Combination of Medium-High-Hydrostatic-Pressure Treatment with Post-/Pre-Heat Treatment for Pasteurization of Bacillus subtilis Spore Suspended in Soy Milk
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strain and Growth Conditions
2.2. Plant Material Sample Preparation
2.3. Pre-/Post-Heat Treatment
2.4. Medium-/High-Hydrostatic-Pressure Treatment
2.5. Bacterial Count and Calculation of D Value, Z Value, Activation Energy, and Frequency Factor
2.6. Statistical Analysis
3. Results and Discussion
3.1. Pasteurization of B. subtilis Spore by Combination of Post-Heat Treatment With and Without MHHP Treatment
3.2. Germination Induction Effect of Combination of MHHP Treatment With and Without Pre-Heat Treatment of B. subtilis Spore
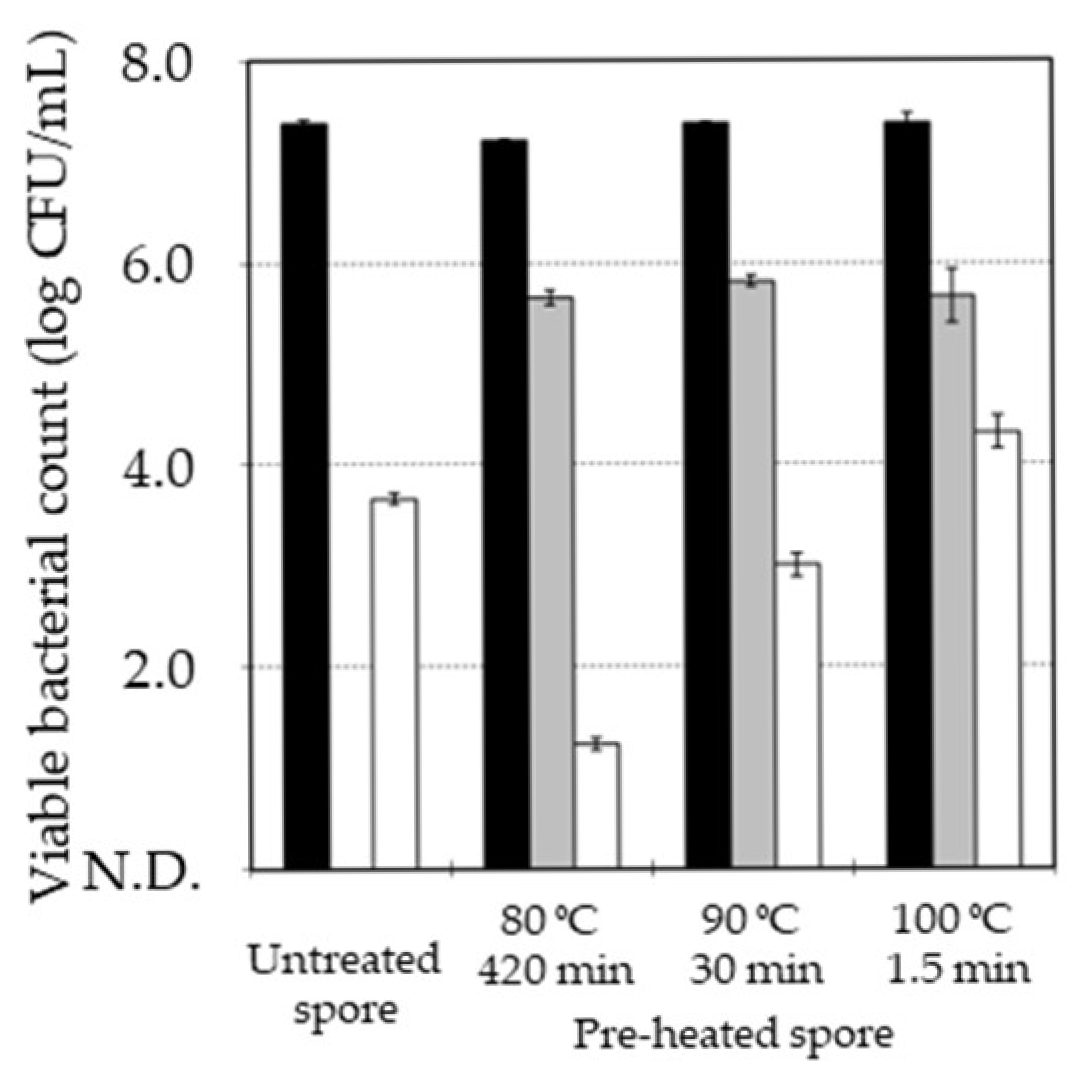
3.3. Effect of Pre-Heat Treatment on Decrease in Heat Resistance of B. subtilis Spore by MHHP Treatment
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nutrizio, M.; Dukić, J.; Sabljak, J.; Samardžija, A.; Fučkar, V.B.; Djekić, I.; Jambark, A.R. Upcycling of Food By-Products and Waste: Nonthermal Green Extractions and Life Cycle Assessment Approach. Sustainability 2024, 16, 9143. [Google Scholar] [CrossRef]
- Andersson, A.; Rönner, U.; Granum, P.E. What problems does the food industry have with the spore-forming pathogens Bacillus cereus and Clostridium perfringens? Int. J. Food Microbiol. 1995, 28, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Guillermo, C.; Santiago, C.; Pilar, M. Physiology of the inactivation of vegetative bacteria by thermal treatments: Mode of action, influence of environmental factors and inactivation kinetics. Foods 2017, 6, 107. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, W.L.; Munakata, N.; Horneck, G.; Melosh, H.J.; Setlow, P. Resistance of Bacillus endospores to extreme terrestrial and extraterrestrial environments. Microbiol. Mol. Biol. Rev. 2000, 64, 548–572. [Google Scholar] [CrossRef] [PubMed]
- Setlow, P. Spores of Bacillus subtilis: Their resistance to and killing by radiation, heat and chemicals. J. Appl. Microbiol. 2006, 101, 514–525. [Google Scholar] [CrossRef] [PubMed]
