kS(II)c, pH | pH-dependent rate constant for chemical sulfide oxidation ((g S m−3)1−n1c (g O2 m−3)−n2c h−1) | |
| kH2Sc | Rate constant for chemical sulfide oxidation of molecular sulfide, H2S ((g S m−3)1−n1c (g O2 m−3)−n2c h−1) | |
| kHS-c | Rate constant for chemical sulfide oxidation of ionic sulfide, HS–((g S m−3)1−n1c (g O2 m−3)−n2c h−1) | |
| Ka1 | The first dissociation constant for sulfide | |
| kS(II)b, pH | pH-dependent rate constant for biological sulfide oxidation ((g S m−3)1−n1c (g O2 m−3)−n2c h−1) | |
| kS(II)b, pHopt | Maximum rate constant for biological sulfide oxidation at the pHopt value ((g S m−3)1−n1c (g O2 m−3)−n2c h−1) | |
| pHopt | Optimum pH value for activity of sulfide oxidation (–) | |
| fS(II), pH | Factor for relative pH dependency (–) | |
| ωS(II)b | Constant that determines the shape of the activity curve for sulfide oxidation versus pH (–) | |
| μHw,NO3 | Maximum specific anoxic growth rate for heterotrophic biomass in the water phase (day−1) | 2–6 |
| KSw | Saturation constant for readily biodegradable substrate in the water phase (g COD m−3) | 0.5–2.0 |
| K1/2 | 1/2-order reaction rate constant per unit area of biofilm surface (g NO3 N0.5 m−0.5 h−1) | 6 |
| Ksf | Saturation constant for readily biodegradable substrate (g COD m−3) |
| Maximum Information Coefficient | Low Salinity 25 °C | Low Salinity 35 °C | Medium Salinity 25 °C | Medium Salinity 35 °C | High Salinity 25 °C | High Salinity 35 °C |
|---|---|---|---|---|---|---|
| COD | 0.99 | 0.47 | 0.99 | 0.47 | 0.47 | 0.99 |
| VFA | 0.29 | 0.47 | 0.52 | 0.52 | 0.2 | 0.99 |
| Sulfate | 0.29 | 0.47 | 0.52 | 0.52 | 0.47 | 0.2 |
| Sulfide | 0.47 | 0.47 | 0.99 | 0.47 | 0.52 | 0.47 |
| Pearson Coefficient | Low Salinity 25 °C | Low Salinity 35 °C | Medium Salinity 25 °C | Medium Salinity 35 °C | High Salinity 25 °C | High Salinity 35 °C |
|---|---|---|---|---|---|---|
| COD | −0.88 | −0.89 | −0.92 | −0.58 | −0.4 | −0.9 |
| VFA | −0.56 | −0.81 | −0.86 | 0.049 | −0.1 | −0.82 |
| Sulfate | 0.22 | −0.023 | −0.21 | 0.47 | 0.12 | −0.15 |
| Sulifde | 0.85 | 0.42 | −0.97 | −0.31 | 0.77 | 0.46 |
| PCA | COD and VFA | COD and Sulfide | Sulfate and Sulfide | |||
|---|---|---|---|---|---|---|
| Principal component contribution | 62.866 | 91.751 | 53.008 | |||
| Single sample | COD | VFA | COD | Sulfide | Sulfate | Sulfide |
| Sample principal component | 0.793 | 0.793 | −0.958 | 0.958 | 0.687 | 0.687 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, Z.; Xu, Z.; Chen, Y.; Zhao, B.; Wang, C.; Yu, Z.; Zhou, J. Dynamic Modeling of the Sulfur Cycle in Urban Sewage Pipelines Under High-Temperature and High-Salinity Conditions. Microorganisms 2025, 13, 1534. https://doi.org/10.3390/microorganisms13071534
Cao Z, Xu Z, Chen Y, Zhao B, Wang C, Yu Z, Zhou J. Dynamic Modeling of the Sulfur Cycle in Urban Sewage Pipelines Under High-Temperature and High-Salinity Conditions. Microorganisms. 2025; 13(7):1534. https://doi.org/10.3390/microorganisms13071534
Chicago/Turabian StyleCao, Zhiwei, Zhen Xu, Yufeng Chen, Bingxuan Zhao, Chenxu Wang, Zuozhou Yu, and Jingya Zhou. 2025. "Dynamic Modeling of the Sulfur Cycle in Urban Sewage Pipelines Under High-Temperature and High-Salinity Conditions" Microorganisms 13, no. 7: 1534. https://doi.org/10.3390/microorganisms13071534
APA StyleCao, Z., Xu, Z., Chen, Y., Zhao, B., Wang, C., Yu, Z., & Zhou, J. (2025). Dynamic Modeling of the Sulfur Cycle in Urban Sewage Pipelines Under High-Temperature and High-Salinity Conditions. Microorganisms, 13(7), 1534. https://doi.org/10.3390/microorganisms13071534






