In Vitro Efficacy of Thymbra capitata (L.) Cav. Essential Oil Against Olive Phytopathogenic Fungi
Abstract
1. Introduction
1.1. Background
1.2. Aim of the Study
2. Materials and Methods
2.1. Chemicals
2.2. Essential Oils
2.3. GC–MS Analysis
2.4. Fungal Strains
2.5. Substrates
2.6. Test Design
2.6.1. First Screening
2.6.2. Dose Test
2.6.3. Carvacrol Comparison
2.7. Test Execution
2.8. Statistical Analysis
3. Results
3.1. GC–MS Analysis of EOs
3.2. First Screening and Selection of TcOE
3.3. TcEO Dosage Test
3.4. Comparison Between TcEO and CAR
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CAR | Carvacrol |
| CTRL | Control |
| EOs | Essential Oils |
| GC | Gas Chromatography |
| EgEO | Eucalyptus globulus Labill. Essential Oil |
| LSD | Least Significant Difference |
| MFC | Minimum Fungicidal Concentration |
| MIC | Minimum Inhibitory Concentration |
| MpEO | Mentha piperita L. Essential Oil |
| MS | Mass Spectrometry |
| PDA | Potato Dextrose Agar |
| RIcal | Retention Indices Calculated |
| RIref | Retention Indices Reference |
| TcEO | Thymbra capitata Essential Oil |
| VOCs | Organic Volatile Compounds |
References
- FAO. World Food and Agriculture. In Statistical Yearbook; FAO: Quebec City, QC, Canada, 2024. [Google Scholar]
- Willer, H.; Trávníček, J.; Schlatter, S. The World of Organic Agriculture. Statistics and Emerging Trends. 2024. Available online: https://www.fibl.org/en/shop-en/1747-organic-world-2024 (accessed on 21 February 2025).
- Fenibo, E.O.; Ijoma, G.N.; Matambo, T. Biopesticides in sustainable agriculture: A critical sustainable development driver governed by green chemistry principles. Front. Sustain. Food Syst. 2021, 5, 619058. [Google Scholar] [CrossRef]
- Chouhan, S.; Sharma, K.; Guleria, S. Antimicrobial activity of some essential oils—Present status and future perspectives. Medicines 2017, 4, 58. [Google Scholar] [CrossRef]
- Abers, M.; Schroeder, S.; Goelz, L.; Sulser, A.; Rose, T.S.; Puchalski, K.; Langland, J. Antimicrobial activity of the volatile substances from essential oils. BMC Complement. Med. Ther. 2021, 21, 124. [Google Scholar] [CrossRef]
- Mutlu-Ingok, A.; Catalkaya, G.; Capanoglu, E.; Karbancioglu-Guler, F. Antioxidant and antimicrobial activities of fennel, ginger, oregano and thyme essential oils. Food Front. 2021, 2, 508–518. [Google Scholar] [CrossRef]
- Puvača, N.; Milenković, J.; Galonja Coghill, T.; Bursić, V.; Petrović, A.; Tanasković, S.; Pelić, M.; Ljubojević Pelić, D.; Miljković, T. Antimicrobial Activity of Selected Essential Oils against Selected Pathogenic Bacteria: In Vitro Study. Antibiotics 2021, 10, 546. [Google Scholar] [CrossRef] [PubMed]
- Chebbac, K.; Ghneim, H.K.; El Moussaoui, A.; Bourhia, M.; El Barnossi, A.; Benziane Ouaritini, Z.; Salamatullah, A.M.; Alzahrani, A.; Aboul-Soud, M.A.M.; Giesy, J.P.; et al. Antioxidant and Antimicrobial Activities of Chemically-Characterized Essential Oil from Artemisia aragonensis Lam. against Drug-Resistant Microbes. Molecules 2022, 27, 1136. [Google Scholar] [CrossRef]
- Grahovac, M.; Hrustić, J.; Tanović, B.; Inđić, D.; Vuković, S.; Mihajlović, M.; Gvozdenac, S. In vitro effects of essential oils on Colletotrichum spp. Agric. For. 2012, 57, 7–15. [Google Scholar]
- Da Cruz Cabral, L.; Fernández Pinto, V.; Patriarca, A. Control of infection of tomato fruits by Alternaria and mycotoxin production using plant extracts. Eur. J. Plant Pathol. 2016, 145, 363–373. [Google Scholar] [CrossRef]
