A Halophilic Bacterium for Bioremediation of Saline–Alkali Land: The Triadic and Synergetic Response Mechanism of Oceanobacillus picturae DY09 to Salt Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Strain and Culture Conditions
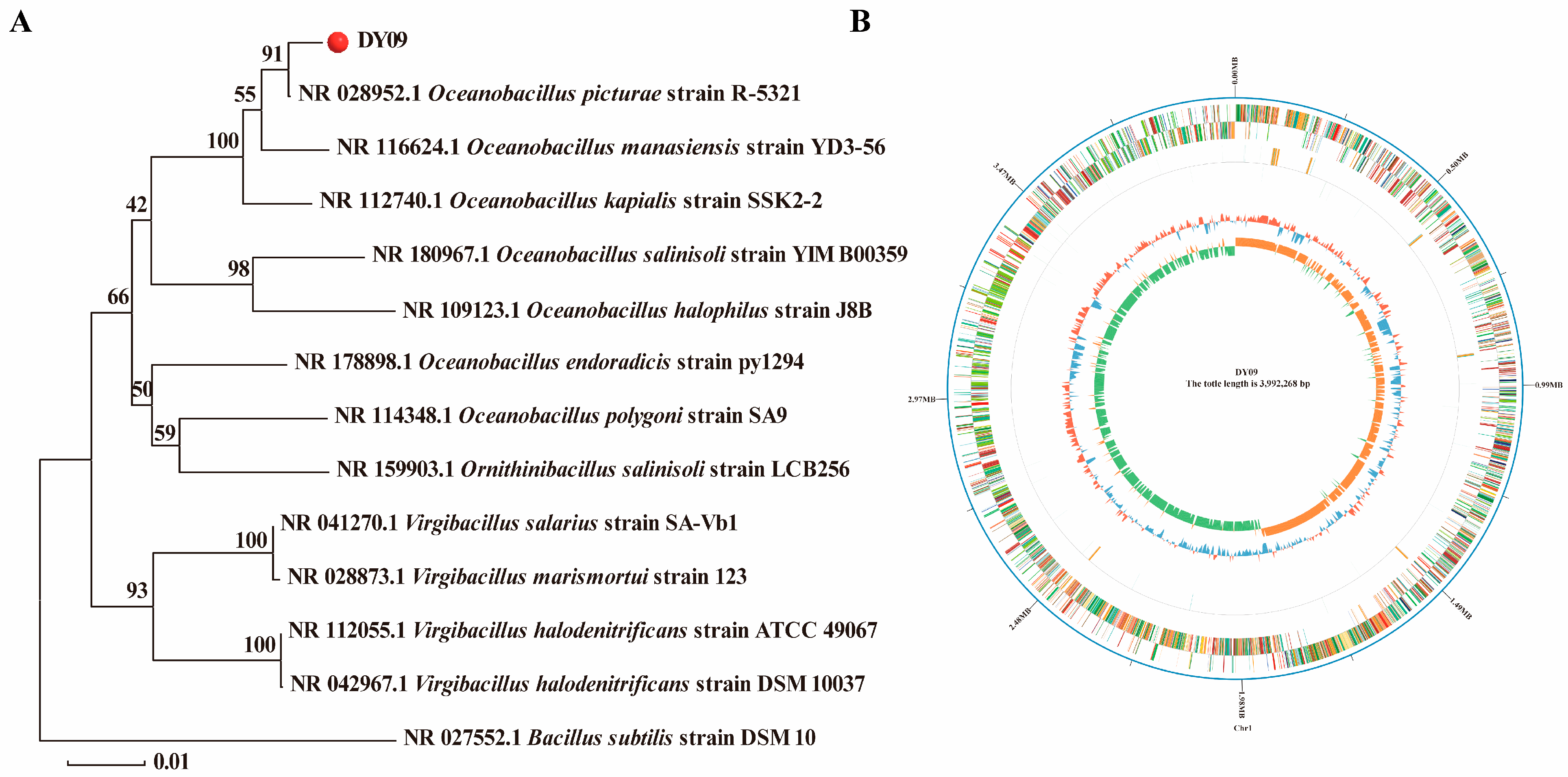
2.2. Phylogenetic Analyses
2.3. Whole-Genome Sequencing, Assembly, and Annotation
2.4. RNA Extraction, Library Preparation, and Sequencing
2.5. RNA-Seq Data Analysis
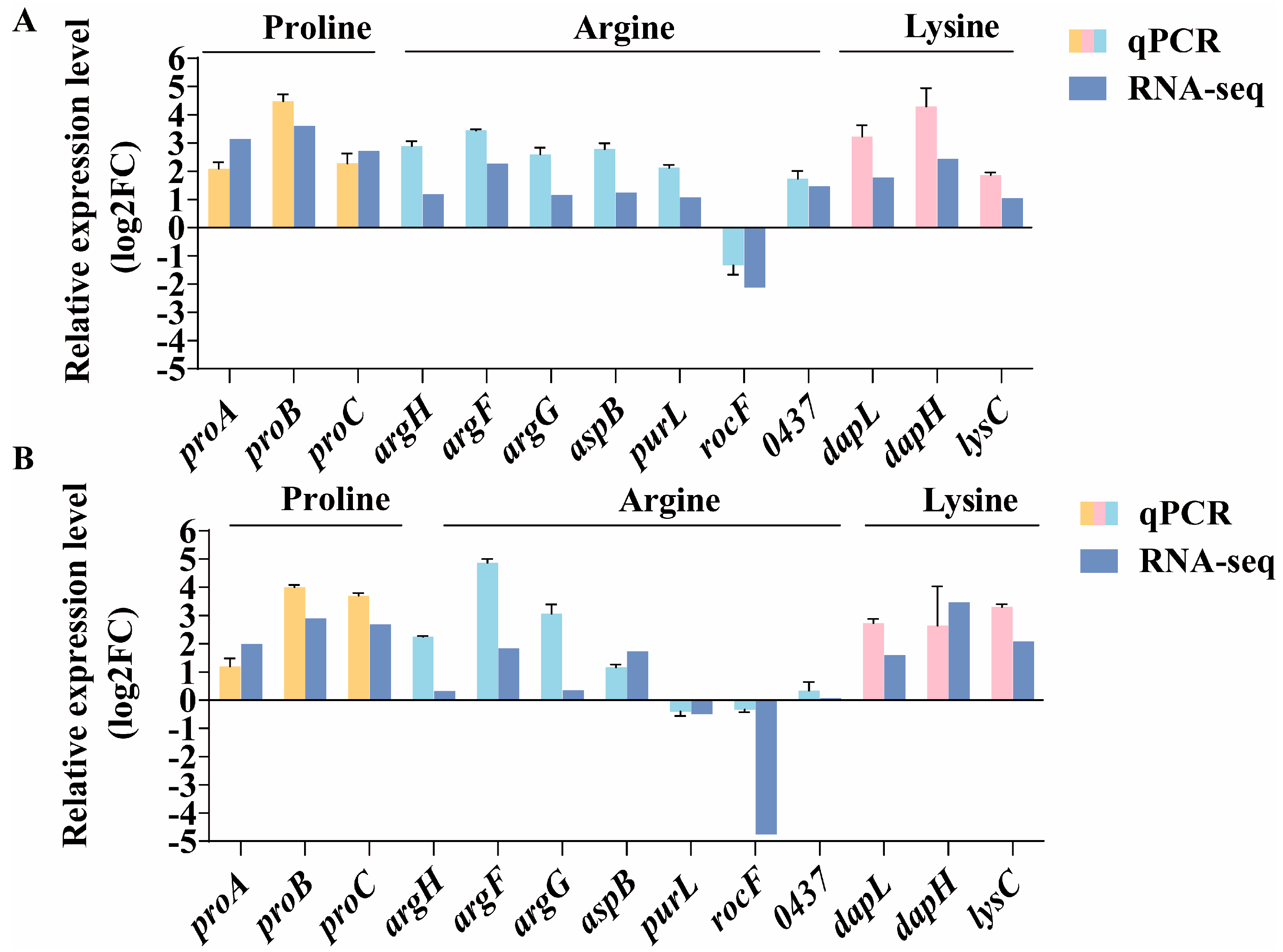
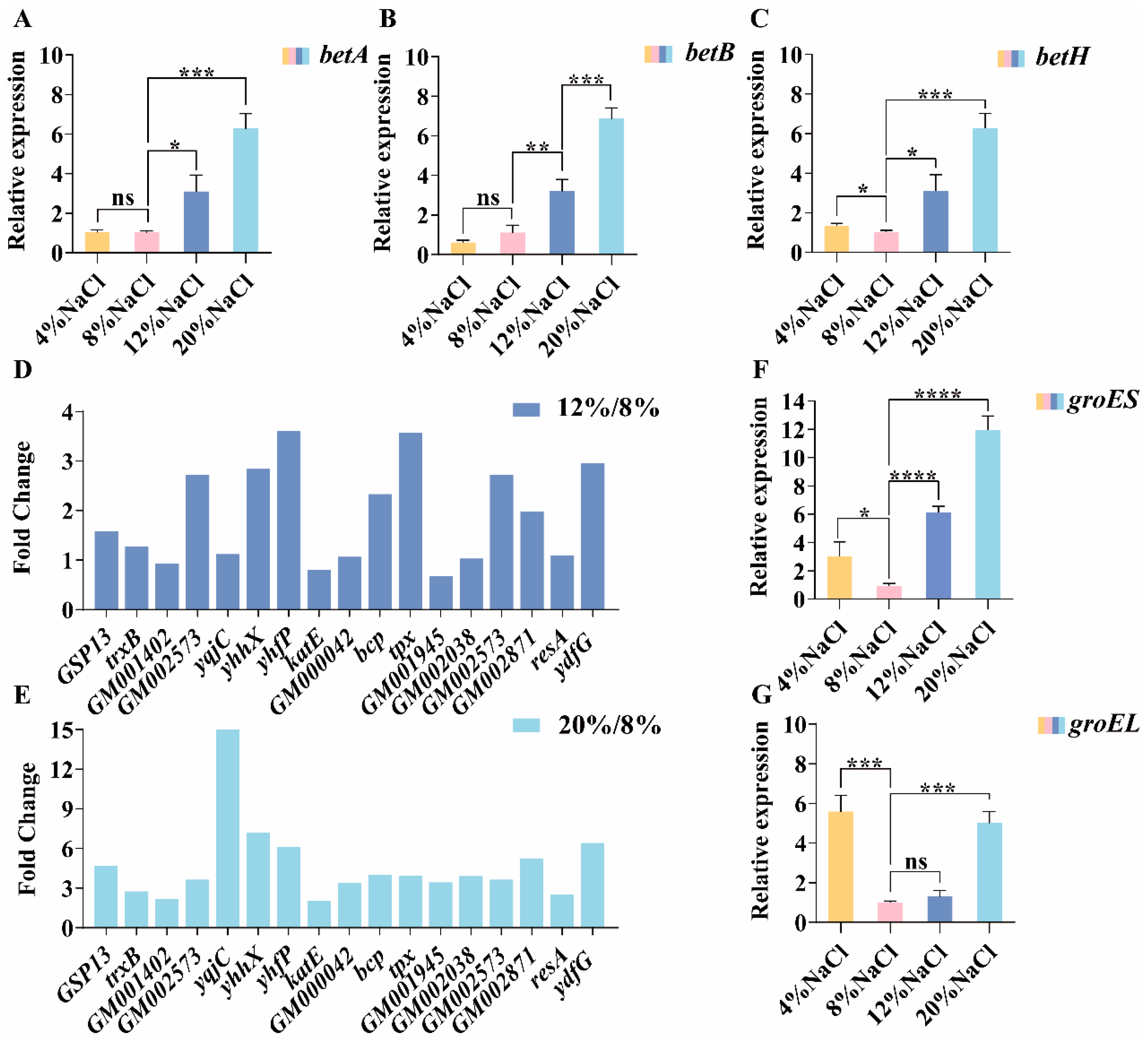
2.6. Validation of RNA-Seq Data by qPCR Analysis
3. Result
3.1. Whole-Genome Sequencing Analysis of the DY09 Strain
3.2. The Morphology and Growth Conditions of DY09 Strain
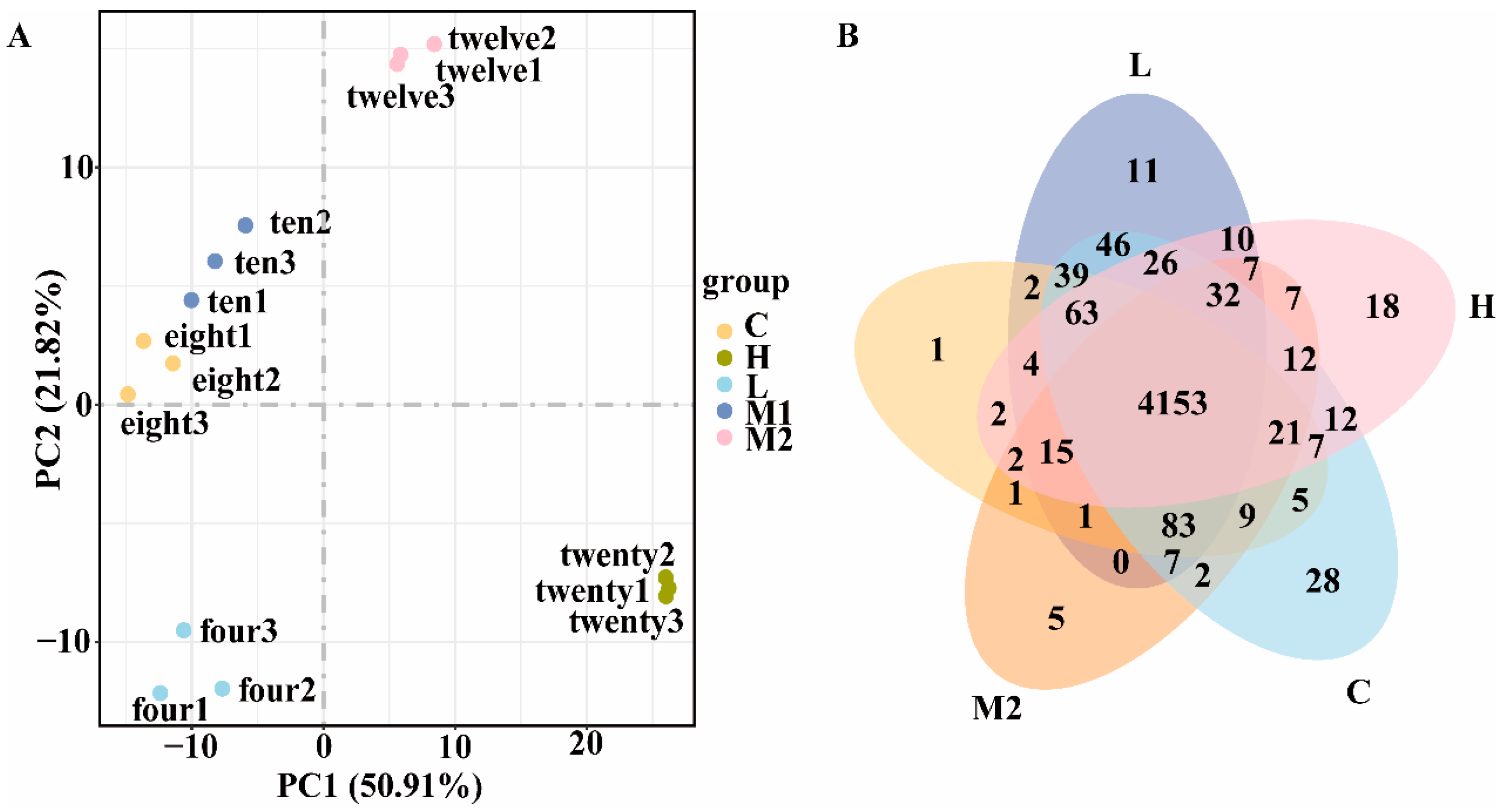
3.3. RNA-Seq Analysis of Transcriptome Expression of DY09 Strain Under Different Salt Stresses
3.4. Analysis of Differential Genes of DY09 Strain Under Different Salt Stresses
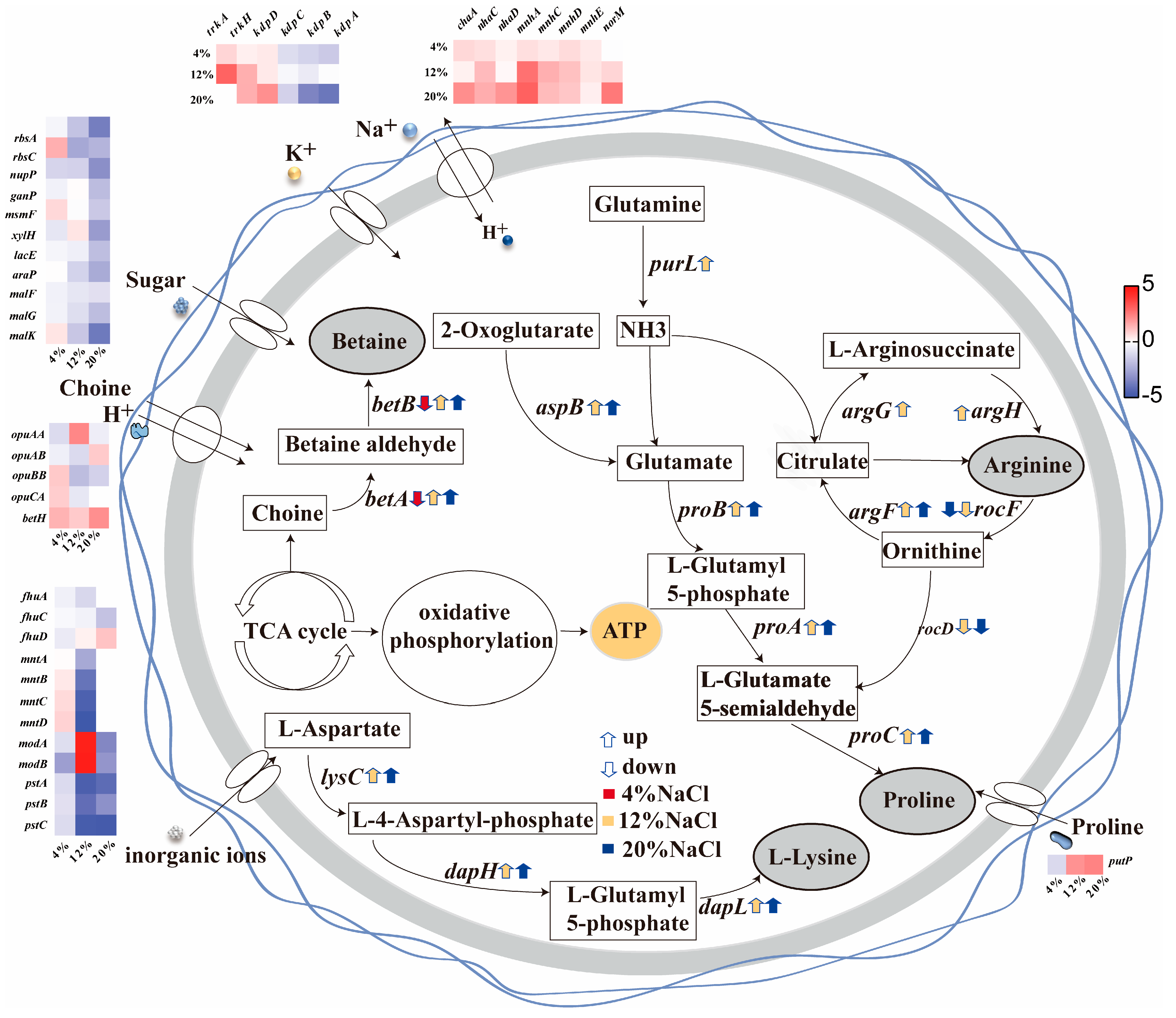
3.5. The Role of Transmembrane Transport in Osmotic Adaptation
3.6. The Role of Amino Acids in Osmotic Adaptation
3.7. The Role of Glycine Betaine in Osmotic Adaptation
3.8. Other Differential Genes Participating in the Adaptation to Salt Stress
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hassani, A.; Azapagic, A.; Shokri, N. Global predictions of primary soil salinization under changing climate in the 21st century. Nat. Commun. 2021, 12, 6663. [Google Scholar] [CrossRef] [PubMed]
- Kurochkina, G.N. The Effect of Preadsorbed Polyelectrolytes on the Surface Properties and Dispersity of Clay Minerals and Soils. Prot. Met. Phys. Chem. Surf. 2019, 55, 266–276. [Google Scholar] [CrossRef]
- Sadeghi, E.; Nasrabadi, R.G.; Movahedi, S.A.; Etesami, H. Actinobacterial and fungal strain-enriched wheat straw as an effective strategy for alleviating the effect of salinity stress on soil chemical and biochemical properties. Chem. Biol. Technol. Agric. 2022, 90, 2345. [Google Scholar] [CrossRef]
