Abstract
In this study, Ni2+ and Cd2+ resistant Pseudomonas aeruginosa 18, Enterobacter ludwigii 11Uz, and Enterobacter cloacae Uz_5 strains were isolated from soils contaminated with heavy metals in the Samarkand and Kashkadarya regions (Uzbekistan), and tested to remove Ni2+ and Cd2+ ions from the environment via biosorption. The biosorption capacity of these strains was observed under in vitro conditions. The biosorption process was highly dependent on the growing conditions, with the highest biosorption rate observed after 300 min of incubation at pH 7.0, and 40 °C. The presence of functional groups such as S=O, NH2, and COOH in the biosorbing microorganisms was confirmed by IR spectroscopy. The adsorption capacity decreased when the initial metal concentration was increased and was enhanced with higher microbial biomass. Enterobacter ludwigii 11Uz strain was found to alter the toxic oxidation state of Ni2+ and Cd2+ cations, while Pseudomonas aeruginosa 18 and Enterobacter cloacae Uz_5 strains reduced the toxicity of Ni2+ cations only by changing their oxidation state. It was confirmed in our studies that the three selected bacterial strains actively participated in the detoxification of Cd2+ through the synthesis of cysteine amino acid.
1. Introduction
Heavy metal pollution is a significant concern due to its persistence and non-biodegradable nature. Soil pollution with heavy metals, especially in Uzbekistan, occurs mainly as a result of amounts of pesticides and contaminants being present in mineral fertilizers. Soil surveys carried out in various locations in Uzbekistan pointed out that the highest amount of copper (1580 mg/kg, i.e., 526 times higher than the permissible limit—PL) and molybdenum (639 mg/kg, i.e., about 64 times higher than the PL) were observed in the soil collected from an old agricultural field (Tashkent region) [1]. Increased concentrations of heavy metals such as zinc, lead, copper, and chromium have been measured in the vicinity of solid waste landfills in the Tashkent region [2]. Analysis of heavy metal soil pollution in the Jizzakh region showed a slight increase in the PL for copper, zinc, chromium, nickel, cobalt, and arsenic [3]. In the soils of the Kashkadarya region of Uzbekistan, the lead content and chromium content exceed the maximum PL by 1.4–1.5 times and 1.16 times, respectively [1]. The Ni content in irrigated soils of that region was on average 7.25 or 10.30 times higher than the PL in the 0–30 cm and 51–80 cm layers, respectively. The Cd content in the deep layer of soil (51–80 cm) was 1.5 times higher than the PL (0.75 mg/kg) [4]. Ni content in the soil upper layer (0–30 cm) of pastures is about 1.5 times higher than the PL, providing evidence that mineral fertilizers are among the main source of heavy metal pollution. Since Cd is one of the most dangerous metals for human health and Ni is found in high amounts in Uzbek soils, these two pollutants were considered for studying microbiological remediation in the present work.
Currently, remediation strategies are classified into two main categories, namely passive and active. Active methods—including soil washing, chemical extraction, and electrokinetic remediation—can be quickly applied and easier to control but often require significant financial investment, large amounts of reagents, and may generate toxic secondary wastes [5]. In contrast, passive methods, such as bioremediation utilizing microorganisms, plants (phytoremediation), or enzymatic processes to degrade or immobilize heavy metals, have raised interest due to their ecological compatibility, cost-effectiveness, and potential for in situ application [6]. Combining microorganisms and plants or various types of microorganisms is a potentially more efficient approach, though its success depends on the species of organisms involved in the process [7]. However, their effectiveness can be limited by factors such as metal bioavailability, soil properties, and microbial activity [8].
Among the latter methods, microbial biosorption offers numerous advantages, including activity within a wide range of pH values and temperatures, strong adsorption capacity, ease of metal separation from the microorganism, cost-effectiveness, and environmental safety [9]. Microorganisms can selectively adsorb both low and high concentrations of heavy metal ions [10], a process which is primarily governed by physical adsorption, ion exchange, complex formation, and bioaccumulation. Due to its negative charge, the cell wall is the main structural component of bacteria cells that interacts with metal ions such as Cd2+ and Ni2+ ions, leading to their immobilization [11]. Therefore, biosorbed metals can also be recovered and their usefulness recovered, a process that cannot occur for those separated by chemical or physical methods [12].
Biosorption efficiency is higher in living bacterial biomass compared to dead biomass and is typically dependent on nutrient availability, cell age, and other environmental factors [10]. However, live bacterial biomass is generally more prone to metal toxicity effects than dead bacterial biomass [12]. These effects are counterbalanced through various mechanisms, including the transformation, bioreduction, or biotransformation of heavy metals [13]. Furthermore, living cells can adapt to metal-contaminated environments by genetically altering their physiological, biochemical, and structural characteristics [14,15,16].
The reduction and oxidation of heavy metal ions by microorganisms can affect their solubility and toxicity, since these features are related to the metal oxidation state [17]. Chemolithotrophic bacteria play a major role in redox processes, enhancing the mobilization of metals [18]. Depending on the chemical characteristics of the environment, heavy metals can be oxidized, reduced, or precipitated into less toxic forms (e.g., sulfides or oxides) by enzymatic activity, which significantly contributes to the resistance of microorganisms to heavy metal toxicity [19,20,21]. A variety of soil bacteria that show resistance to various heavy metals, particularly species belonging to the genera Pseudomonas, Bacillus, Rhizobium, Shewanella, and Desulfovibrio, have been exploited for the bioremediation of contaminated soils [6]. They contribute to the detoxification of metals through mechanisms such as the biosorption, bioaccumulation, enzymatic transformation, precipitation, and reduction of metal ions to less toxic or immobilized forms [22]. To remain viable and active in a contaminated environment, these bacteria must adapt to the chemical and physical stresses caused by heavy metal poisoning. Viability is often supported by mechanisms such as efflux pumps, metal-binding proteins (e.g., metallothionins), and the formation of biofilms that protect against harsh conditions [23]. Once bacteria have accumulated heavy metals in their biomass, the fate of this biomass becomes a major concern for remediation efficiency and environmental safety [18,19,20].
Soils studied for microbial heavy metal remediation are characterized by a moderate pH (typically 6.0–8.0), porosity, sufficient moisture, and organic matter. Effective bioremediation depends not only on the bacterial species and their metabolic capacity but also on soil conditions that support microbial viability and optimize mass transfer processes [12,21].
In this study, experiments were carried out to identify the mechanism involved in the detoxification of Cd2+ and Ni2+ ions by three strains of Ni- and Cd-resistant bacteria that were isolated from polluted soils, with the aim to enhance the understanding and efficiency of bacterial bioremediation/biosorption and detoxification for use in practical applications. For this reason, the biosorption capacity was evaluated under varying environmental conditions (pH, temperature, incubation time, and biomass concentration). The findings could thus contribute to the optimization of biosorption parameters, essential for the development of sustainable and efficient bioremediation technologies (i.e., production and formulation of the strains and their application in polluted fields).
2. Materials and Methods
2.1. Bacteria Strains
Cd- and Ni-resistant strains were isolated from Cd- and Ni-contaminated soil located in the Kashkadarya region (38°20′36.1″ N 66°26′45.3″ E) and Samarkand Region (39°41′11.0″N 66°48′19.8″E. The isolated strains were identified by 16S rDNA sequence analysis and registered in the NCBI GenBank database as Pseudomonas aeruginosa 18, Enterobacter ludwigii 11Uz, and Enterobacter cloacae Uz_5. Currently, these strains are stored in the “Industrially Important Microorganisms Collection” of the Institute of Microbiology, Tashkent.
