Cyanobacteria and Soil Restoration: Bridging Molecular Insights with Practical Solutions
Abstract
1. Introduction
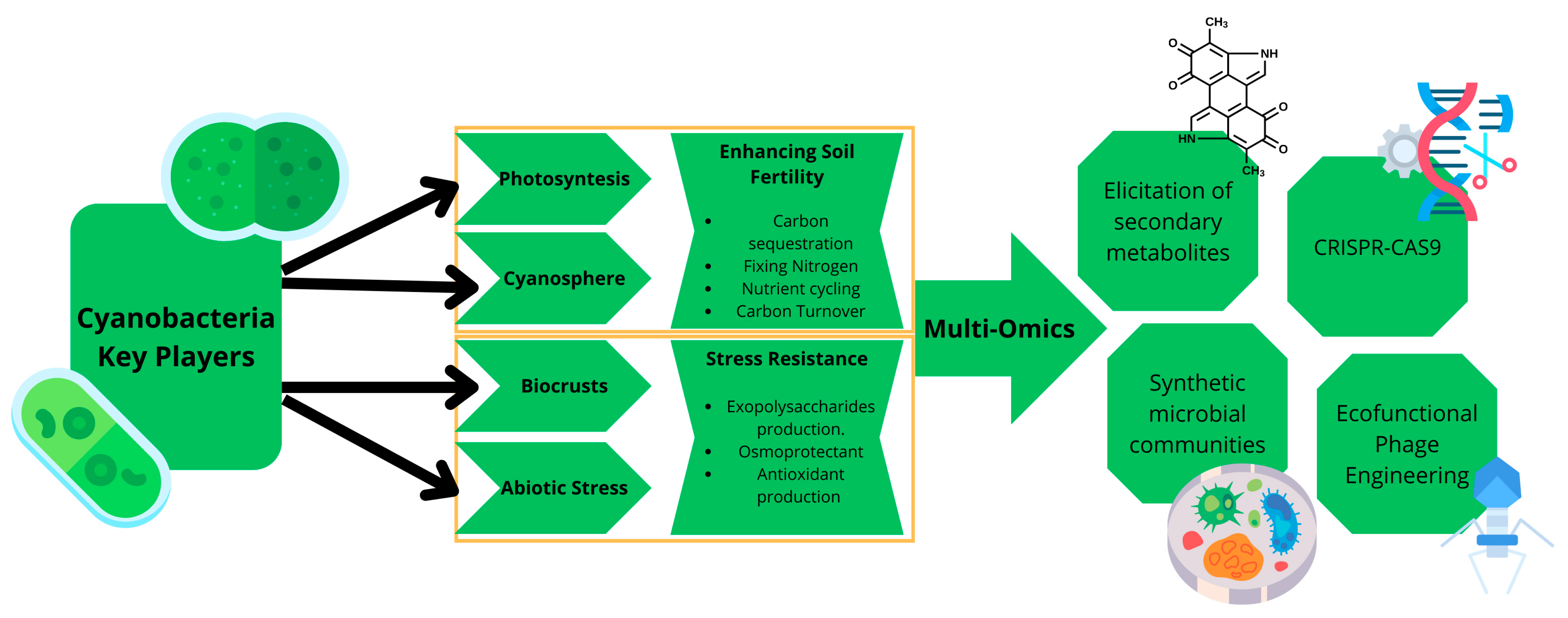
2. Cyanobacteria: Key Players for Improving Heal Soils
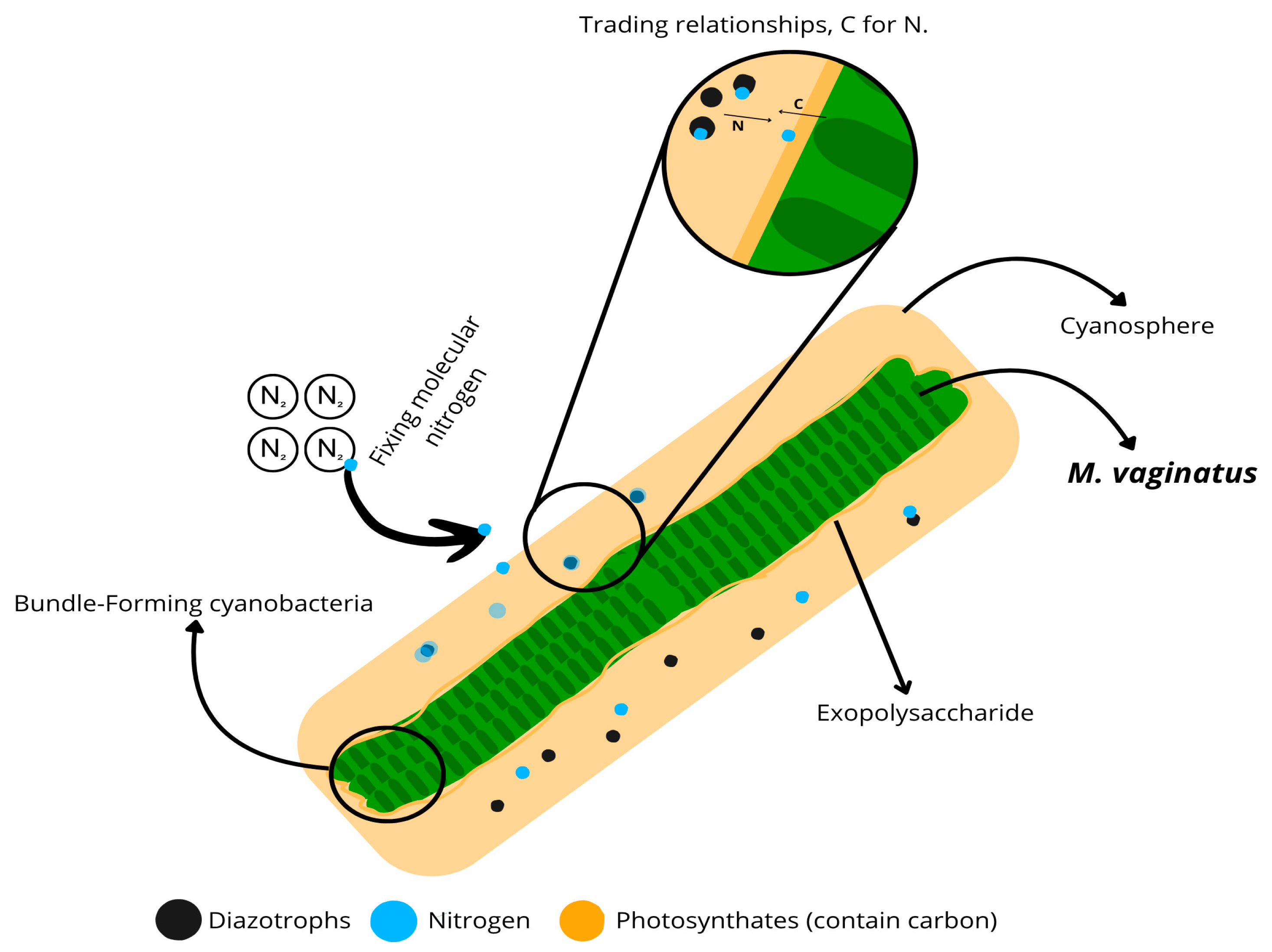
2.1. Photosynthesis, Carbon Sequestration, and Nitrogen Fixation: Enhancing Soil Fertility
2.2. EPS and Biocrust Formation: Improving Soil Structure and Stability
2.3. Stress Resilience and Functional Adaptability in Harsh Environments
2.3.1. Desiccation Tolerance and Protective Genes
2.3.2. Salinity Resistance
2.4. Microbial Interactions in the Cyanosphere: Promoting Ecosystem Function
3. The Role of Sequencing Technologies in Understanding Cyanobacteria Dynamics in Soils
Unlocking the Biotechnological Potential of Cyanobacteria with Bioinformatics Tools
4. Future Prospects and Challenges
4.1. State of the Art of Cyanophages’ Ability in Response to Stress and Metabolic Innovation
4.2. New Advances and Challenges in Soil Cyanobacteria Multi-Omics: Towards a Comprehensive Understanding of Their Role in Soil Restoration
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Olsson, L.; Barbosa, H.; Bhadwal, S.; Cowie, A.; Delusca, K.; Flores-Renteria, D.; Hermans, K.; Jobbagy, E.; Kurz, W.; Li, D.; et al. Land Degradation. In Climate Change and Land: An IPCC Special Report on Climate Change, Desertification, Land Degradation, Sustainable Land Management, Food Security, and Greenhouse Gas Fluxes in Terrestrial Ecosystems; IPCC: Geneva, Switzerland, 2022; pp. 345–436. [Google Scholar] [CrossRef]
- Key Messages|Global Symposium on Soil Erosion|Food and Agriculture Organization of the United Nations. Available online: https://www.fao.org/about/meetings/soil-erosion-symposium/key-messages/en/ (accessed on 13 May 2025).
- Rumpel, C.; Amiraslani, F.; Bossio, D.; Henry, C.; Espinoza, F.; Koutika, A.; Shirato, A.O.; Sall, N.; Varela-Ortega, C. The Role of Soil Carbon Sequestration in Enhancing Human Resilience in Tackling Global Crises Including Pandemics. Soil Security 2022, 8, 100069. [Google Scholar] [CrossRef]
- Food and Agriculture Organization. FAO Publications Catalogue 2023; FAO: Rome, Italy, 2023. [Google Scholar] [CrossRef]
- Food and Agriculture Organization. The Third Report on The State of the World’s Plant Genetic Resources for Food and Agriculture; FAO: Rome, Italy, 2025. [Google Scholar] [CrossRef]
- Eisenstein, M. Natural Solutions for Agricultural Productivity. Nature 2020, 588, S58–S59. [Google Scholar] [CrossRef]
- Bai, Z.; Liu, L.; Obersteiner, M.; Mosnier, A.; Chen, X.; Yuan, Z.; Ma, L. Agricultural Trade Impacts Global Phosphorus Use and Partial Productivity. Nat. Food 2023, 4, 762–773. [Google Scholar] [CrossRef] [PubMed]
- Yetgin, A.; History, A. Exploring the Link between Soil Microbial Diversity and Nutritional Deficiencies. J. Agric. Prod. 2023, 4, 81–90. [Google Scholar] [CrossRef]
- Meng, S.; Peng, T.; Liu, X.; Wang, H.; Huang, T.; Gu, J.-D.; Hu, Z. Ecological Role of Bacteria Involved in the Biogeochemical Cycles of Mangroves Based on Functional Genes Detected through GeoChip 5.0. mSphere 2022, 7, e0093621. [Google Scholar] [CrossRef] [PubMed]
- Upendar, G.; Singh, S.; Chakrabarty, J.; Chandra Ghanta, K.; Dutta, S.; Dutta, A. Sequestration of Carbon Dioxide and Production of Biomolecules Using Cyanobacteria. J. Environ. Manag. 2018, 218, 234–244. [Google Scholar] [CrossRef]
- Li, Y.; Shi, X.; Ling, Q.; Li, S.; Wei, J.; Xin, M.; Xie, D.; Chen, X.; Liu, K.; Yu, F. Bacterial Extracellular Polymeric Substances: Impact on Soil Microbial Community Composition and Their Potential Role in Heavy Metal-Contaminated Soil. Ecotoxicol. Environ. Saf. 2022, 240, 113701. [Google Scholar] [CrossRef]