- Tang, Y.; Wang, Z.; Zhang, T. Soil salinity estimation in Shule River Basin using support vector regression model. Land Degrad. Dev. 2023, 34, 4094–4108. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, S.; Zhao, Z.; Zhang, K.; Tian, C.; Mai, W. Progress of Euhalophyte Adaptation to Arid Areas to Remediate Salinized Soil. Agriculture 2023, 13, 704. [Google Scholar] [CrossRef]
- Baloch, M.Y.J.; Zhang, W.; Sultana, T.; Akram, M.; Shoumik, B.A.A.; Khan, M.; Farooq, M.A. Utilization of sewage sludge to manage saline–alkali soil and increase crop production: Is it safe or not? Environ. Technol. Innov. 2023, 32, 103266. [Google Scholar] [CrossRef]
- Wang, G.; Wang, X.; Ma, H.; Fan, H.; Lin, F.; Chen, J.; Chai, T.; Wang, H. PcWRKY11, an II-d WRKY Transcription Factor from Polygonum cuspidatum, Enhances Salt Tolerance in Transgenic Arabidopsis thaliana. Int. J. Mol. Sci. 2022, 23, 4357. [Google Scholar] [CrossRef]
- Wang, S.; Guo, K.; Luo, L.; Zhang, R.; Xu, W.; Song, Y.; Zhao, Z. Fattening in Saline and Alkaline Water Improves the Color, Nutritional and Taste Quality of Adult Chinese Mitten Crab Eriocheir sinensis. Foods 2022, 11, 2573. [Google Scholar] [CrossRef]
- Li, W.; Wang, Z.; Zhang, J.; Zong, R. Soil salinity variations and cotton growth under long-term mulched drip irrigation in saline-alkali land of arid oasis. Irrig. Sci. 2021, 40, 103–113. [Google Scholar] [CrossRef]
- Ran, X.; Huang, X.; Wang, X.; Liang, H.; Wang, Y.; Li, J.; Huo, Z.; Liu, B.; Ma, C. Ion absorption, distribution and salt tolerance threshold of three willow species under salt stress. Front. Plant Sci. 2022, 13, 969896. [Google Scholar] [CrossRef]
- Kudakwashe, M.; Qiang, L.I.U.; Shuai, W.U. Plant- and microbe-assisted biochar amendment technology for petroleum hydrocarbon remediation in saline-sodic soils: A review. Pedosphere 2021, 32, 211–221. [Google Scholar] [CrossRef]
- Li-Ping, L.; Xiao-Hua, L.; Hong-Bo, S.; Zhao-Pu, L.; Ya, T.; Quan-Suo, Z.; Jun-Qin, Z. Ameliorants improve saline–alkaline soils on a large scale in northern Jiangsu Province, China. Ecol. Eng. 2015, 81, 328–334. [Google Scholar] [CrossRef]