- Ross, A.I.V.; Griffiths, M.W.; Mittal, G.S.; Deeth, H.C. Combining nonthermal technologies to control foodborne microorganisms. Int. J. Food Microbiol. 2003, 89, 125–138. [Google Scholar] [CrossRef] [PubMed]
- Black, E.P.; Koziol-Dube, K.; Guan, D.; Wei, J.; Setlow, B.; Cortezzo, D.E.; Hoover, D.G.; Setlow, P. Factors influencing germination of Bacillus subtilis spores via activation of nutrient receptors by high pressure. Appl. Environ. Microbiol. 2005, 71, 5879–5887. [Google Scholar] [CrossRef] [PubMed]
- Black, E.P.; Setlow, P.; Hocking, A.D.; Stewart, C.M.; Kelly, A.L.; Hoover, D.G. Response of spores to high-pressure processing. Compr. Rev. Food Sci. Food Saf. 2007, 6, 103–119. [Google Scholar] [CrossRef]
- Paidhungat, M.; Setlow, B.; Daniels, W.B.; Hoover, D.; Papafragkou, E.; Setlow, P. Mechanisms of induction of germination of Bacillus subtilis spores by high pressure. Appl. Environ. Microbiol. 2002, 68, 3172–3175. [Google Scholar] [CrossRef] [PubMed]
- Reineke, K.; Doehner, I.; Schlumbach, K.; Baier, D.; Mathys, A.; Knorr, D. The different pathways of spore germination and inactivation in dependence of pressure and temperature. Innov. Food Sci. Emerg. Technol. 2012, 13, 31–41. [Google Scholar] [CrossRef]
- Reinek, K.; Mathys, A.; Heinz, V.; Knorr, D. Mechanisms of endospore inactivation under high pressure. Trends Microbiol. 2013, 21, 296–304. [Google Scholar] [CrossRef] [PubMed]
- Wuytack, E.Y.; Boven, S.; Michiels, C.W. Comparative study of pressure-induced germination of Bacillus subtilis spores at low and high pressures. Appl. Environ. Microbiol. 1998, 64, 3220–3224. [Google Scholar] [CrossRef] [PubMed]
- Wuytack, E.Y.; Soons, J.; Poschet, F.; Michiels, C.W. Comparative study of pressure- and nutrient-induced germination of Bacillus subtilis spores. Appl. Environ. Microbiol. 2000, 66, 257–261. [Google Scholar] [CrossRef] [PubMed]
- Ogino, H.; Nishiumi, T. Sterilization of heat-resistant spores by a combination of high-pressure and subsequent heat treatment. Rev. High Press. Sci. Technol. 2015, 25, 334–342. (In Japanese) [Google Scholar] [CrossRef]
- Berg, R.W.; Sandine, W.E. Activation of bacterial spores. A review. J. Food Prot. 1970, 33, 435–441. [Google Scholar]