- Wei, Z.; Niu, S.; Wei, Y.; Liu, Y.; Xu, Y.; Yang, Y.; Zhang, P.; Zhou, Q.; Wang, J.J. The Role of Extracellular Polymeric Substances (EPS) in Chemical-Degradation of Persistent Organic Pollutants in Soil: A Review. Sci. Total Environ. 2024, 912, 168877. [Google Scholar] [CrossRef]
- Singh, R.P.; Yadav, P.; Kujur, R.; Pandey, K.D.; Gupta, R.K. Cyanobacteria and Salinity Stress Tolerance. In Cyanobacterial Lifestyle and Its Applications in Biotechnology; Academic Press: Cambridge, MA, USA, 2022; pp. 253–280. [Google Scholar] [CrossRef]
- Singh, V.K.; Jha, S.; Rana, P.; Mishra, S.; Kumari, N.; Singh, S.C.; Anand, S.; Upadhye, V.; Sinha, R.P. Resilience and Mitigation Strategies of Cyanobacteria under Ultraviolet Radiation Stress. Int. J. Mol. Sci. 2023, 24, 12381. [Google Scholar] [CrossRef]
- Yadav, P.; Singh, R.P.; Rana, S.; Joshi, D.; Kumar, D.; Bhardwaj, N.; Gupta, R.K.; Kumar, A. Mechanisms of Stress Tolerance in Cyanobacteria under Extreme Conditions. Stresses 2022, 2, 531–549. [Google Scholar] [CrossRef]
- Yadav, P.; Singh, R.P.; Hashem, A.; Abd_Allah, E.F.; Santoyo, G.; Kumar, A.; Gupta, R.K.; Yadav, P.; Singh, R.P.; Hashem, A.; et al. Enhancing Biocrust Development and Plant Growth through Inoculation of Desiccation-Tolerant Cyanobacteria in Different Textured Soils. Microorganisms 2023, 11, 2507. [Google Scholar] [CrossRef] [PubMed]
- Liao, K.; Chen, C.; Ye, W.; Zhu, J.; Li, Y.; She, S.; Wang, P.; Tao, Y.; Lv, A.; Wang, X.; et al. The Adaptability, Distribution, Ecological Function and Restoration Application of Biological Soil Crusts on Metal Tailings: A Critical Review. Sci. Total Environ. 2024, 927, 172169. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhao, Y.; Xu, W.; Wang, N.; Zhang, Z. Acquiring High-Quality and Sufficient Propagules/Fragments for Cyanobacteria Crust Inoculation and Restoration of Degraded Soils in a Sandy Desert. Land Degrad. Dev. 2023, 34, 1593–1597. [Google Scholar] [CrossRef]
- Adessi, A.; De Philippis, R.; Rossi, F. Drought-Tolerant Cyanobacteria and Mosses as Biotechnological Tools to Attain Land Degradation Neutrality. Web Ecol. 2021, 21, 65–78. [Google Scholar] [CrossRef]
- Velichko, N.; Smirnova, S.; Averina, S.; Pinevich, A. A Survey of Antarctic Cyanobacteria. Hydrobiologia 2021, 848, 2627–2652. [Google Scholar] [CrossRef]
- Garcia-Pichel, F.; Lombard, J.; Soule, T.; Dunaj, S.; Wu, S.H.; Wojciechowski, M.F. Timing the Evolutionary Advent of Cyanobacteria and the Later Great Oxidation Event Using Gene Phylogenies of a Sunscreen. mBio 2019, 10, e00561-19. [Google Scholar] [CrossRef]
- Demoulin, C.F.; Lara, Y.J.; Cornet, L.; François, C.; Baurain, D.; Wilmotte, A.; Javaux, E.J. Cyanobacteria Evolution: Insight from the Fossil Record. Free Radic. Biol. Med. 2019, 140, 206–223. [Google Scholar] [CrossRef] [PubMed]
- Bhatnagar, A.; Makandar, M.B.; Garg, M.K.; Bhatnagar, M. Community Structure and Diversity of Cyanobacteria and Green Algae in the Soils of Thar Desert (India). J. Arid. Environ. 2008, 72, 73–83. [Google Scholar] [CrossRef]
- Lumian, J.E.; Jungblut, A.D.; Dillon, M.L.; Hawes, I.; Doran, P.T.; Mackey, T.J.; Dick, G.J.; Grettenberger, C.L.; Sumner, D.Y. Metabolic Capacity of the Antarctic Cyanobacterium Phormidium Pseudopriestleyi That Sustains Oxygenic Photosynthesis in the Presence of Hydrogen Sulfide. Genes 2021, 12, 426. [Google Scholar] [CrossRef]