- Yılmaz, A.; Ermiş, E.; Boyraz, N. Investigation of in vitro and in vivo antifungal activities of different plant essential oils against postharvest apple rot diseases—Colletotrichum gloeosporioides, Botrytis cinerea and Penicillium expansum. J. Food Saf. Food Qual.-Arch. Fur Leb. 2016, 67, 122–131. [Google Scholar] [CrossRef]
- España, M.D.; Arboleda, J.W.; Ribeiro, J.A.; Abdelnur, P.V.; Guzman, J.D. Eucalyptus leaf byproduct inhibits the anthracnose-causing fungus Colletotrichum gloeosporioides. Ind. Crops Prod. 2017, 108, 793–797. [Google Scholar] [CrossRef]
- Tomazoni, E.Z.; Pauletti, G.F.; da Silva Ribeiro, R.T.; Moura, S.; Schwambach, J. In vitro and in vivo activity of essential oils extracted from Eucalyptus staigeriana, Eucalyptus globulus and Cinnamomum camphora against Alternaria solani Sorauer causing early blight in tomato. Sci. Hortic. 2017, 223, 72–77. [Google Scholar] [CrossRef]
- França, K.R.S.; Silva, T.L.; Cardoso, T.A.L.; Ugulino, A.L.N.; Rodrigues, A.P.M.; Júnior, A.F. In vitro effect of essential oil of peppermint (Mentha × piperita L.) on the mycelial growth of Alternaria alternata. J. Exp. Agric. Int. 2018, 26, 1–7. [Google Scholar] [CrossRef]
- Morkeliūnė, A.; Rasiukevičiūtė, N.; Šernaitė, L.; Valiuškaitė, A. The use of essential oils from thyme, sage and peppermint against Colletotrichum acutatum. Plants 2021, 10, 114. [Google Scholar] [CrossRef]
- Akhlaghi, M.; Ziaolhagh, H.R.; Babakhanzade, E.; Rafiee, V. Evaluation of antifungal effect of thyme and peppermint essential oils and their major monoterpenes in controlling Verticillium fungus on pistachios. J. Plant Prod. Res. 2022, 29, 183–199. [Google Scholar]
- Pedrotti, C.; Franzoi, C.; Rosa, M.T.S.; Trentin, T.R.; Vilasboa, J.; Scariot, F.J.; Schwambach, J. Antifungal activity of essential oil from Eucalyptus staigeriana against Alternaria alternata causing leaf spot and black rot in table grapes. An. Acad. Bras. Ciências 2022, 94, e20200394. [Google Scholar] [CrossRef]
- Hajji-Hedfi, L.; Rhouma, A.; Hlaoua, W.; Dmitry, K.E.; Jaouadi, R.; Zaouali, Y.; Rebouh, N.Y. Phytochemical characterization of forest leaves extracts and application to control apple postharvest diseases. Sci. Rep. 2024, 14, 2014. [Google Scholar] [CrossRef] [PubMed]
- Boutagayout, A.; Lahlali, R.; El Baghazaoui, R.; Bouiamrine, E.H.; Radah, A.; Benabderrahmane, A.; Belmalha, S. GC-MS, FTIR analysis and effects of thyme (Thymbra capitata L. Cav) and rosemary (Rosmarinus officinalis L.) essential oils on the growth and pathogenicity of Botrytis fabae. Int. J. Environ. Stud. 2025, 82, 501–522. [Google Scholar] [CrossRef]
- Salgueiro, L.R.; Pinto, E.; Gonçalves, M.J.; Pina-Vaz, C.; Cavaleiro, C.; Rodrigues, A.G.; Martinez-de-Oliveira, J. Chemical composition and antifungal activity of the essential oil of Thymbra capitata. Planta Med. 2004, 70, 572–575. [Google Scholar] [CrossRef]
- Almeida, L.; Lopes, N.; Gaio, V.; Cavaleiro, C.; Salgueiro, L.; Silva, V.; Cerca, N. Thymbra capitata essential oil has a significant antimicrobial activity against methicillin-resistant Staphylococcus aureus pre-formed biofilms. Lett. Appl. Microbiol. 2022, 74, 787–795. [Google Scholar] [CrossRef]
- Eurostat. Eurostat Statistics Explained. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Agricultural_production_-_orchards#Olive_trees (accessed on 22 January 2025).