2.2. Preparation of Selected Bacteria Biomass
The strains were cultivated in a liquid peptone medium at 28 °C for 36 h on an orbital shaker (IKA KS 4000i control, Thermo Fisher Scientific Inc., Waltham, MA, USA) at 120 rpm; at the end of the exponential growth phase, bacterial cells were collected by centrifugation at 6000/8000 rpm for 5/15 min (Eppendorf 5810 R, Hamburg, Germany). The biomass was washed three times with a sterile phosphate-buffered solution (pH 7.2), followed by distilled water to remove the residual growth medium. The biomass was then used for the experiments.
2.3. Determination of Bacterial Biosorption Capacity Under Variable Environmental Conditions
Biosorption experiments were performed by incubating the bacterial cells with the cadmium chloride (CdCl2) or nickel sulfate (NiSO4 × 7H2O), at concentrations of 24.6 mg/L and 200 mg/L, respectively. These concentrations were selected to reflect both environmental relevance (50–80 cm layer of soils have of 0.75 mg/kg Cd, 41.2 mg/kg Ni) and relative toxicity: cadmium is significantly more toxic than nickel and is strictly regulated at lower permissible limits in environmental and health guidelines. The biosorption capacity was determined under varying environmental conditions, including various pH, temperature, incubation time, and cell biomass concentration. The effect of the initial pH on bacterial biosorption was evaluated in the range of 3.0 to 8.0. The pH of the biosorption medium was adjusted using 0.1 M HCl or NaOH solutions before the addition of bacterial cells. This range was chosen based on the known sensitivity of biosorption efficiency to pH, which influences both metal ion speciation and the ionization state of functional groups on the bacterial cell surface (e.g., carboxyl, hydroxyl, and phosphate groups). At lower pH values, competition from excess H+ ions can inhibit metal binding, while at higher pH values, metals may precipitate as hydroxides, distorting biosorption measurements. The temperature range for the biosorption experiments was set from 15 °C to 40 °C. The experiments were carried out in a temperature-controlled shaker incubator to ensure uniform temperature conditions. This range encompasses typical environmental conditions found in Uzbekistan regions characterized by polluted soils and allows for the evaluation of thermal effects on metal uptake kinetics and biomass integrity. The effect of incubation time on biosorption was tested at different time points: 6, 12, 24, 48, and 72 h. At each time interval, samples were withdrawn, and the residual metal concentration in the solution was measured.
The effect of cell biomass concentration on biosorption capacity was tested by varying the bacterial cell concentration between 0.5 g/L and 3.0 g/L. For each concentration, the biosorption experiment was carried out at the optimal temperature, pH, and incubation time as determined in the previous experiments.
For all biosorption experiments, various amounts of bacterial biomass were added to 25 mL of growth medium to which a known concentration of the specific metal ion was added. The mixture was incubated under constant shaking conditions (150 rpm) at the specified temperature. After the desired incubation time, the suspension was sampled and filtered through a 0.22 µm membrane, and the concentration of Ni2+/Cd2+ in the filtrate was measured using an optical emission spectrometer (Perkin Elmer Avio 200, Waltham, MA, USA). To determine metal loss related to processes other than biosorption, a control sample containing only the growth medium and the heavy metals solutions without bacterial biomass were used. The results were expressed as the bacterial biosorption percentage using the following equation:
where Ci and Cf represent the initial and final concentrations of the heavy metals, respectively [12,20].
Biosorption Percentage (%) = (Ci − Cf)/Ci × 100
2.4. Study of Redox Process Reducing the Toxicity of Heavy Metal Cations
The ability of the selected bacteria strains to convert Cd2+ cations into the non-toxic CdS form was determined in Nutrient-Limited Medium (P2). Initially, the bacteria were cultivated in the P2 medium without cysteine and Cd salt at 37 °C for 24 h. Then, 1 mM of CdCl2 salt was added, and the mixture was incubated for 6 h. After 6 h of incubation, if a yellow-green precipitate was formed in the medium, it was concluded that the Cd2+ cations were converted to non-toxic CdS through a reaction of bacteria-produced amino acid cysteine, producing the colored sulfide group (CdS) [24].
2.5. Observation of Oxidation–Reduction of Ni2+ and Cd2+ by Bacteria
The change in the oxidation state of Ni2+ and Cd2+ as a result of the redox activity of the bacteria was determined colorimetrically [25]. The selected bacteria were cultured for 48 h in a modified nutrient peptone medium containing appropriate amounts of Ni2+ and Cd2+ based on the MIC (minimum inhibitory concentration). In our previous studies, the MIC values of Pseudomonas aeruginosa 18, Enterobacter ludwigii 11Uz, and Enterobacter cloacae Uz_5 strains against Ni2+ and Cd2+ cations were determined to be 560, 840, and 840 mg/L and 549, 183, and 183 mg/L, respectively. The MIC values of bacteria against metals were determined by the Agarwal method [26]. The supernatant was separated from the biomass by centrifugation. NaOH, Na2CO3, and Na2HPO4 were added to the separated supernatant in proportions corresponding to the amounts of Ni2+ and Cd2+ in the nutrient medium. These substances served as qualitative analysis indicators for Ni2+ and Cd2+ cations, as their interaction results in the formation of precipitates of various colors. The indicators form a green precipitate upon interaction with Ni2+ and a white precipitate upon exposure to Cd2+ cations [25]. Pure nutrient medium both with and without the addition of Ni2+ and Cd2+ (without bacterial biomass) was used as the control. The absence of precipitate formation was assessed to determine whether the oxidation state of the two cations had changed as a result of bacterial activity, and the toxic form of the metal was lost [27].