- Li, P.-S.; Kong, W.-L.; Wu, X.-Q. Salt Tolerance Mechanism of the Rhizosphere Bacterium JZ-GX1 and its Effects on Tomato Seed Germination and Seedling Growth. Front. Microbiol. 2021, 12, 657238. [Google Scholar] [CrossRef]
- Harding, T.; Roger, A.J.; Simpson, A.G.B. Adaptations to High Salt in a Halophilic Protist: Differential Expression and Gene Acquisitions through Duplications and Gene Transfers. Front. Microbiol. 2017, 8, 944. [Google Scholar] [CrossRef] [PubMed]
- Born, J.; Weitzel, K.; Suess, B.; Pfeifer, F. A synthetic riboswitch to regulate haloarchaeal gene expression. Front. Microbiol. 2021, 12, 696181. [Google Scholar] [CrossRef] [PubMed]
- Dubey, S.; Mishra, S. Efficient Production of Polyhydroxyalkanoate Through Halophilic Bacteria Utilizing Algal Biodiesel Waste Residue. Front. Bioeng. Biotechnol. 2021, 9, 624859. [Google Scholar] [CrossRef] [PubMed]
- Hänelt, I.; Müller, V. Molecular Mechanisms of Adaptation of the Moderately Halophilic Bacterium Halobacillis halophilus to Its Environment. Life 2013, 3, 234–243. [Google Scholar] [CrossRef]
- Chen, D.-D.; Fang, B.-Z.; Manzoor, A.; Liu, Y.-H.; Li, L.; Mohamad, O.A.A.; Shu, W.-S.; Li, W.-J. Revealing the salinity adaptation mechanism in halotolerant bacterium Egicoccus halophilus EGI 80432T by physiological analysis and comparative transcriptomics. Appl. Microbiol. Biotechnol. 2021, 105, 2497–2511. [Google Scholar] [CrossRef]
- Louesdon, S.; Charlot-Rougé, S.; Juillard, V.; Tourdot-Maréchal, R.; Béal, C. Osmotic stress affects the stability of freeze-dried Lactobacillus buchneri R1102 as a result of intracellular betaine accumulation and membrane characteristics. J. Appl. Microbiol. 2014, 117, 196–207. [Google Scholar] [CrossRef]
- Zhao, S.; Zhang, Q.; Hao, G.; Liu, X.; Zhao, J.; Chen, Y.; Zhang, H.; Chen, W. The protective role of glycine betaine in Lactobacillus plantarum ST-III against salt stress. Food Control 2014, 44, 208–213. [Google Scholar] [CrossRef]