- Nostro, A.; Papalia, T. Antimicrobial activity of carvacrol: Current progress and future prospectives. Recent Pat. Antiinfect. Drug Discov. 2012, 7, 28–35. [Google Scholar] [CrossRef]
- Ultee, A.; Bennik, M.H.J.; Moezelaar, R. The phenolic hydroxyl group of carvacrol is essential for action against the food-borne pathogen Bacillus cereus. Appl. Environ. Microbiol. 2002, 68, 1561–1568. [Google Scholar] [CrossRef] [PubMed]
- Chaillot, J.; Tebbji, R.; Boone, C.; Brown, G.W.; Bellaoui, M.; Sellam, A. The monoterpene carvacrol generates endoplasmic reticulum stress in the pathogenic fungus Candida albicans. Antimicrob. Agents Chemother. 2015, 59, 4584–4592. [Google Scholar] [CrossRef] [PubMed]
- Ali, T.; Majeed, S.T.; Majeed, R.; Bashir, R.; Mir, S.A.; Jan, I.; Andrabi, K.I. Recent advances in the pharmacological properties and molecular mechanisms of carvacrol. Rev. Bras. Farmacogn. 2024, 34, 35–47. [Google Scholar] [CrossRef]
- Van den Dool, H.; Kratz, P.D. A generalization of the retention index system including linear temperature programmed gas-liquid partition chromatography. J. Chromatogr. 1963, 11, 463–471. [Google Scholar] [CrossRef]
- Chemistry WebBook. Available online: http://www.nist.gov/index.html (accessed on 16 December 2024).
- Cavalcanti de Albuquerque, C.; Camara, T.R.; Mariano, R.D.L.R.; Willadino, L.; Marcelino Junior, C.; Ulisses, C. Antimicrobial action of the essential oil of Lippia gracilis Schauer. Braz. Arch. Biol. Technol. 2006, 49, 527–535. [Google Scholar] [CrossRef]
- Tian, J.; Ban, X.; Zeng, H.; He, J.; Huang, B.; Wang, Y. Chemical composition and antifungal activity of essential oil from Cicuta virosa L. var. latisecta. Int. J. Food Microbiol. 2011, 145, 464–470. [Google Scholar] [CrossRef]
- Wu, H.S.; Wang, Y.; Zhang, C.Y.; Bao, W.; Ling, N.; Liu, D.Y.; Shen, Q.R. Growth of in vitro Fusarium oxysporum f. sp. niveum in chemically defined media amended with gallic acid. Biol. Res. 2009, 42, 297–304. [Google Scholar] [CrossRef]
- Morana, R.; Romani, A.; Campo, M.; Morana, M.; Romiti, E.; Simone, G. Formulati Innovativi per Agricoltura Biologica e Biodinamica a Carattere Antiossidante, Corroborante, Antimicrobico, Biocida e Repellente a Base di Estratti Naturali Standardizzati in Tannini Idrolizzabili e Condensati. Italian Patent 102020000015442, 1 August 2022. filed 2 October 2020. [Google Scholar]