3. Results
3.1. Effect of Growing and Environmental Conditions on the Biosorption Capacity of the Bacterial Strains
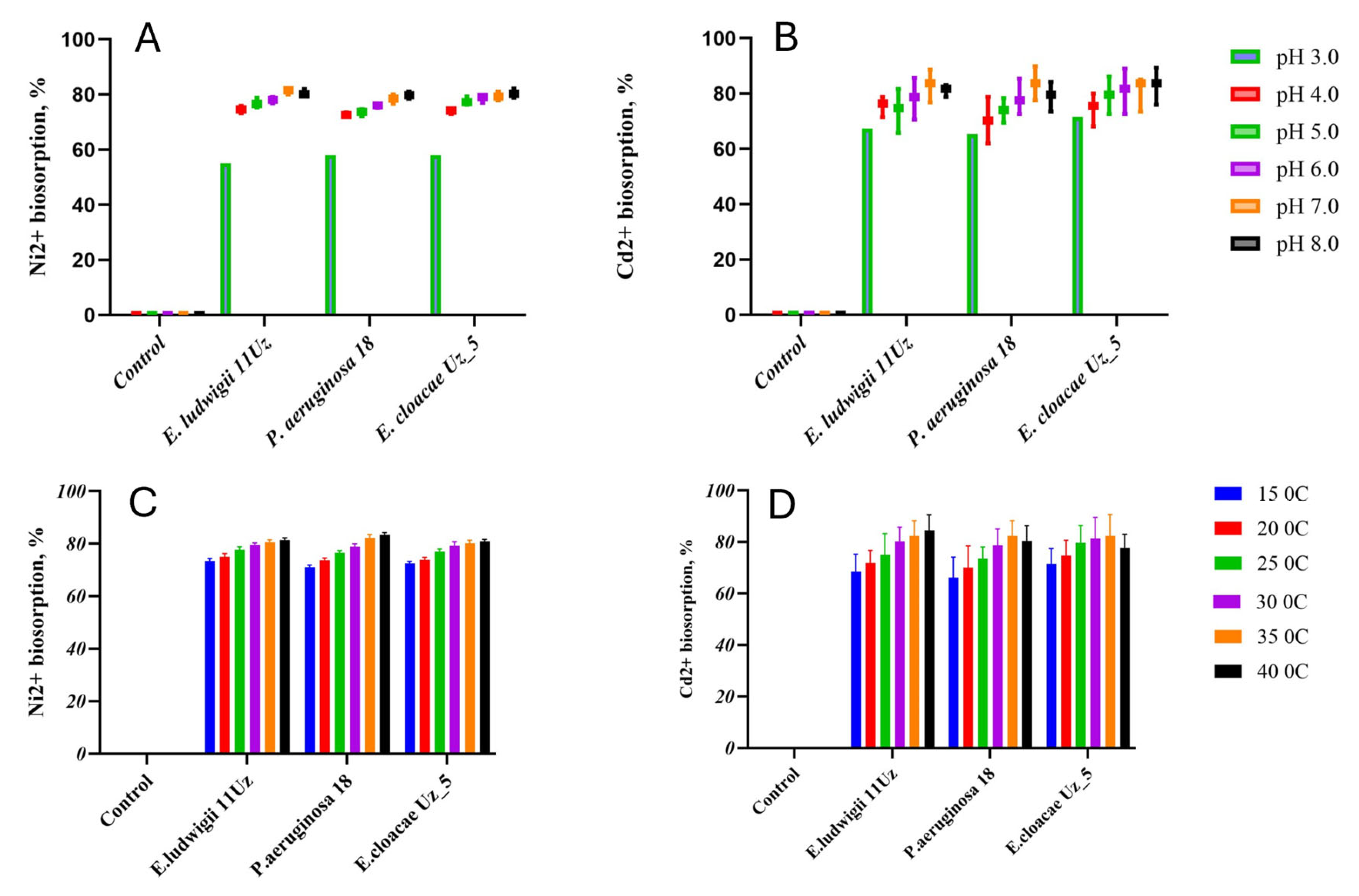
The biosorption capacities of all strains for Ni2+ and Cd2+ were strongly affected by the pH of the solution, generally increasing with the transition from acid to neutral conditions, without further significant changes upon reaching alkaline pH (Figure 1A,B). The biosorption capacity of all strains ranged from 58% to 83% and 63.8% to 82.7% in cases of Ni2+ and Cd2+, respectively, depending on the pH. The removal of the heavy metal cations increased by about 10% and 20% for Ni2+ and Cd2+, respectively (Figure 1A,B).
Figure 1.
Ni2+ and Cd2+ biosorption capacity of the three selected bacteria strains as affected by various pH (A,B) and temperature (C,D) levels. Means ± SD.
No significant differences in the biosorption of Ni2+ and Cd2+ were observed between 15 °C and 20 °C, but the biosorption capacities of all strains increased with higher temperatures. This pattern was particularly noted with Pseudomonas aeruginosa 18, in which the biosorption capacity increased from 142 mg and 16.6 mg at 15 °C to 167 mg and 20.6 mg at 40 °C for Ni2+ and Cd2+, respectively. The 25 °C increase in the temperature thus induced thus an enhancement in the biosorption capacity of about 12% or 15% for Ni2+ and Cd2+, respectively (Figure 1C,D).
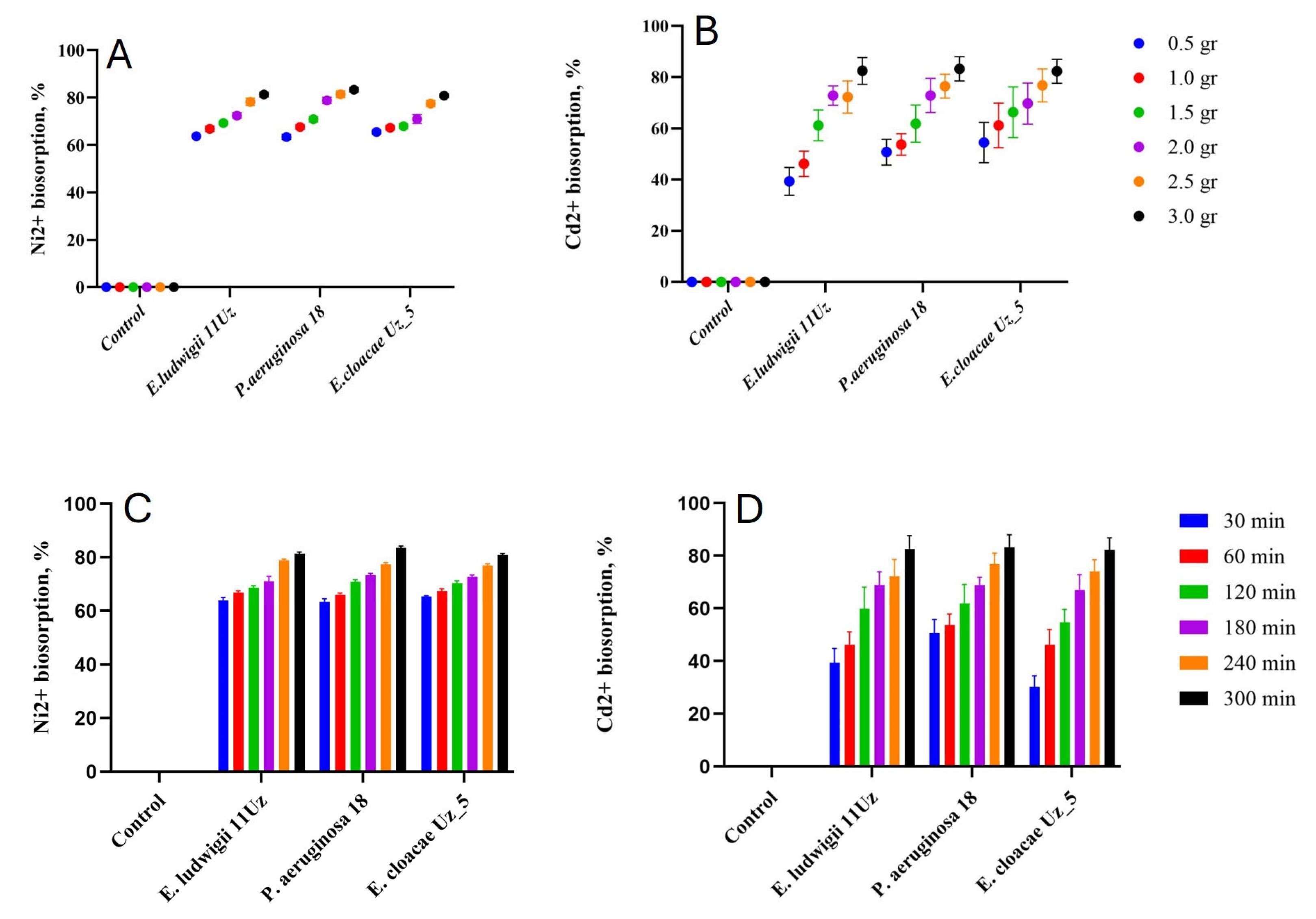
The efficiency of Ni2+ and Cd2+ removal was positively correlated to the amount of bacterial biomass (Figure 2). However, the relation was different for the two cations and was partly affected by the strain. P. aeruginosa 18 was able to increase the Ni2+ biosorption by about 10% when the biomass was increased six-fold, while this increase was more than 30% in case of Cd2+. On average, the three strains biosorption capacity was about 72% or 64% in case of Ni2+ and Cd2+, respectively.