- Yang, S.; Han, X.; Li, J.; Luan, F.; Zhang, S.; Han, D.; Yang, M.; Chen, Q.; Qi, Z. Oceanobacillus picturae alleviates cadmium stress and promotes growth in soybean seedlings. J. Hazard. Mater. 2024, 472, 134568. [Google Scholar] [CrossRef]
- Etesami, H.; Glick, B.R. Halotolerant plant growth–promoting bacteria: Prospects for alleviating salinity stress in plants. Environ. Exp. Bot. 2020, 178, 104124. [Google Scholar] [CrossRef]
- Srivastava, A.K.; Srivastava, R.; Sharma, A.; Bharati, A.P.; Yadav, J.; Singh, A.K.; Tiwari, P.K.; Srivatava, A.K.; Chakdar, H.; Kashyap, P.L.; et al. Transcriptome Analysis to Understand Salt Stress Regulation Mechanism of Chromohalobacter salexigens ANJ207. Front. Microbiol. 2022, 13, 909276. [Google Scholar] [CrossRef] [PubMed]
- Detkova, E.N.; Boltyanskaya, Y.V. Osmoadaptation of haloalkaliphilic bacteria: Role of osmoregulators and their possible practical application. Microbiology 2007, 76, 511–522. [Google Scholar] [CrossRef]
- Csonka, L.N.; Hanson, A.D. Prokaryotic osmoregulation: Genetics and physiology. Annu. Rev. Microbiol. 1991, 45, 569–606. [Google Scholar] [CrossRef]
- Padan, E.; Schuldiner, S. Molecular Physiology of the Na+/H+ Antiporter in Escherichia coli. J. Exp. Biol. 2021, 196, 443–456. [Google Scholar] [CrossRef]
- Padan, E.; Tzubery, T.; Herz, K.; Kozachkov, L.; Rimon, A.; Galili, L. NhaA of Escherichia coli, as a model of a pH-regulated Na+/H+ antiporter. Biochim. Biophys. Acta (BBA)-Bioenerg. 2004, 1658, 2–13. [Google Scholar] [CrossRef]
- Wang, S.; Liu, X.; Qian, H.; Zhang, S.; Lu, L. Calcineurin and Calcium Channel CchA Coordinate the Salt Stress Response by Regulating Cytoplasmic Ca2+ Homeostasis in Aspergillus nidulans. Appl. Environ. Microbiol. 2016, 82, 3420–3430. [Google Scholar] [CrossRef]