- Gakuubi, M.M.; Maina, A.W.; Wagacha, J.M. Antifungal activity of essential oil of Eucalyptus camaldulensis Dehnh. against selected Fusarium spp. Int. J. Microbiol. 2017, 2017, 8761610. [Google Scholar]
- Kottearachchi, N.S.; Sammani, A.; Kelaniyangoda, D.B.; Samarasekara, R. Antifungal activity of essential oils of Ceylon Eucalyptus species for the control of Fusarium solani and Sclerotium rolfsii. Arch. Phytopathol. Plant Prot. 2012, 45, 2026–2035. [Google Scholar] [CrossRef]
- Duduk, N.; Markovic, T.; Vasic, M.; Duduk, B.; Vico, I.; Obradovic, A. Antifungal activity of three essential oils against Colletotrichum acutatum, the causal agent of strawberry anthracnose. J. Essent. Oil Bear. Plants 2015, 18, 529–537. [Google Scholar] [CrossRef]
- Varo, A.; Mulero-Aparicio, A.; Adem, M.; Roca, L.F.; Raya-Ortega, M.C.; López-Escudero, F.J.; Trapero, A. Screening water extracts and essential oils from Mediterranean plants against Verticillium dahliae in olive. Crop Prot. 2017, 92, 168–175. [Google Scholar] [CrossRef]
- Aslam, M.F.; Irshad, G.; Naz, F.; Khan, M.A. Evaluation of the antifungal activity of essential oils against Alternaria alternata causing fruit rot of Eriobotrya japonica. Turk. J. Biochem. 2022, 47, 511–521. [Google Scholar] [CrossRef]
- Saoud, I.; Hamrouni, L.; Gargouri, S.; Amri, I.; Hanana, M.; Fezzani, T.; Jamoussi, B. Chemical composition, weed killer and antifungal activities of Tunisian thyme (Thymus capitatus Hoff. et Link.) essential oils. Acta Aliment. 2013, 42, 417–427. [Google Scholar] [CrossRef]
- Mi, T.; Luo, D.; Li, J.; Qu, G.; Sun, Y.; Cao, S. Carvacrol exhibits direct antifungal activity against stem-end rot disease and induces disease resistance to stem-end rot disease in kiwifruit. Physiol. Mol. Plant Pathol. 2023, 127, 102065. [Google Scholar] [CrossRef]
- Ćosić, J.; Vrandečić, K.; Jurkovic, D. The effect of essential oils on the development of phytopathogenic fungi. Biol. Control. Prev. Food Deterior. 2014, 273–291. [Google Scholar]
- Numpaque, M.A.; Oviedo, L.A.; Gil, J.H.; García, C.M.; Durango, D.L. Thymol and carvacrol: Biotransformation and antifungal activity against the plant pathogenic fungi Colletotrichum acutatum and Botryodiplodia theobromae. Trop. Plant Pathol. 2011, 36, 3–13. [Google Scholar] [CrossRef]
- Kant, R.; Kumar, A. Review on essential oil extraction from aromatic and medicinal plants: Techniques, performance and economic analysis. Sustain. Chem. Pharm. 2022, 30, 100829. [Google Scholar] [CrossRef]