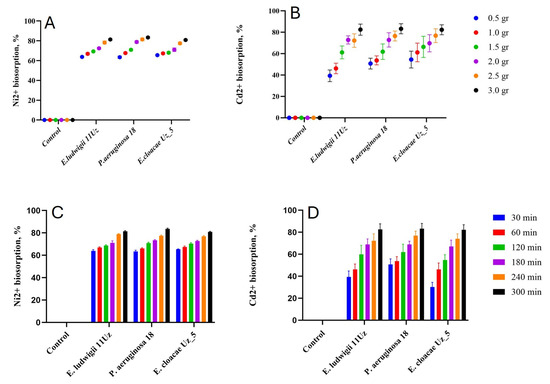
Figure 2.
Ni2+ and Cd2+ biosorption capacity of the three selected bacteria strains as affected by biomass amounts (A,B) and incubation period (C,D). Means ± SD.
The amount of biosorption gradually increased with the duration of the experiment, increasing after approximately 1 and 4 h for Ni2+ and Cd2+, respectively (Figure 2). All three strains responded similarly in terms of biosorption capacity in relation to the incubation time. However, Cd2+ biosorption was more efficient with much longer incubation time, allowing for reductions in the cation concentration of up to 85% in the solution.
3.2. Identification of Bacterial Functional Groups Responsible for Heavy Metal Binding
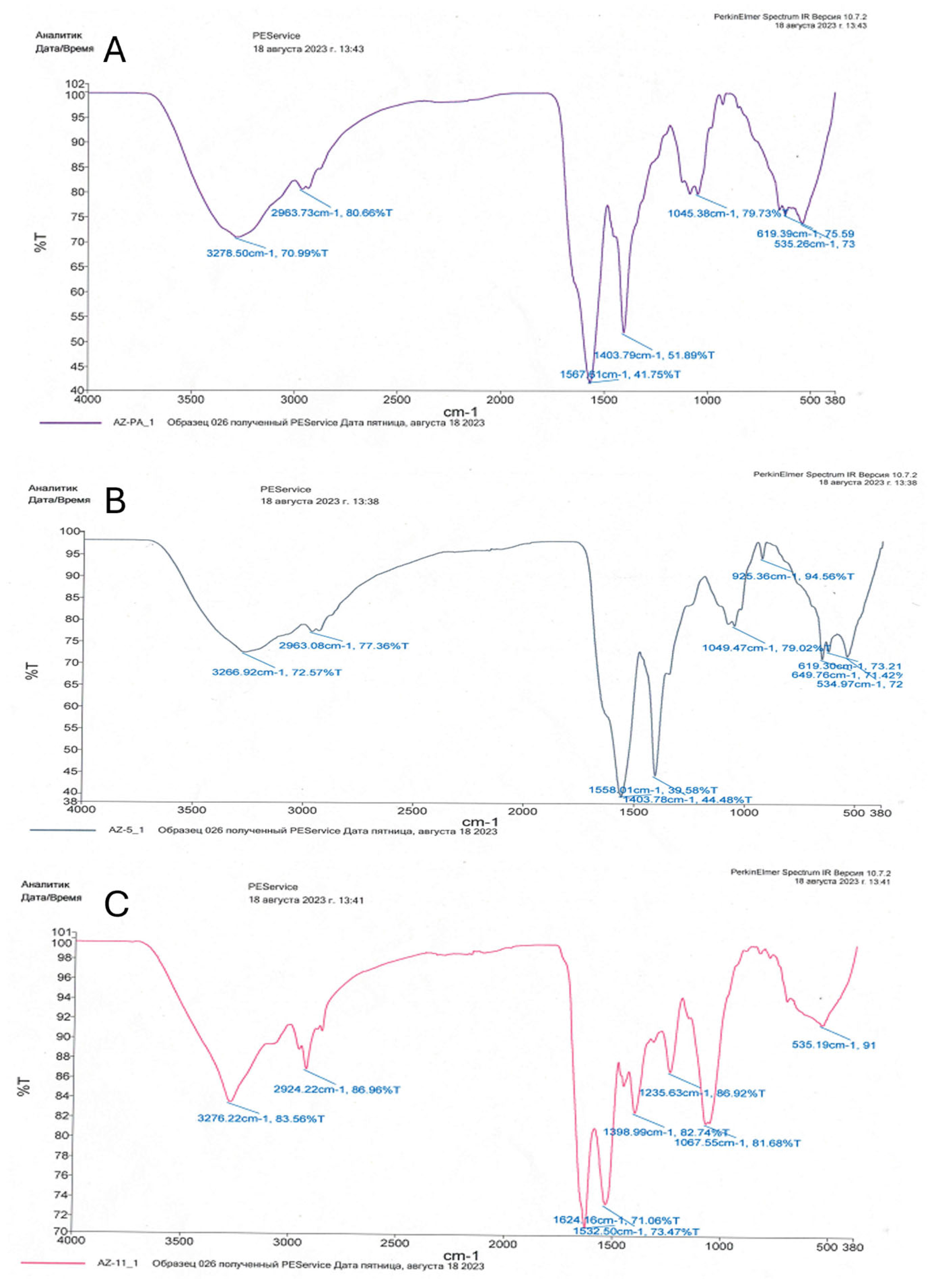
The bioadsorption of heavy metals on the cell surface can be carried out by simple physical methods without disrupting the structural integrity of the cells. FTIR spectroscopy helped in identifying the various functional groups present in bacterial cells in response to heavy metal stress.
The functional groups detected with FTIR analysis were not always present in all three bacteria strains (Table 1, Figure 3). Alcoholic (C-OH), ketonic (C=O), amid (N-H), amino (NH2), and sulfoxide (S=O) groups were common to all. Interestingly, a diverse number of different amino groups was detected for the three strains. Acid (COOH) and aromatic (C-N) groups were detected only in P. aeruginosa and E. cloacae strains, while C-N and P-O groups were identified only in E. ludwigii, as indicated by the presence of specific peaks (Figure 3).

Table 1.
FTIR values determining the functional groups present in the bacterial biomass binding Ni2+ and Cd2+.