- Luu, S.; Cruz-Mora, J.; Setlow, B.; Feeherry, F.E.; Doona, C.J.; Setlow, P. The effects of heat activation on Bacillus spore germination, with nutrients or under high pressure, with or without various germination proteins. Appl. Environ. Microbiol. 2002, 68, 3172–3175. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; Smelt, J.P.P.M.; Vischer, N.O.E.; Vos, A.L.; Setlow, P.; Brul, S. Heat activation and inactivation of bacterial spores: Is there an overlap? Appl. Environ. Microbiol. 2022, 88, e02324-21. [Google Scholar] [CrossRef] [PubMed]
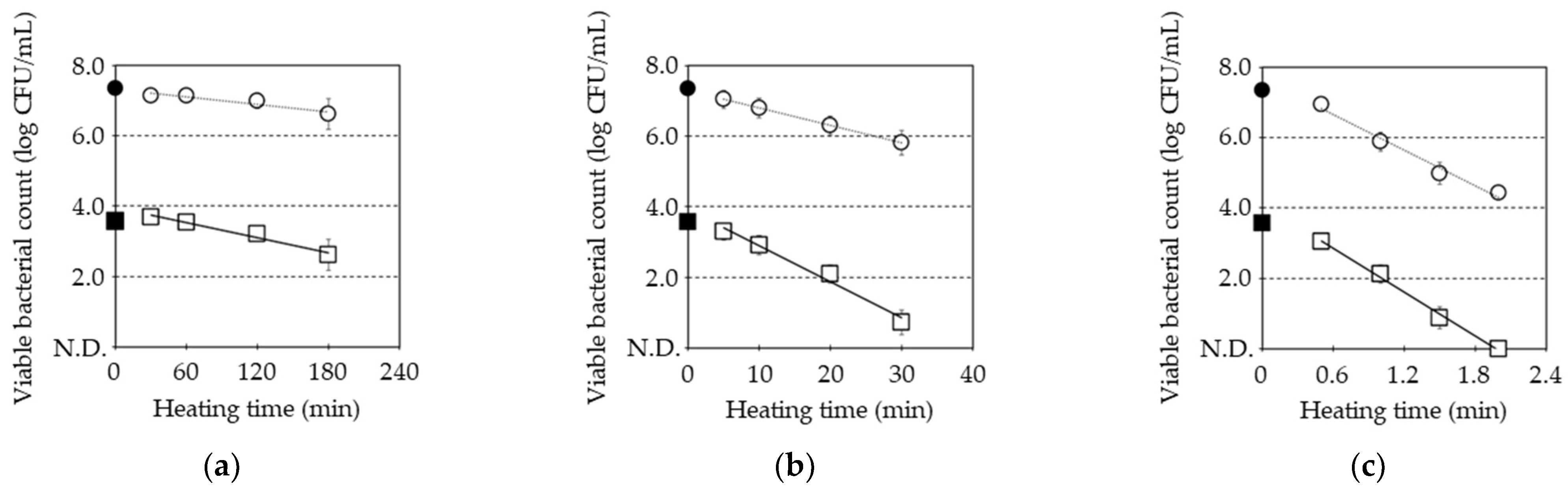

| D Value (Min) | Z Value (°C) | Activation Energy (J·mol−1) | Frequency Factor | |||
|---|---|---|---|---|---|---|
| 80 °C | 87 °C | 100 °C | ||||
| Untreated | 300 ± 76 a | 20 ± 3 a | 0.6 ± 0.0 a | 7.5 ± 0.3 a | 335 ± 14 a | 109 ± 4.7 a |
| MHHP-treated | 150 ± 41 b | 10 ± 2 b | 0.5 ± 0.0 b | 8.2 ± 0.5 a | 309 ± 17 a | 101 ± 5.7 a |
| D Value (min) | Pre-Heat Treatment | ||
|---|---|---|---|
| Untreated | Pre-Heated | ||
| MHHP treatment | Untreated | 23 ± 4 a | 29 ± 4 a |
| MHHP-treated | 11 ± 2 b | 14 ± 1 b | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kazuya, M. Combination of Medium-High-Hydrostatic-Pressure Treatment with Post-/Pre-Heat Treatment for Pasteurization of Bacillus subtilis Spore Suspended in Soy Milk. Microorganisms 2025, 13, 1469. https://doi.org/10.3390/microorganisms13071469
Kazuya M. Combination of Medium-High-Hydrostatic-Pressure Treatment with Post-/Pre-Heat Treatment for Pasteurization of Bacillus subtilis Spore Suspended in Soy Milk. Microorganisms. 2025; 13(7):1469. https://doi.org/10.3390/microorganisms13071469
Chicago/Turabian StyleKazuya, Morimatsu. 2025. "Combination of Medium-High-Hydrostatic-Pressure Treatment with Post-/Pre-Heat Treatment for Pasteurization of Bacillus subtilis Spore Suspended in Soy Milk" Microorganisms 13, no. 7: 1469. https://doi.org/10.3390/microorganisms13071469
APA StyleKazuya, M. (2025). Combination of Medium-High-Hydrostatic-Pressure Treatment with Post-/Pre-Heat Treatment for Pasteurization of Bacillus subtilis Spore Suspended in Soy Milk. Microorganisms, 13(7), 1469. https://doi.org/10.3390/microorganisms13071469