| Compound | RIcal | RIref | EgEO | MpEO | TcEO |
|---|---|---|---|---|---|
| α-pinene | 1030 | 1026 | 2.3 | 1.0 | 0.8 |
| α-thujene | 1030 | 1030 | 0.0 | 0.0 | 0.3 |
| camphene | 1067 | 1065 | 0.0 | 0.0 | 0.1 |
| β-pinene | 1122 | 1118 | 0.1 | 1.2 | 0.1 |
| sabinene | 1130 | 1126 | 0.0 | 0.7 | 0.0 |
| myrcene | 1168 | 1167 | 0.2 | 0.4 | 0.6 |
| α-phellandrene | 1177 | 1177 | 0.3 | 0.2 | 0.0 |
| α-terpinene | 1180 | 1179 | 0.3 | 0.5 | 0.4 |
| 2,3-dehydro-1,8-cineole | 1202 | 1197 | 0.1 | 0.0 | 0.0 |
| limonene | 1185 | 1199 | 3.8 | 3.0 | 0.4 |
| 1,8-cineole | 1225 | 1221 | 86.7 | 7.0 | 0.1 |
| β-ocimene | 1235 | 1245 | 0.3 | 0.5 | 0.0 |
| γ-terpinene | 1268 | 1254 | 0.3 | 0.5 | 0.2 |
| p-cymene | 1286 | 1281 | 1.2 | 0.6 | 13.3 |
| terpinolene | 1290 | 1305 | 0.0 | 0.3 | 0.0 |
| allo-ocimene | 1383 | 1377 | 0.1 | 0.0 | 0.0 |
| germacrene D | 1410 | 1409 | 0.0 | 2.0 | 0.0 |
| menthone | 1470 | 1476 | 0.0 | 25.5 | 0.0 |
| isomenthone | 1480 | 1484 | 0.0 | 4.2 | 0.0 |
| linalool | 1545 | 1544 | 0.1 | 0.4 | 1.6 |
| menthyl acetate | 1555 | 1561 | 0.0 | 4.0 | 0.0 |
| 4-ol-terpinen | 1617 | 1612 | 0.5 | 1.7 | 1.4 |
| β-caryophillene | 1631 | 1625 | 0.0 | 3.6 | 3.0 |
| menthol isomer | 1610 | 1644 | 0.0 | 5.3 | 0.0 |
| menthol isomer | 1641 | 1644 | 0.0 | 35.9 | 0.0 |
| alloaromandrene | 1640 | 1645 | 0.3 | 0.0 | 0.0 |
| α-humulene | 1658 | 1667 | 0.0 | 0.5 | 0.1 |
| α-terpineol | 1675 | 1695 | 2.7 | 0.0 | 0.2 |
| citral | 1698 | 1695 | 0.1 | 0.0 | 0.0 |
| (-)borneol | 1706 | 1704 | 0.0 | 0.0 | 0.5 |
| piperitone | 1705 | 1710 | 0.0 | 0.9 | 0.0 |
| α-terpinyl acetate | 1722 | 1721 | 0.7 | 0.0 | 0.0 |
| thymol | 2155 | 2154 | 0.0 | 0.1 | 0.9 |
| carvacrol | 2230 | 2225 | 0.0 | 0.0 | 76.1 |
| Fungal Strains | Percentage (%) of Inhibition | ||
|---|---|---|---|
| EgEO 0.1 g/L | MpEO 0.1 g/L | TcEO 0.1 g/L | |
| Alternaria sp. | 3.60 ± 4.59 a | 10.3 ± 3.25 b | 66.05 ± 2.47 c |
| Arthrinium marii | 13.20 ± 11.83 b | 1.30 ± 1.64 a | 99.57 ± 1.06 c |
| Colletotrichum acutatum | 4.94 ± 1.98 a | 7.71 ± 2.28 b | 35.85 ± 5.54 c |
| Fomitiporia mediterranea | −0.41 ± 6.34 a | 5.10 ± 4.68 b | 28.93 ± 3.60 c |
| Fusarium solani | 2.25 ± 3.27 a | 2.82 ± 3.15 a | 47.04 ± 15.04 b |
| Verticillium dahliae | −1.63 ± 2.93 a | 1.60 ± 2.93 b | 68.89 ± 4.78 c |
| Fungal Strains | Percentage (%) of Inhibition | ||||
|---|---|---|---|---|---|