- Bolay, P.; Schlüter, S.; Grimm, S.; Riediger, M.; Hess, W.R.; Klähn, S. The Transcriptional Regulator RbcR Controls Ribulose-1,5-Bisphosphate Carboxylase/Oxygenase (RuBisCO) Genes in the Cyanobacterium Synechocystis sp. PCC 6803. New Phytol. 2022, 235, 432–445. [Google Scholar] [CrossRef]
- Rae, B.D.; Long, B.M.; Whitehead, L.F.; Förster, B.; Badger, M.R.; Price, G.D. Cyanobacterial Carboxysomes: Microcompartments That Facilitate CO2 Fixation. J. Mol. Microbiol. Biotechnol. 2013, 23, 300–307. [Google Scholar] [CrossRef]
- Savir, Y.; Noor, E.; Milo, R.; Tlusty, T. Cross-Species Analysis Traces Adaptation of Rubisco toward Optimality in a Low-Dimensional Landscape. Proc. Natl. Acad. Sci. USA 2010, 107, 3475–3480. [Google Scholar] [CrossRef]
- Gale, G.A.R.; Osorio, A.A.S.; Mills, L.A.; Wang, B.; Lea-Smith, D.J.; McCormick, A.J. Emerging Species and Genome Editing Tools: Future Prospects in Cyanobacterial Synthetic Biology. Microorganisms 2019, 7, 409. [Google Scholar] [CrossRef] [PubMed]
- Ooms, M.D.; Dinh, C.T.; Sargent, E.H.; Sinton, D. Photon Management for Augmented Photosynthesis. Nat. Commun. 2016, 7, 12699. [Google Scholar] [CrossRef] [PubMed]
- Al-Najjar, M.A.A.; de Beer, D.; Kühl, M.; Polerecky, L. Light Utilization Efficiency in Photosynthetic Microbial Mats. Environ. Microbiol. 2012, 14, 982–992. [Google Scholar] [CrossRef]
- Schubert, M.G.; Tang, T.-C.; Goodchild-Michelman, I.M.; Ryon, K.A.; Henriksen, J.R.; Chavkin, T.; Wu, Y.; Miettinen, T.P.; Van Wychen, S.; Dahlin, L.R.; et al. Cyanobacteria Newly Isolated from Marine Volcanic Seeps Display Rapid Sinking and Robust, High-Density Growth. Appl. Environ. Microbiol. 2024, 90, e0084124. [Google Scholar] [CrossRef]
- Piatka, D.R.; Frank, A.H.; Köhler, I.; Castiglione, K.; van Geldern, R.; Barth, J.A.C. Balance of Carbon Species Combined with Stable Isotope Ratios Show Critical Switch towards Bicarbonate Uptake during Cyanobacteria Blooms. Sci. Total Environ. 2022, 807, 151067. [Google Scholar] [CrossRef] [PubMed]
- Tamura, M.; Suseela, V. Warming and Labile Substrate Addition Alter Enzyme Activities and Composition of Soil Organic Carbon. Front. For. Glob. Chang. 2021, 4, 691302. [Google Scholar] [CrossRef]
- Chen, X.; You, M.; Han, X.; Lu, X.; Zou, W.; Yan, J. Native Soil Organic-Carbon Contents Shape Distinct Bacterial Communities Associated with Priming Effect. Pedobiologia 2022, 95, 150842. [Google Scholar] [CrossRef]
- Velmurugan, R.; Incharoensakdi, A. Overexpression of Glucose-6-Phosphate Isomerase in Synechocystis sp. PCC 6803 with Disrupted Glycogen Synthesis Pathway Improves Exopolysaccharides Synthesis. Algal Res. 2021, 57, 102357. [Google Scholar] [CrossRef]
- Eisenhut, M.; Von Wobeser, E.A.; Jonas, L.; Schubert, H.; Ibelings, B.W.; Bauwe, H.; Matthijs, H.C.P.; Hagemann, M. Long-Term Response toward Inorganic Carbon Limitation in Wild Type and Glycolate Turnover Mutants of the Cyanobacterium Synechocystis sp. Strain PCC 6803. Plant Physiol. 2007, 144, 1946–1959. [Google Scholar] [CrossRef] [PubMed]
- Bozieva, A.M.; Khasimov, M.K.; Rao, M.S.; Sinetova, M.A.; Voloshin, R.A.; Dunikov, D.O.; Tsygankov, A.A.; Leong, Y.K.; Chang, J.S.; Allakhverdiev, S.I.; et al. Optimizing Cyanobacterial Hydrogen Production: Metabolic and Genetic Strategies with Glycerol Supplementation. Front. Energy Res. 2025, 13, 1547215. [Google Scholar] [CrossRef]
- Kramer, B.J.; Hem, R.; Gobler, C.J. Elevated CO2 Significantly Increases N2 Fixation, Growth Rates, and Alters Microcystin, Anatoxin, and Saxitoxin Cell Quotas in Strains of the Bloom-Forming Cyanobacteria, Dolichospermum. Harmful Algae 2022, 120, 102354. [Google Scholar] [CrossRef] [PubMed]