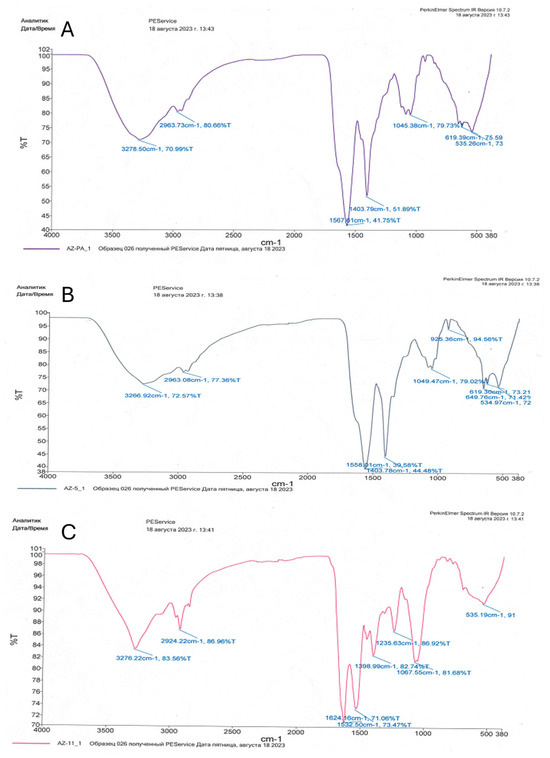
Figure 3.
IR spectroscopy of bacterial biomass: (A)—Pseudomonas aeruginosa 18; (B)—Enterobacter cloacae Uz_5; (C)—Enterobacter ludwigii 11 Uz.
The C-S, CH2/CH3, -CH, and P=S groups, do not participate in the binding of heavy metal cations in bacterial biomass, and the presence of any these groups was not detected.
3.3. Monitoring the Redox Process That Reduces the Toxicity of Heavy Metal Cations
The addition of indicators led to the formation of green and white precipitates when no bacterial cells were present in the medium (control), indicating that the Ni2+ and Cd2+ ions in the nutrient medium were in the +2 oxidation state (Table 2).

Table 2.
Capacity of the three bacterial strains to transform Ni2+ and Cd2+ cations into non-toxic forms.
P. aeruginosa 18 and E. cloacae Uz_5 strains were only able to alter the oxidation state of Ni2+ cations, changing them from the +2 oxidation state, while they did not affect the Cd2+ cations (Table 2). On the other hand, E. ludwigii 11Uz was able to detoxify the substrate by modifying the +2 oxidation state of both Ni2+ and Cd2+ cations.
After 6 h of incubation, a yellow-green precipitate was visually observed in the cultures of all three bacterial strains exposed to Cd2+. This observation suggested a possible involvement of cysteine synthesis in the detoxification process.
4. Discussion
The study showed that pH significantly influenced the biosorption capacity of all tested bacterial strains for both Ni2+ and Cd2+. The uptake of both metals increased as the pH shifted from acidic to neutral conditions. For both Ni2+ and Cd2+ biosorption increased by about 25%. Cd2+ biosorption exhibited a slightly higher sensitivity to pH change compared to Ni2+, increasing by 20% when changing from acidic to neutral/alkaline pH, compared to 10% for Ni2+. This suggests that Cd2+ biosorption may be more affected by changes in ion exchange capacity and surface charge distribution. This trend aligns with findings from previous studies, where pH has been proved to highly affect microorganisms’ biosorption efficiency of Cd2+ and Ni2+ [28,29]. This phenomenon can be explained by the competition between protons and Ni2+ and Cd2+ ions for the negatively charged functional group binding sites on the cell surface: as the pH increases, the number of protons decreases, and the number of binding sites for Ni2+ and Cd2+ increases [30,31]. Furthermore, as the pH increases, the deprotonation of binding sites, reduced electronic repulsion, and enhanced attraction of Ni2+ and Cd2+ cations may cause bacterial surface functional groups to become negatively charged [32,33]. Tests were not conducted at pH values above 8.0 due to the potential formation of metal hydroxide or oxide complexes as a result of hydrolysis under alkaline conditions.
Temperature is another crucial factor that influenced biosorption. No significant differences were found between 15 °C and 20 °C, but the capacities increased at higher temperatures, particularly in P. aeruginosa 18. This corresponds to an average increase in efficiency of ~12% and ~15% for Ni2+ and Cd2+, respectively. Importantly, the temperature-dependent trends were observed across all strains, highlighting a conserved biological response, although the strain-specific variation in the absolute uptake values suggests differences in thermotolerance or surface binding chemistry. Temperature-dependent biosorption enhancement has been reported in other microbial systems [34]. High temperatures can increase the kinetic energy of the solution and the surface activity, the diffusion rates, and, possibly, the permeability of cell walls, facilitating ion exchange [35]. However, excessive temperatures may denature cell wall proteins, limiting biosorption. The observed enhancement suggests that the process includes both passive surface binding and energy-dependent mechanisms and it has been proposed that the biosorption process is endothermic in nature [36].
The duration of exposure also affected metal removal efficiency, with equilibrium being reached faster for Ni2+ (1 h) than for Cd2+ (4 h). Cd2+ biosorption continued to improve with extended incubation, allowing up to 85% removal, which was ~50% higher than what was observed at 30 min. This could reflect a multi-phase binding process, including an initial rapid surface binding followed by a slower intracellular sequestration or complexation phase. Furthermore, the slower saturation for Cd2+ may reflect its larger ionic radius or lower diffusion coefficient, which has also been documented in other bacterial systems [9,36].
The biosorption capacity of E. cloacae Uz_5, E. ludwigii 11Uz, and P. aeruginosa 18 strains remained steady after 5 h of incubation, indicating that approximately 3 g/L of bacterial cells became saturated with Ni and Cd after 5 h, which can thus be considered the optimal immobilization time to assess such capacity. Therefore, the effect of bacterial biomass on Ni and Cd immobilization efficiency was measured at the optimal immobilization time until 6 h. The biosorption processes requiring a short time would be highly advantageous for practical applications. Slight fluctuations in the biosorption rate may result in the desorption or release of metal ions from the bacterial cells
The biosorption of both Ni2+ and Cd2+ was positively correlated with the amount of bacterial biomass, highlighting the importance of surface area and the density of available binding sites in determining biosorption capacity [37]. However, this relationship was not uniform across metals or bacterial strains. P. aeruginosa 18 demonstrated a notable increase in Cd2+ uptake with the increase in biomass—over 30%—while the same increase in biomass led to only a 10% improvement in Ni2+ uptake. This indicates that Cd2+ removal may benefit more from the availability of additional sorption sites, or that Cd2+ has a higher affinity for certain functional groups that become more abundant with increased biomass.
Increasing the bacterial cell biomass in a 150 mL Cd and Ni solution did not significantly improve Cd and Ni immobilization efficiency, indicating that a high quantity of microbial cells in a limited heavy metal solution does not enhance the immobilization of metal ions. Therefore, finding the optimal balance between the amount of biosorbent and the volume of the heavy metal solution is important. In this study, it was determined that 3 g/L of bacterial cells was the most optimal amount for a 150 mL Ni solution with a concentration of 200 mg/L.
Overall, the average biosorption capacities across all strains were higher for Ni2+ (72%) than for Cd2+ (64%). The variation between strains in terms of biosorption efficiency points to differences in cell surface chemistry and the presence of specific functional groups, as corroborated by infrared (IR) spectroscopy. IR spectroscopy has a high sensitivity for detecting functional groups and changes in bacterial components such as lipids and proteins [38].