- Foreman, S.; Ferrara, K.; Hreha, T.N.; Duran-Pinedo, A.E.; Frias-Lopez, J.; Barquera, B. Genetic and Biochemical Characterization of the Na+/H+ Antiporters of Pseudomonas aeruginosa. J. Bacteriol. 2021, 203, e0028421. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, B.; Wang, L.; Yang, S. The short C-terminal hydrophilic domain of NhaH Na+/H+ antiporter from Halobacillus dabanensis with roles in resistance to salt and in pH sensing. Sci. Bull. 2008, 53, 3311–3316. [Google Scholar] [CrossRef]
- Yang, L.F.; Jiang, J.Q.; Zhao, B.S.; Zhang, B.; Feng, D.Q.; Lu, W.D.; Wang, L.; Yang, S.S. A Na+/H+ antiporter gene of the moderately halophilic bacterium Halobacillus dabanensis D-8T: Cloning and molecular characterization. FEMS Microbiol. Lett. 2006, 255, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Kixmüller, D.; Strahl, H.; Wende, A.; Greie, J.-C. Archaeal transcriptional regulation of the prokaryotic KdpFABC complex mediating K(+) uptake in H. salinarum. Extremophiles 2011, 15, 643–652. [Google Scholar] [CrossRef]
- Kraegeloh, A.; Amendt, B.; Kunte, H.J. Potassium transport in a halophilic member of the bacteria domain: Identification and characterization of the K+ uptake systems TrkH and TrkI from Halomonas elongata DSM 2581T. J. Bacteriol. 2005, 187, 1036–1043. [Google Scholar] [CrossRef]
- Liu, L.; Si, L.; Meng, X.; Luo, L. Comparative transcriptomic analysis reveals novel genes and regulatory mechanisms of Tetragenococcus halophilus in response to salt stress. J. Ind. Microbiol. Biotechnol. 2015, 42, 601–616. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Wegner, C.-E.; Liesack, W. Short-Term Exposure of Paddy Soil Microbial Communities to Salt Stress Triggers Different Transcriptional Responses of Key Taxonomic Groups. Front. Microbiol. 2017, 8, 400. [Google Scholar] [CrossRef] [PubMed]
- Saum, S.H.; Müller, V. Salinity-dependent switching of osmolyte strategies in a moderately halophilic bacterium: Glutamate induces proline biosynthesis in Halobacillus halophilus. J. Bacteriol. 2007, 189, 6968–6975. [Google Scholar] [CrossRef]
- Saum, S.H.; Müller, V. Regulation of osmoadaptation in the moderate halophile Halobacillus halophilus: Chloride, glutamate and switching osmolyte strategies. Saline Syst. 2008, 4, 4. [Google Scholar] [CrossRef]