- Galetović, A.; Peña, G.; Fernández, N.; Urrutia, M.; Flores, N.; Gómez-Silva, B.; Di Ruggiero, J.; Shene, C.; Bustamante, M. Cellulose Synthase in Atacama Cyanobacteria and Bioethanol Production from Their Exopolysaccharides. Microorganisms 2023, 11, 2668. [Google Scholar] [CrossRef]
- Tamagnini, P.; Leitão, E.; Oliveira, P.; Ferreira, D.; Pinto, F.; Harris, D.J.; Heidorn, T.; Lindblad, P. Cyanobacterial Hydrogenases: Diversity, Regulation and Applications. FEMS Microbiol. Rev. 2007, 31, 692–720. [Google Scholar] [CrossRef] [PubMed]
- Milton, R.D.; Abdellaoui, S.; Khadka, N.; Dean, D.R.; Leech, D.; Seefeldt, L.C.; Minteer, S.D. Nitrogenase Bioelectrocatalysis: Heterogeneous Ammonia and Hydrogen Production by MoFe Protein. Energy Environ. Sci. 2016, 9, 2550–2554. [Google Scholar] [CrossRef]
- Forchhammer, K.; Selim, K.A. Carbon/Nitrogen Homeostasis Control in Cyanobacteria. FEMS Microbiol. Rev. 2019, 44, 33. [Google Scholar] [CrossRef]
- Patova, E.; Novakovskaya, I.; Gusev, E.; Martynenko, N. Diversity of Cyanobacteria and Algae in Biological Soil Crusts of the Northern Ural Mountain Region Assessed through Morphological and Metabarcoding Approaches. Diversity 2023, 15, 1080. [Google Scholar] [CrossRef]
- Tian, C.; Ju, M.; Eldridge, D.J.; Bu, C.; Bai, X.; Li, Y.; Guo, Q. Exogenous Microorganisms Promote Moss Biocrust Restoration and Shape Microbiomes in a Sandy Desert. Plant Soil 2023, 491, 421–437. [Google Scholar] [CrossRef]
- Li, X.; Hui, R.; Tan, H.; Zhao, Y.; Liu, R.; Song, N. Biocrust Research in China: Recent Progress and Application in Land Degradation Control. Front. Plant Sci. 2021, 12, 751521. [Google Scholar] [CrossRef]
- Rubio, C.; Lázaro, R. Patterns in Biocrust Recovery over Time in Semiarid Southeast Spain. Front. Microbiol. 2023, 14, 1184065. [Google Scholar] [CrossRef]
- Kumar, A.; Mukhia, S.; Kumar, R. Production, Characterisation, and Application of Exopolysaccharide Extracted from a Glacier Bacterium Mucilaginibacter sp. ERMR7:07. Process Biochem. 2022, 113, 27–36. [Google Scholar] [CrossRef]
- Barrera, A.; Acuña-Rodríguez, I.S.; Ballesteros, G.I.; Atala, C.; Molina-Montenegro, M.A. Biological Soil Crusts as Ecosystem Engineers in Antarctic Ecosystem. Front. Microbiol. 2022, 13, 755014. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Cao, C.; Peng, M.; Xu, X.; Zhang, P.; Yu, Q.; Sun, T. Diversity of Nitrogen-Fixing, Ammonia-Oxidizing, and Denitrifying Bacteria in Biological Soil Crusts of a Revegetation Area in Horqin Sandy Land, Northeast China. Ecol. Eng. 2014, 71, 71–79. [Google Scholar] [CrossRef]
- Roncero-Ramos, B.; Román, J.R.; Acién, G.; Cantón, Y. Towards Large Scale Biocrust Restoration: Producing an Efficient and Low-Cost Inoculum of N-Fixing Cyanobacteria. Sci. Total Environ. 2022, 848, 157704. [Google Scholar] [CrossRef]
- Sharma, V.; Prasanna, R.; Hossain, F.; Muthusamy, V.; Nain, L.; Das, S.; Shivay, Y.S.; Kumar, A. Priming Maize Seeds with Cyanobacteria Enhances Seed Vigour and Plant Growth in Elite Maize Inbreds. 3 Biotech 2020, 10, 154. [Google Scholar] [CrossRef] [PubMed]
- Cruz, J.D.; Delattre, C.; Felpeto, A.B.; Pereira, H.; Pierre, G.; Morais, J.; Petit, E.; Silva, J.; Azevedo, J.; Elboutachfaiti, R.; et al. Bioprospecting for Industrially Relevant Exopolysaccharide-Producing Cyanobacteria under Portuguese Simulated Climate. Sci. Rep. 2023, 13, 13561. [Google Scholar] [CrossRef]
- Karimi, A.; Tahmourespour, A.; Hoodaji, M. The Formation of Biocrust and Improvement of Soil Properties by the Exopolysaccharide-Producing Cyanobacterium: A Biogeotechnological Study. Biomass Convers. Biorefinery 2023, 13, 15489–15499. [Google Scholar] [CrossRef]