Bacterial cells consist of lipids, proteins, and carbohydrates, which contain chemically active functional groups (such as PO43−, OH−, S2−, CO2−, and SO42−) known to contribute to metal ion chelation. These functional groups participate in metal-microbe interactions and are involved in the metal adsorption mechanism on bacterial surfaces [23,39]. For example, the presence of these functional groups was noted in Bacillus sp. MC3B-22, Microbacterium sp. MC3B-10, and Bacillus sp. strains, which were able to remove 75% of Cd2+ from the environment [40]. These functional groups including hydroxyl, carboxyl, phosphate, and amino can form electrostatic, ion-dipole, and coordination interactions with metal ions, leading to their immobilization on bacterial surfaces [41] and thus ensuring biosorption capacity [42].
The alteration of the oxidation–reduction state of heavy metal ions through reduction or oxidation reactions can effectively reduce their toxicity [43]. This protective mechanism can be managed by detoxifying enzymes regulated by the specific resistance genes of microorganisms. For example, bacteria like Bacillus sp. exhibit resistance to mercury ions through the action of mercury reductase [44].
Beyond passive biosorption, certain bacterial strains showed evidence of metabolic detoxification pathways—an ability to chemically alter or reduce the toxicity of heavy metals through redox transformation or bioprecipitation. The colorimetric redox assay indicated that P. aeruginosa 18 and E. cloacae Uz_5 could alter the oxidation state of Ni2+, but not Cd2+. In contrast, E. ludwigii 11Uz was capable of reducing both Ni2+ and Cd2+, indicating a broader detoxification capacity.
A promising pathway for Cd2+ detoxification involves the synthesis of non-toxic CdS, a sulfur-containing amino acid. The visual formation of yellow-green precipitates after 6 h of incubation with Cd2+ suggested the biosynthesis of cadmium sulfide (CdS), a relatively insoluble and less toxic compound [24]. This biotransformation is likely mediated by cysteine’s sulfhydryl (-SH) groups, which can bind with Cd2+ to form CdS nanoparticles [24,45]. Heavy metals form insoluble complexes with thiol groups, which play an important role in coordinating the antioxidant defense systems of living organisms. They are strong antioxidants that act as electron acceptors, reducing unstable free radicals through oxidation [46]. Important enzymes in microbial metabolism often contain sulfhydryl (SH) groups, and heavy metals such as Cd2+, Ag2+, and Hg2+ can bind to these groups, which inhibits the activity of metals [47]. On metal-resistant bacterial surfaces, anion groups such as S2− and PO43− are present, which easily bind with Cd2+, thereby reducing the phytoavailability of metal ions [48]. Although not quantitatively confirmed in this study, this mechanism is consistent with the previous literature and warrants further validation to quantify cysteine production and CdS formation through spectrophotometric and molecular assay.
The findings presented in this work have clear implications for practical applications. The rapid biosorption kinetics (within 1–4 h), enhanced metal uptake under neutral to slightly alkaline conditions, and effective detoxification mechanisms, make these bacterial strains strong candidates for bio-based remediation technologies. For instance, the use of E. ludwigii in Cd2+-rich environments could be particularly beneficial due to its dual biosorption and redox conversion capabilities. Moreover, optimizing biomass dosage could enhance biosorption performance while minimizing the process costs in industrial-scale bioreactors, constructed wetlands, or in situ soil treatment.
5. Conclusions
This study highlights the significant potential of selected bacterial strains in the biosorption and detoxification of heavy metal ions, particularly Ni2+ and Cd2+. The experimental results demonstrated that the biosorption capacity of Pseudomonas aeruginosa 18, Enterobacter ludwigii 11Uz, and Enterobacter cloacae Uz_5 was influenced by various environmental factors such as pH, temperature, and biosorbent dosage. Among these strains, Enterobacter ludwigii 11Uz exhibited the most effective capacity to reduce the toxic 2+ oxidation state of Ni2+ and Cd2+ ions, transforming them into less harmful forms.
Additionally, cysteine played a crucial role in the detoxification process by facilitating the transformation of Cd2+ into a less toxic CdS precipitate. This suggests that the selected bacterial strains possess intrinsic mechanisms to not only biosorb but also detoxify heavy metals, making them promising candidates for bioremediation applications. The involvement of functional groups such as hydroxyl, amine, carboxyl, phosphate, and sulfur in the biosorption process further emphasizes the biochemical versatility of these bacteria.
These findings suggest that these bacterial strains isolated from soils in Uzbekistan can be effectively utilized for the remediation of environments contaminated with Ni2+ and Cd2+, offering a sustainable alternative to traditional methods of heavy metal removal. The formulation and application methods most suitable for exploiting these strains are currently under development. These local bacterial strains could be used as unexpensive, environmentally friendly, and efficient biosorbents for cleaning Ni2+ and Cd2+ contaminated environments without polluting the surroundings.
Based on these findings, future research should explore the molecular and genetic basis of heavy metal biosorption and redox transformation in these strains. Expanding the study to include mixed-metal systems and more complex real-world effluents will help assess the robustness and selectivity of these bacterial strains under environmentally relevant conditions. In this context, the development of a consortium with these strains with proper formulation may allow for the enhancement or combination of desirable traits across strains, which can be tailored for specific bioremediation applications in heavy metal-contaminated environments.
Author Contributions