- Cheng, Z.; Lin, M.; Rikihisa, Y. Ehrlichia chaffeensis proliferation begins with NtrY/NtrX and PutA/GlnA upregulation and CtrA degradation induced by proline and glutamine uptake. mBio 2014, 5, e02141. [Google Scholar] [CrossRef]
- Dingemans, J.; Monsieurs, P.; Yu, S.-H.; Crabbé, A.; Förstner, K.U.; Malfroot, A.; Cornelis, P.; Houdt, R.V. Effect of Shear Stress on Pseudomonas aeruginosa Isolated from the Cystic Fibrosis Lung. mBio 2016, 7, e00813-16. [Google Scholar] [CrossRef]
- Mao, Y.; Chen, N.; Cao, M.; Chen, R.; Guan, X.; Wang, D. Functional Characterization and Evolutionary Analysis of Glycine-Betaine Biosynthesis Pathway in Red Seaweed Pyropia yezoensis. Mar. Drugs 2019, 17, 70. [Google Scholar] [CrossRef]
- Cao, Y.; Dever, K.; Sivasankaran, S.K.; Nguyen, S.V.; Macori, G.; Naithani, A.; Gopinath, G.R.; Tall, B.; Lehner, A.; Stephan, R.; et al. Alterations in the transcriptional landscape allows differential desiccation tolerance in different strains of Cronobacter sakazakii. Appl. Environ. Microbiol. 2021, 87, e0083021. [Google Scholar] [CrossRef] [PubMed]
- Deole, R.; Hoff, W.D. A potassium chloride to glycine betaine osmoprotectant switch in the extreme halophile Halorhodospira halophila. Sci. Rep. 2020, 10, 3383. [Google Scholar] [CrossRef] [PubMed]
- Huan, R.; Huang, J.; Liu, D.; Wang, M.; Liu, C.; Zhang, Y.; Yi, C.; Xiao, D.; He, H. Genome Sequencing of Mesonia algae K4-1 Reveals Its Adaptation to the Arctic Ocean. Front. Microbiol. 2019, 10, 2812. [Google Scholar] [CrossRef] [PubMed]
- Schuster, C.F.; Bellows, L.E.; Tosi, T.; Campeotto, I.; Corrigan, R.M.; Freemont, P.; Gründling, A. The second messenger c-di-AMP inhibits the osmolyte uptake system OpuC in Staphylococcus aureus. Sci. Signal. 2016, 9, ra81. [Google Scholar] [CrossRef]
- Smith, L.T.; Pocard, J.A.; Bernard, T.; Rudulier, D.L. Osmotic control of glycine betaine biosynthesis and degradation in Rhizobium meliloti. J. Bacteriol. 1988, 170, 3142–3149. [Google Scholar] [CrossRef]