- Hartmann, M.; Six, J. Soil Structure and Microbiome Functions in Agroecosystems. Nat. Rev. Earth Environ. 2022, 4, 4–18. [Google Scholar] [CrossRef]
- Nguyen, H.T.; Pham, T.T.; Nguyen, P.T.; Le-Buanec, H.; Rabetafika, H.N.; Razafindralambo, H.L. Advances in Microbial Exopolysaccharides: Present and Future Applications. Biomolecules 2024, 14, 1162. [Google Scholar] [CrossRef]
- Madsen, M.A.; Semerdzhiev, S.; Twigg, J.D.; Moss, C.; Bavington, C.D.; Amtmann, A. Environmental Modulation of Exopolysaccharide Production in the Cyanobacterium Synechocystis 6803. Appl. Microbiol. Biotechnol. 2023, 107, 6121. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Ding, M.; Wang, M.; Yang, S.; Ma, X.; Hu, J.; Song, F.; Wang, L.; Liang, W. Proteome Profiling Reveals Changes in Energy Metabolism, Transport and Antioxidation during Drought Stress in Nostoc Flagelliforme. BMC Plant Biol. 2022, 22, 162. [Google Scholar] [CrossRef] [PubMed]
- Han, P.P.; Guo, R.J.; Shen, S.G.; Yan, R.R.; Wu, Y.K.; Yao, S.Y.; Wang, H.Y.; Jia, S.R. Proteomic Profiling of Nostoc Flagelliforme Reveals the Common Mechanism in Promoting Polysaccharide Production by Different Light Qualities. Biochem. Eng. J. 2018, 132, 68–78. [Google Scholar] [CrossRef]
- Roncero-Ramos, B.; Savaglia, V.; Durieu, B.; Van de Vreken, I.; Richel, A.; Wilmotte, A. Ecophysiological and Genomic Approaches to Cyanobacterial Hardening for Restoration. J. Phycol. 2024, 60, 465–482. [Google Scholar] [CrossRef]
- Han, C.F.; Liu, S.T.; Yan, R.R.; Li, J.; Chen, N.; Zhang, L.L.; Jia, S.R.; Han, P.P. Salicylic Acid and Jasmonic Acid Increase the Polysaccharide Production of Nostoc Flagelliforme via the Regulation of the Intracellular NO Level. Foods 2023, 12, 915. [Google Scholar] [CrossRef]
- Wu, S.; Yu, K.; Li, L.; Wang, L.; Liang, W. Enhancement of Exopolysaccharides Production and Reactive Oxygen Species Level of Nostoc Flagelliforme in Response to Dehydration. Environ. Sci. Pollut. Res. 2021, 28, 34300–34308. [Google Scholar] [CrossRef]
- Santos, M.; Pacheco, C.C.; Yao, L.; Hudson, E.P.; Tamagnini, P. Crispri as a Tool to Repress Multiple Copies of Extracellular Polymeric Substances (Eps)-Related Genes in the Cyanobacterium Synechocystis sp. Pcc 6803. Life 2021, 11, 1198. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Chen, L.; Wang, Y.; Sun, T.; Zhang, W. Current Advances in CRISPR-Cas-Mediated Gene Editing and Regulation in Cyanobacteria. Blue Biotechnol. 2024, 1, 9. [Google Scholar] [CrossRef]
- Potts, M. The Anhydrobiotic Cyanobacterial Cell. Physiol. Plant. 1996, 97, 788–794. [Google Scholar] [CrossRef]
- Li, C.; Zhang, X.; Ye, T.; Li, X.; Wang, G. Protection and Damage Repair Mechanisms Contributed To the Survival of Chroococcidiopsis sp. Exposed To a Mars-Like Near Space Environment. Microbiol. Spectr. 2022, 10, e03440-22. [Google Scholar] [CrossRef]
- Li, C.; Chen, Z.; Chen, L.; Wang, G. The Adaptation Mechanism of Desert Soil Cyanobacterium Chroococcidiopsis sp. to Desiccation. Plant Physiol. Biochem. 2025, 219, 109414. [Google Scholar] [CrossRef] [PubMed]
- Elbein, A.D.; Pan, Y.T.; Pastuszak, I.; Carroll, D. New Insights on Trehalose: A Multifunctional Molecule. Glycobiology 2003, 13, 17R–27R. [Google Scholar] [CrossRef]
- McDonald, M.D.; Owusu-Ansah, C.; Ellenbogen, J.B.; Malone, Z.D.; Ricketts, M.P.; Frolking, S.E.; Ernakovich, J.G.; Ibba, M.; Bagby, S.C.; Weissman, J.L. What Is Microbial Dormancy? Trends Microbiol. 2024, 32, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Balskus, E.P.; Walsh, C.T. The Genetic and Molecular Basis for Sunscreen Biosynthesis in Cyanobacteria. Science 1979 2010, 329, 1653–1656. [Google Scholar] [CrossRef]