| TcEO 0.05 g/L | TcEO 0.10 g/L | TcEO 0.20 g/L | TcEO 0.30 g/L | EC50 g/L | |
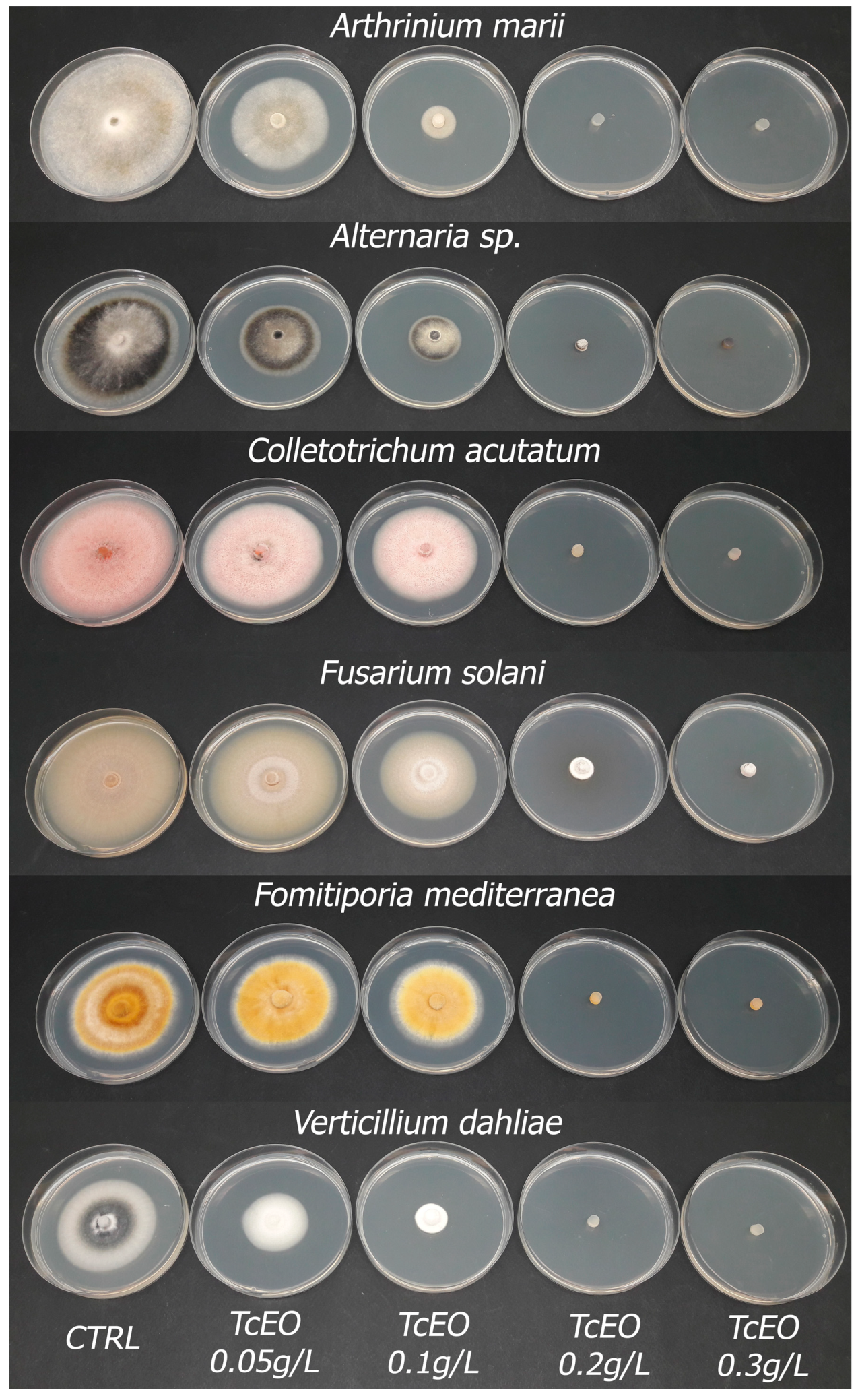
| Alternaria sp. | 24.39 ± 21.97 a | 53.69 ± 12.19 b | 98.27 ± 1.06 c | 100.00 ± 0.00 c * | 0.09 ± 0.06 |
| Arthrinium marii | 58.49 ± 23.27 a | 89.57 ± 8.48 b | 100.00 ± 0.00 c ** | 100.00 ± 0.00 c ** | 0.05 ± 0.03 |
| Colletotrichum acutatum | 18.78 ± 1.52 a | 25.71 ± 0.61 b | 97.85 ± 3.93 c | 100.00 ± 0.00 d * | 0.128 ± 0.005 |
| Fomitiporia mediterranea | 6.46 ± 2.19 a | 12.06 ± 3.30 b | 100.00 ± 0.00 c ** | 100.00 ± 0.00 c ** | 0.13 ± 0.05 |
| Fusarium solani | 8.27 ± 6.14 a | 25.24 ± 14.17 b | 75.12 ± 14.25 c | 93.50 ± 5.80 d | 0.14 ± 0.01 |
| Verticillium dahliae | 29.16 ± 8.99 a | 65.40 ± 5.88 b | 97.06 ± 3.60 c | 100.00 ± 0.00 d ** | 0.08 ± 0.03 |
| Fungal Strains | Percentage (%) of Inhibition | |
|---|---|---|
| TcEO 0.10 g/L | CAR 0.07 g/L | |
| Alternaria sp. | 53.69 ± 12.19 a | 60.16 ± 11.17 a |
| Arthrinium marii | 89.57 ± 8.48 a | 91.67 ± 9.86 a |
| Colletotrichum acutatum | 25.71 ± 0.61 a | 28.24 ± 1.25 b |
| Fomitiporia mediterranea | 12.06 ± 3.30 a | 6.95 ± 3.00 a |
| Fusarium solani | 25.24 ± 14.17 a | 26.86 ± 15.54 a |
| Verticillium dahliae | 65.40 ± 5.88 a | 65.79 ± 10.51 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simone, G.; Campo, M.; Urciuoli, S.; Moncini, L.; Giorgini, M.; Ieri, F.; Vignolini, P. In Vitro Efficacy of Thymbra capitata (L.) Cav. Essential Oil Against Olive Phytopathogenic Fungi. Microorganisms 2025, 13, 1503. https://doi.org/10.3390/microorganisms13071503
Simone G, Campo M, Urciuoli S, Moncini L, Giorgini M, Ieri F, Vignolini P. In Vitro Efficacy of Thymbra capitata (L.) Cav. Essential Oil Against Olive Phytopathogenic Fungi. Microorganisms. 2025; 13(7):1503. https://doi.org/10.3390/microorganisms13071503
Chicago/Turabian StyleSimone, Gabriele, Margherita Campo, Silvia Urciuoli, Lorenzo Moncini, Maider Giorgini, Francesca Ieri, and Pamela Vignolini. 2025. "In Vitro Efficacy of Thymbra capitata (L.) Cav. Essential Oil Against Olive Phytopathogenic Fungi" Microorganisms 13, no. 7: 1503. https://doi.org/10.3390/microorganisms13071503
APA StyleSimone, G., Campo, M., Urciuoli, S., Moncini, L., Giorgini, M., Ieri, F., & Vignolini, P. (2025). In Vitro Efficacy of Thymbra capitata (L.) Cav. Essential Oil Against Olive Phytopathogenic Fungi. Microorganisms, 13(7), 1503. https://doi.org/10.3390/microorganisms13071503