Conceptualization, A.U. and E.M.; Methodology, I.K., and L.A.; Software, A.U.; Formal analysis, G.K.; Investigation, L.C.; Resources, I.K.; Data curation, E.M. and L.C.; Writing—Original Manuscript Preparation, A.U.; Writing—Review and editing, E.M., G.K. and L.C.; Visualization, L.A.; Project Administration, I.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was was carried out with the basic funding of the Academy of Sciences of the Republic of Uzbekistan and supported by sub-project titled Modernization of the Institute of Microbiology of the Academy of Sciences of the Republic of Uzbekistan to create an Accredited Microbiological Testing Laboratory (contract number PRIM-01-06) within the framework of the Public Research Institutes Modernization Program (PRIM).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Acknowledgments
We acknowledge the Institute Microbiology of Academy Sciences of the Republic of Uzbekistan, which carried out a basic topic, for creating sufficient conditions to the experiments in the laboratory and Plant Protection and Quarantine Scientific Research Institute of Uzbekistan for conducting joint research. This publication has been produced within the framework of the Grant PRIM 01-24 titled “Modernization of the Institute of Microbiology of the Academy of Sciences of the Republic of Uzbekistan to create an Accredited Microbiological Testing Laboratory (AMTL)”, funded under the MUNIS Project, supported by the World Bank and the Government of the Republic of Uzbekistan. The statements do not necessarily reflect the official position of the World Bank and the Government of the Republic of Uzbekistan.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Karimov, H.N.; Uzakov, Z.Z.; Khushmurodov, J.P.; Usmonova, D.A.; Mallaeva, D.A. Pollution of irrigated soils and their biological treatment. Sci. Rev. Biol. Sci. 2021, 2, 34–40. [Google Scholar]
- Zhabbarov, Z.A.; Atoeva, G.R.; Sayitov, S.S. Pollution of soils with heavy metals around the landfill of municipal solid waste in Tashkent. Sci. Rev. Biol. Sci. 2021, 2, 17–23. [Google Scholar]
- Berdieva, D.S. Soil contamination with heavy metals in the sh. Rashidovsky district of Jizzakh region and methods of their decrease from the soil composition. E3S Web Conf 2021, 265, 03007. [Google Scholar] [CrossRef]
- Karimov, K.N.; Kadirova, G.K.; Riskiev, R.R.; Usmanova, D.A.; Mallaeva, D.A. Pollution of agricultural soils with mobile metals. J. Agric. Biol. Sci. 2024, 5, 76–84. [Google Scholar]
- Wang, Y.; Luo, Y.; Zeng, G.; Wu, X.; Wu, B.; Li, X.; Xu, H. Characteristics and in situ remediation effects of heavy metal immobilizing bacteria on cadmium and nickel co-contaminated soil. Ecotoxicol. Environ. Saf. 2020, 192, 110294. [Google Scholar] [CrossRef]
- Rahman, Z.; Singh, V.P. Bioremediation of toxic heavy metals (THMs) contaminated sites: Concepts, applications and challenges. Environ. Sci. Pollut. Res. 2020, 27, 27563–27581. [Google Scholar] [CrossRef]
- Kang, C.-H.; Kwon, Y.-J.; So, J.-S. Bioremediation of heavy metals by using bacterial mixtures. Ecol. Eng. 2016, 89, 64–69. [Google Scholar] [CrossRef]
- Hassan, M.U.; Chattha, M.U.; Khan, I.; Chattha, M.B.; Aamer, M.; Nawaz, M.; Ali, A.; Khan, M.A.U.; Khan, T.A. Nickel toxicity in plants: Reasons, toxic effects, tolerance mechanisms, and remediation possibilities—A review. Environ. Sci. Pollut. Res. 2019, 26, 12673–12688. [Google Scholar] [CrossRef]
- Nnaji, N.D.; Onyeaka, H.; Miri, T.; Ugwa, C. Bioaccumulation for heavy metal removal: A review. SN Appl. Sci. 2023, 5, 125. [Google Scholar] [CrossRef]
- Pathak, A.; Agarwal, M.; Rathore, R.S.; Chauhan, A. Gene Determinants for Mercury Bioremediation as Revealed by Draft Genome Sequence Analysis of Stenotrophomonas sp. Strain MA5. Microbiol. Resour. Announc. 2019, 8, e00130-19. [Google Scholar] [CrossRef]
- Tan, H.; Wang, C.; Zeng, G.; Luo, Y.; Li, H.; Xu, H. Bioreduction and biosorption of Cr(VI) by a novel Bacillus sp. CRB-B1 strain. J. Hazard. Mater. 2020, 15, 121628. [Google Scholar] [CrossRef] [PubMed]
- Pham, V.H.T.; Kim, J.; Chang, S.; Chung, W. Bacterial biosorbents, an efcient heavy metals green clean-up strategy: Prospects, challenges, and opportunities. Microorganisms 2022, 10, 610. [Google Scholar] [CrossRef]
- Parihar, J.K.; Parihar, P.K.; Pakade, Y.B.; Katnoria, J.K. Bioaccumulation potential of indigenous plants for heavy metal phytoremediation in rural areas of Shaheed Bhagat Singh Nagar, Punjab (India). Environ. Sci. Pollut. Res. Int. 2021, 28, 2426–2442. [Google Scholar] [CrossRef]
- Wood, B.W. Nickel deficiency symptoms are influenced by foliar Zn:Ni or Cu:Ni concentration ratio. Acta Hortic. 2010, 868, 163–170. [Google Scholar] [CrossRef]
- Maňkovská, B.; Godzik, B.; Badea, O.; Shparyk, Y.; Moravčík, P. Chemical and morphological characteristics of key tree species of the Carpathian Mountains. Environ. Pollut. 2004, 130, 41–54. [Google Scholar] [CrossRef]
- Usmonkulova, A.; Shonakhunov, T.; Kadirova, G. Activity of nitrogen-fixing cyanobacteria under salinity and heavy metals stress. J. Pharm. Negat. Results 2022, 13, 355–363. [Google Scholar] [CrossRef]
- Kang, S.M.; Shahzad, R.; Bilal, S.; Khan, A.L.; Park, Y.G.; Lee, K.E.; Lee, I.J. Indole3-acetic-acid and ACC deaminase producing Leclercia adecarboxylata MO1 improves Solanum lycopersicum L. growth and salinity stress tolerance by endogenous secondary metabolites regulation. BMC Microbiol. 2019, 19, 80. [Google Scholar] [CrossRef]
- Enshaei, M.; Khanafari, A.; Akhavan, S.A. Metallothionein induction in two species of Pseudomonas exposed to cadmium and copper contamination. Iran. J. Environ. Health Sci. Eng. 2010, 7, 287–298. [Google Scholar]
- Meng, D.; Li, J.; Liu, T.; Liu, Y.; Yan, M.; Hu, J.; Li, X.; Liu, X.; Liang, Y.; Liu, H.; et al. Effects of redox potential on soil cadmium solubility: Insight into microbial community. J. Environ. Sci. 2019, 75, 224–232. [Google Scholar] [CrossRef]
- Essa, A.M.M.; Al Abboud, M.A.; Khatib, S.I. Metal transformation as a strategy for bacterial detoxification of heavy metals. J. Basic Microbiol. 2018, 58, 17–29. [Google Scholar] [CrossRef]
- Liu, M.; Hu, Z.; Fan, Y.; Hua, B.; Yang, W.; Pang, S.; Mao, R.; Zhang, Y.; Bai, K.; Fadda, C.; et al. Effects of leguminous green manure–crop rotation on soil enzyme activity and stoichiometry. J. Plant Ecol. 2024, 17, rtae065. [Google Scholar] [CrossRef]
- Patil, A.; Chakraborty, S.; Yadav, Y.; Sharma, B.; Singh, S.; Arya, M. Bioremediation strategies and mechanisms of bacteria for resistance against heavy metals: A review. Bioremediat. J. 2024, 1–33. [Google Scholar] [CrossRef]
- Pagnucco, G.; Overfield, D.; Chamlee, Y.; Shuler, C.; Kassem, A.; Opara, S.; Najaf, H.; Abbas, L.; Coutinho, O.; Fortuna, A. Metal tolerance and biosorption capacities of bacterial strains isolated from an urban watershed. Front. Microbiol. 2023, 14, 1278886. [Google Scholar] [CrossRef] [PubMed]