- Cánovas, D.; Vargas, C.; Csonka, L.N.; Ventosa, A.; Nieto, J.J. Osmoprotectants in Halomonas elongata: High-affinity betaine transport system and choline-betaine pathway. J. Bacteriol. 1996, 178, 7221–7226. [Google Scholar] [CrossRef]
- Cánovas, D.; Vargas, C.; Kneip, S.; Morón, M.-J.; Ventosa, A.; Bremer, E.; Nieto, J.J. Genes for the synthesis of the osmoprotectant glycine betaine from choline in the moderately halophilic bacterium Halomonas elongata DSM 3043. Microbiology 2000, 146, 455–463. [Google Scholar] [CrossRef]
- Altobelli, G.G.; Van Noorden, S.; Cimini, V. Copper/Zinc-Superoxide Dismutase in Human Epidermis: An Immunochemical Study. Front. Med. 2019, 6, 258. [Google Scholar] [CrossRef]
- Feng, X.; Sun, W.; Kong, L.; Gao, H. Distinct Roles of Shewanella oneidensis Thioredoxin in Regulation of Cellular Responses to Hydrogen and Organic Peroxides. Appl. Environ. Microbiol. 2019, 85, e01700-19. [Google Scholar] [CrossRef]
- Haydari, M.; Maresca, V.; Rigano, D.; Taleei, A.; Shahnejat-Bushehri, A.A.; Hadian, J.; Sorbo, S.; Guida, M.; Manna, C.; Piscopo, M.; et al. Salicylic Acid and Melatonin Alleviate the Effects of Heat Stress on Essential Oil Composition and Antioxidant Enzyme Activity in Mentha × piperita and Mentha arvensis L. Antioxidants 2019, 8, 547. Antioxidants 2019, 8, 547. [Google Scholar] [CrossRef]
- Ma, J.; Wang, J.; Wang, Q.; Shang, L.; Zhao, Y.; Zhang, G.; Ma, Q.; Hong, S.; Gu, C. Physiological and transcriptional responses to heat stress and functional analyses of PsHSPs in tree peony (Paeonia suffruticosa). Front. Plant Sci. 2022, 13, 926900. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Hong, L.; Wang, Y.; Yu, J.; Yang, J.; Yang, J.; Zhang, H.; Perrett, S. Kinetics of the conformational cycle of Hsp70 reveals the importance of the dynamic and heterogeneous nature of Hsp70 for its function. Proc. Natl. Acad. Sci. USA 2020, 117, 7814–7823. [Google Scholar] [CrossRef]