- Hübschmann, T.; Yamamoto, H.; Gieler, T.; Murata, N.; Börner, T. Red and Far-Red Light Alter the Transcript Profile in the Cyanobacterium Synechocystis sp. PCC 6803: Impact of Cyanobacterial Phytochromes. FEBS Lett. 2005, 579, 1613–1618. [Google Scholar] [CrossRef]
- Oren, N.; Raanan, H.; Kedem, I.; Turjeman, A.; Bronstein, M.; Kaplan, A.; Murik, O. Desert Cyanobacteria Prepare in Advance for Dehydration and Rewetting: The Role of Light and Temperature Sensing. Mol. Ecol. 2019, 28, 2305–2320. [Google Scholar] [CrossRef]
- Xu, H.F.; Dai, G.Z.; Bai, Y.; Shang, J.L.; Zheng, B.; Ye, D.M.; Shi, H.; Kaplan, A.; Qiu, B.S. Coevolution of Tandemly Repeated Hlips and RpaB-like Transcriptional Factor Confers Desiccation Tolerance to Subaerial Nostoc Species. Proc. Natl. Acad. Sci. USA 2022, 119, e2211244119. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, R.; Durán, P. Natural Holobiome Engineering by Using Native Extreme Microbiome to Counteract the Climate Change Effects. Front. Bioeng. Biotechnol. 2020, 8, 568. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, S.; Gaurav, A.K.; Srivastava, S.; Verma, J.P. Plant Growth-Promoting Bacteria: Biological Tools for the Mitigation of Salinity Stress in Plants. Front. Microbiol. 2020, 11, 523350. [Google Scholar] [CrossRef]
- Bratovcic, A.; Bratovcic, A. Different Approaches to Reduce Salinity in Salt-Affected Soils and Enhancing Salt Stress Tolerance in Plants. Agric. Sci. 2024, 15, 830–847. [Google Scholar] [CrossRef]
- El-Ramady, H.; Prokisch, J.; Mansour, H.; Bayoumi, Y.A.; Shalaby, T.A.; Veres, S.; Brevik, E.C. Review of Crop Response to Soil Salinity Stress: Possible Approaches from Leaching to Nano-Management. Soil Syst. 2024, 8, 11. [Google Scholar] [CrossRef]
- Kageyama, H.; Waditee-Sirisattha, R. Osmoprotectant Molecules in Cyanobacteria: Their Basic Features, Biosynthetic Regulations, and Potential Applications. In Cyanobacterial Physiology: From Fundamentals to Biotechnology; Academic Press: Cambridge, MA, USA, 2022; pp. 113–123. [Google Scholar] [CrossRef]
- Waditee-Sirisattha, R.; Kageyama, H. Halotolerance, Stress Mechanisms, and Circadian Clock of Salt-Tolerant Cyanobacteria. Appl. Microbiol. Biotechnol. 2023, 107, 1129–1141. [Google Scholar] [CrossRef]
- Dong, Z.; Sun, T.; Zhang, W.; Chen, L. Improved Salt Tolerance of Synechococcus Elongatus PCC 7942 by Heterologous Synthesis of Compatible Solute Ectoine. Front. Microbiol. 2023, 14, 1123081. [Google Scholar] [CrossRef]
- Mudtham, N.A.; Promariya, A.; Duangsri, C.; Maneeruttanarungroj, C.; Ngamkala, S.; Akrimajirachoote, N.; Powtongsook, S.; Salminen, T.A.; Raksajit, W. Exogenous Trehalose Improves Growth, Glycogen and Poly-3-Hydroxybutyrate (PHB) Contents in Photoautotrophically Grown Arthrospira Platensis under Nitrogen Deprivation. Biology 2024, 13, 127. [Google Scholar] [CrossRef] [PubMed]
- Duan, H.; Liu, W.; Zhou, L.; Han, B.; Huo, S.; El-Sheekh, M.; Dong, H.; Li, X.; Xu, T.; Elshobary, M. Improving Saline-Alkali Soil and Promoting Wheat Growth by Co-Applying Potassium-Solubilizing Bacteria and Cyanobacteria Produced from Brewery Wastewater. Front. Environ. Sci 2023, 11, 1170734. [Google Scholar] [CrossRef]
- Nelson, C.; Giraldo-Silva, A.; Garcia-Pichel, F. A Symbiotic Nutrient Exchange within the Cyanosphere Microbiome of the Biocrust Cyanobacterium, Microcoleus Vaginatus. ISME J. 2021, 15, 282–292. [Google Scholar] [CrossRef] [PubMed]