- Mahle, R.; Kumbhakar, P.; Pramanik, A.; Kumbhakar, P.; Sahoo, S.; Mukherjee, R.; Tiwary, C.S.; Banerjee, R. Probing the bacterial detoxification of cadmium to form cadmium sulfide quantum dots and the underlying mechanism. Mater. Adv. 2020, 1, 1168–1175. [Google Scholar] [CrossRef]
- Amonov, M.R.; Shirinova, Q.G. Analytical chemistry in diagrams and tables [Analitik kimyo sxema va jadvallarda]. In Reference Book; BuxDU: Bukhara, Uzbekistan, 2019; p. 285. [Google Scholar]
- Agarwal, M.; Rathore, R.S.; Chauhan, A. A Rapid and High Throughput MIC Determination Method to Screen Uranium Resistant Microorganisms. Methods Protoc. 2020, 3, 21. [Google Scholar] [CrossRef]
- Govarthanan, M.; Park, S.-H.; Park, Y.-J.; Myung, H.; Krishnamurthy, R.R.; Lee, S.-H.; Lovanh, N.; Kamala-Kannan, S.; Oh, B.-T. Lead biotransformation potential of allochthonous Bacillus sp. SKK11 with sesame oil cake extract in mine soil. RSC Adv. 2015, 5, 54564–54570. [Google Scholar] [CrossRef]
- Mejias Carpio, I.E.; Ansari, A.; Rodrigues, D.F. Relationship of biodiversity with heavy metal tolerance and sorption capacity: A meta-analysis approach. Environ. Sci. Technol. 2018, 52, 184–194. [Google Scholar] [CrossRef]
- Green-Ruiz, C.; Rodriguez-Tirado, V.; Gomez-Gil, B. Cadmium and zinc removal from aqueous solutions by Bacillus Jeotgali: pH, salinity and temperature effects. Bioresour. Technol. 2008, 99, 3864–3870. [Google Scholar] [CrossRef]
- Agarwal, M.; Pathak, A.; Rathore, R.S.; Prakash, O.; Singh, R.; Jaswal, R.; Seaman, J.; Chauhan, A. Proteogenomic Analysis of Burkholderia Species Strains 25 and 46 Isolated from Uraniferous Soils Reveals Multiple Mechanisms to Cope with Uranium Stress. Cells 2018, 7, 269. [Google Scholar] [CrossRef]
- Andy, A.K.; Masih, S.A.; Gour, V.S. Isolation, screening and characterization of plant growth promoting rhizobacteria from rhizospheric soils of selected pulses. Biocatal. Agric. Biotechnol. 2020, 27, 101685. [Google Scholar] [CrossRef]
- Gupta, K.; Chatterjee, C.; Gupta, B. Isolation and characterization of heavy metal tolerant Gram-positive bacteria with bioremedial properties from municipal waste rich soil of Kestopur canal (Kolkata), West Bengal, India. Biologia 2012, 67, 827–836. [Google Scholar] [CrossRef]
- Edulamudi, P.; Antony Masilamani, A.J.; Vanga, U.R.; Divi, V.R.S.G.; Konada, V.M. Nickel tolerance and biosorption potential of rhizobia associated with horse gram [Macrotyloma uniflorum (Lam.) Verdc.]. Int. J. Phytoremediation 2021, 23, 1184–1190. [Google Scholar] [CrossRef] [PubMed]
- Kara, A.K.; Fakıoğlu, Ö.; Kotan, R.; Atamanalp, M.; Alak, G. The investigation of bioremediation potential of Bacillus subtilis and B. thuringiensis isolates under controlled conditions in freshwater. Arch. Microbiol. 2021, 203, 2075–2085. [Google Scholar] [CrossRef]
- Halim, M.A.; Rahman, M.M.; Megharaj, M.; Naidu, R. Cadmium Immobilization in the Rhizosphere and Plant Cellular Detoxification: Role of Plant-Growth-Promoting Rhizobacteria as a Sustainable Solution. J. Agric. Food Chem. 2020, 68, 13497–13529. [Google Scholar] [CrossRef] [PubMed]
- Rocco, D.H.E.; Freire, B.M.; Oliveira, T.J.; Alves, P.L.M.; Júnior, J.M.d.O.; Batista, B.L.; Grotto, D.; Jozala, A.F. Bacillus subtilis as an efective tool for bioremediation of lead, copper and cadmium in water. Discov. Appl. Sci. 2024, 6, 430. [Google Scholar] [CrossRef]
- Ayangbenro, A.S.; Babalola, O.O. A New Strategy for Heavy Metal Polluted Environments: A Review of Microbial Biosorbents. Int. J. Environ. Res. Public Health 2017, 14, 94. [Google Scholar] [CrossRef]
- Kassem, A.; Abbas, L.; Coutinho, O.; Opara, S.; Najaf, H.; Kasperek, D.; Pokhrel, K.; Li, X.; Tiquia-Arashiro, S. Applications of Fourier Transform-Infrared spectroscopy in microbial cell biology and environmental microbiology: Advances, challenges, and future perspectives. Front. Microbiol. 2023, 21, 1304081. [Google Scholar] [CrossRef]
- Ma, Y.; Bantec, T.N.; Oliveira, R.S.; Coutinho, A.; Zhang, C.; Freitas, H. The role of bacteria in metal bioaccumulation and biosorption In Advances in Microbe-Assisted Phytoremediation of Polluted Sites; Bauddh, K., Ma, Y., Eds.; Elsevier: New York, NY, USA, 2022; pp. 103–112. [Google Scholar]
- Camacho-Chab, J.C.; Castañeda-Chávez, M.D.R.; Chan-Bacab, M.J.; Aguila-Ramírez, R.N.; Galaviz-Villa, I.; Bartolo-Pérez, P.; Lango-Reynoso, F.; Tabasco-Novelo, C.; Gaylarde, C.; Ortega-Morales, B.O. Biosorption of Cadmium by Non-Toxic Extracellular Polymeric Substances (EPS) Synthesized by Bacteria from Marine Intertidal Biofilms. Int. J. Environ. Res. Public Health 2018, 15, 314. [Google Scholar] [CrossRef]
- Tunali, S.; Çabuk, A.; Akar, T. Removal of lead and copper ions from aqueous solutions by bacterial strain isolated from soil. Chem. Eng. J. 2006, 115, 203–211. [Google Scholar] [CrossRef]
- Nascimento, T.L.S.; Oliveira, K.F.S.; Junior, J.O.D.; Pimenta, A.S.; Melo, D.M.A.; Melo, M.A.F.; Braga, R.M. Biosorption of nickel and cadmium using Pachira aquatica Aubl. peel biochar. Sci. Rep. 2024, 14, 5086. [Google Scholar] [CrossRef]
- Nascimento, F.X.; Rossi, M.J.; Glick, B.R. Role of ACC deaminase in stress control of leguminous plants. In Plant Growth Promoting Actinobacteria; Subramanian, G., Ed.; Springer Science: Berlin/Heidelberg, Germany, 2016; pp. 179–192. [Google Scholar]
- Pande, V.; Pandey, S.C.; Sati, D.; Bhatt, P.; Samant, M. Microbial Interventions in Bioremediation of Heavy Metal Contaminants in Agroecosystem. Front. Microbiol. 2022, 6, 824084. [Google Scholar] [CrossRef] [PubMed]
- Afzal, A.M.; Rasool, M.H.; Waseem, M.; Aslam, B. Assessment of heavy metal tolerance and biosorptive potential of Klebsiella variicola isolated from industrial effluents. AMB Express 2017, 7, 184. [Google Scholar] [CrossRef] [PubMed]
- Salbitani, G.; Maresca, V.; Cianciullo, P.; Bossa, R.; Carfagna, S.; Basile, A. Non-Protein Thiol Compounds and Antioxidant Responses Involved in Bryophyte Heavy-Metal Tolerance. Int. J. Mol. Sci. 2023, 10, 5302. [Google Scholar] [CrossRef] [PubMed]
- Fein, J.B.; Yu, Q.; Nam, J.; Yee, N. Bacterial cell envelope and extracellular sulfhydryl binding sites: Their roles in metal binding and bioavailability. Chem. Geol. 2019, 521, 28–38. [Google Scholar] [CrossRef]
- Usmonkulova, A.A.; Kadirova, G.K.H.; Khusanov, T.S.; Shonakhunov, T.E.; Shukurov, N. Determination of local bacteria synthesizing ACC deaminase on plant growth indicators under nickel and cadmium stress conditions. SABRAO J. Breed. Genet. 2024, 56, 2033–2044. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).