- Zhu, Y.; Gagaoua, M.; Mullen, A.M.; L. Kelly, A.; Sweeney, T.; Cafferky, J.; Viala, D.; M. Hamill, R. A Proteomic Study for the Discovery of Beef Tenderness Biomarkers and Prediction of Warner–Bratzler Shear Force Measured on Longissimus thoracis Muscles of Young Limousin-Sired Bulls. Foods 2021, 10, 952. [Google Scholar] [CrossRef] [PubMed]
- Caldas, T.; Demont-Caulet, N.; Ghazi, A.; Richarme, G. Thermoprotection by glycine betaine and choline. Microbiology 1999, 145, 2543–2548. [Google Scholar] [CrossRef] [PubMed]
- Diamant, S.; Eliahu, N.; Rosenthal, D.; Goloubinoff, P. Chemical chaperones regulate molecular chaperones in vitro and in cells under combined salt and heat stresses. J. Biol. Chem. 2001, 276, 39586–39591. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nie, T.; Wang, L.; Liu, Y.; Fu, S.; Wang, J.; Cui, K.; Wang, L. A Halophilic Bacterium for Bioremediation of Saline–Alkali Land: The Triadic and Synergetic Response Mechanism of Oceanobacillus picturae DY09 to Salt Stress. Microorganisms 2025, 13, 1474. https://doi.org/10.3390/microorganisms13071474
Nie T, Wang L, Liu Y, Fu S, Wang J, Cui K, Wang L. A Halophilic Bacterium for Bioremediation of Saline–Alkali Land: The Triadic and Synergetic Response Mechanism of Oceanobacillus picturae DY09 to Salt Stress. Microorganisms. 2025; 13(7):1474. https://doi.org/10.3390/microorganisms13071474
Chicago/Turabian StyleNie, Tianying, Liuqing Wang, Yilan Liu, Siqi Fu, Jiahui Wang, Kunpeng Cui, and Lu Wang. 2025. "A Halophilic Bacterium for Bioremediation of Saline–Alkali Land: The Triadic and Synergetic Response Mechanism of Oceanobacillus picturae DY09 to Salt Stress" Microorganisms 13, no. 7: 1474. https://doi.org/10.3390/microorganisms13071474
APA StyleNie, T., Wang, L., Liu, Y., Fu, S., Wang, J., Cui, K., & Wang, L. (2025). A Halophilic Bacterium for Bioremediation of Saline–Alkali Land: The Triadic and Synergetic Response Mechanism of Oceanobacillus picturae DY09 to Salt Stress. Microorganisms, 13(7), 1474. https://doi.org/10.3390/microorganisms13071474