- Ataeian, M.; Liu, Y.; Kouris, A.; Hawley, A.K.; Strous, M. Ecological Interactions of Cyanobacteria and Heterotrophs Enhances the Robustness of Cyanobacterial Consortium for Carbon Sequestration. Front. Microbiol. 2022, 13, 780346. [Google Scholar] [CrossRef]
- Hooda, S.; Malik, G.; Saini, P.; Grewall, A.; Pandey, V.C. Cyanobacteria as a Potential Bioasset for Restoring Degraded Land. Land Degrad. Dev. 2023, 34, 3435–3450. [Google Scholar] [CrossRef]
- Campos, J.R.; de Aviz, R.O.; Silva, D.E.O.; Costa, R.M.; Rocha, S.M.B.; de Alcantara Neto, F.; de Medeiros, E.V.; Mendes, L.W.; de Pereira, A.P.A.; Araujo, A.S.F. Short-Term Effects of Restoration on Soil Biological Properties in Degraded Lands of the Brazilian Semiarid Region. Restor. Ecol. 2025, e70060. [Google Scholar] [CrossRef]
- Dadzie, F.A.; Moles, A.T.; Erickson, T.E.; Slavich, E.; Muñoz-Rojas, M. Native Bacteria and Cyanobacteria Can Influence Seedling Emergence and Growth of Native Plants Used in Dryland Restoration. J. Appl. Ecol. 2022, 59, 2983–2992. [Google Scholar] [CrossRef]
- Qian, L.; Wu, L.; Yang, L.; Zhang, Z. Inoculation Concentration Modulating the Secretion and Accumulation Pattern of Exopolysaccharides in Desert Cyanobacterium Microcoleus Vaginatus. Biotechnol. Appl. Biochem. 2021, 68, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Qi, S.; Wang, J.; Zhang, Y.; Naz, M.; Afzal, M.R.; Du, D.; Dai, Z. Omics Approaches in Invasion Biology: Understanding Mechanisms and Impacts on Ecological Health. Plants 2023, 12, 1860. [Google Scholar] [CrossRef]
- Overy, D.P.; Bell, M.A.; Habtewold, J.; Helgason, B.L.; Gregorich, E.G. “Omics” Technologies for the Study of Soil Carbon Stabilization: A Review. Front. Environ. Sci. 2021, 9, 617952. [Google Scholar] [CrossRef]
- Sharuddin, S.S.; Ramli, N.; Yusoff, M.Z.M.; Muhammad, N.A.N.; Ho, L.S.; Maeda, T. Advancement of Metatranscriptomics towards Productive Agriculture and Sustainable Environment: A Review. Int. J. Mol. Sci. 2022, 23, 3737. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Zhou, X.; Rensing, C.; Liesack, W.; Zhu, Y.G. Soil Microbial Ecology through the Lens of Metatranscriptomics. Soil Ecol. Lett. 2023, 6, 230217. [Google Scholar] [CrossRef]
- Rene, E.R.; Zappi, M.; Gonzalez-Gonzalez, L.M.; De-Bashan, L.E. The Potential of Microalgae–Bacteria Consortia to Restore Degraded Soils. Biology 2023, 12, 693. [Google Scholar] [CrossRef]
- Rebello, S.; Nathan, V.K.; Sindhu, R.; Binod, P.; Awasthi, M.K.; Pandey, A. Bioengineered Microbes for Soil Health Restoration: Present Status and Future. Bioengineered 2021, 12, 12839–12853. [Google Scholar] [CrossRef]
- Márquez-Godoy, J.N.; González-Escobedo, R.; Márquez-Godoy, J.N.; González-Escobedo, R. Tecnologías Ómicas Para La Exploración de La Biocostra Del Suelo. Terra Latinoam. 2022, 40, 1062. [Google Scholar] [CrossRef]
- Pound, H.L.; Martin, R.M.; Sheik, C.S.; Steffen, M.M.; Newell, S.E.; Dick, G.J.; McKay, R.M.L.; Bullerjahn, G.S.; Wilhelm, S.W. Environmental Studies of Cyanobacterial Harmful Algal Blooms Should Include Interactions with the Dynamic Microbiome. Environ. Sci. Technol. 2021, 55, 12776–12779. [Google Scholar] [CrossRef]
- Tu, C.; Dong, X.; Yang, H.; Chang, Y.; Xu, Z.; Che, F.; Wang, S.; Huang, W. Characterization of Phosphate Solubilizing Bacteria in the Sediments of Eutrophic Lakes and Their Potential for Cyanobacterial Recruitment. Chemosphere 2024, 352, 141276. [Google Scholar] [CrossRef]
- Ji, X.; Wu, T.; Xiao, J.; Yang, K.; Sun, Z.; Yang, T.; Hu, R. Strong Spring Winds Accelerated the Recruitment and Reinvasion of Cyanobacteria. Environ. Sci. Pollut. Res. 2021, 28, 16855–16866. [Google Scholar] [CrossRef] [PubMed]
- Kust, A.; Zorz, J.; Paniker, C.C.; Bouma-Gregson, K.; Krishnappa, N.; Banfield, J.F.; Diamond, S. Model Cyanobacterial Consortia Reveal a Consistent Core Microbiome Independent of Inoculation Source or Cyanobacterial Host Species. bioRxiv 2023, 2023, 570939. [Google Scholar] [CrossRef]
- Lebre, P.H.; De Maayer, P.; Cowan, D.A. Xerotolerant Bacteria: Surviving through a Dry Spell. Nat. Rev. Microbiol. 2017, 15, 285–296. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Han, Y.; Lan, S.; Hu, C. Metagenomic Insight Into Patterns and Mechanism of Nitrogen Cycle During Biocrust Succession. Front. Microbiol. 2021, 12, 633428. [Google Scholar] [CrossRef]
- Nelson, C.; Garcia-Pichel, F. Beneficial Cyanosphere Heterotrophs Accelerate Establishment of Cyanobacterial Biocrust. Appl. Environ. Microbiol. 2021, 87, e01236-21. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.; Hu, Y.; Kosina, S.M.; Van Goethem, M.W.; Tringe, S.G.; Bowen, B.P.; Northen, T.R. Conservation of Beneficial Microbes between the Rhizosphere and the Cyanosphere. New Phytol. 2023, 240, 1246–1258. [Google Scholar] [CrossRef]
- Chamizo, S.; Rodríguez-Caballero, E.; Sánchez-Cañete, E.P.; Domingo, F.; Cantón, Y. Temporal Dynamics of Dryland Soil CO2 Efflux Using High-Frequency Measurements: Patterns and Dominant Drivers among Biocrust Types, Vegetation and Bare Soil. Geoderma 2022, 405, 115404. [Google Scholar] [CrossRef]
- Pascault, N.; Rué, O.; Loux, V.; Pédron, J.; Martin, V.; Tambosco, J.; Bernard, C.; Humbert, J.F.; Leloup, J. Insights into the Cyanosphere: Capturing the Respective Metabolisms of Cyanobacteria and Chemotrophic Bacteria in Natural Conditions? Environ. Microbiol. Rep. 2021, 13, 364–374. [Google Scholar] [CrossRef]
- Chamizo, S.; Mugnai, G.; Rossi, F.; Certini, G.; De Philippis, R. Cyanobacteria Inoculation Improves Soil Stability and Fertility on Different Textured Soils: Gaining Insights for Applicability in Soil Restoration. Front. Environ. Sci. 2018, 6, 369549. [Google Scholar] [CrossRef]
- Rossi, F.; Mugnai, G.; De Philippis, R. Cyanobacterial Biocrust Induction: A Comprehensive Review on a Soil Rehabilitation-Effective Biotechnology. Geoderma 2022, 415, 115766. [Google Scholar] [CrossRef]
- Becerra-Absalón, I.; Johansen, J.R.; Muñoz-Martín, M.A.; Montejano, G. Chroakolemma gen. Nov. (Leptolyngbyaceae, Cyanobacteria) from Soil Biocrusts in the Semi-Desert Central Region of Mexico. Phytotaxa 2018, 367, 201–218. [Google Scholar] [CrossRef]
- Becerra-Absalón, I.; Muñoz-Martín, M.Á.; Montejano, G.; Mateo, P. Differences in the Cyanobacterial Community Composition of Biocrusts from the Drylands of Central Mexico. Are There Endemic Species? Front. Microbiol. 2019, 10, 444841. [Google Scholar] [CrossRef] [PubMed]
- Machado de Lima, N.M.; Muñoz-Rojas, M.; Vázquez-Campos, X.; Branco, L.H.Z. Biocrust Cyanobacterial Composition, Diversity, and Environmental Drivers in Two Contrasting Climatic Regions in Brazil. Geoderma 2021, 386, 114914. [Google Scholar] [CrossRef]
- Dojani, S.; Kauff, F.; Weber, B.; Büdel, B. Genotypic and Phenotypic Diversity of Cyanobacteria in Biological Soil Crusts of the Succulent Karoo and Nama Karoo of Southern Africa. Microb. Ecol. 2014, 67, 286–301. [Google Scholar] [CrossRef] [PubMed]
- Giraldo-Silva, A.; Nelson, C.; Penfold, C.; Barger, N.N.; Garcia-Pichel, F. Effect of Preconditioning to the Soil Environment on the Performance of 20 Cyanobacterial Strains Used as Inoculum for Biocrust Restoration. Restor. Ecol. 2020, 28, S187–S193. [Google Scholar] [CrossRef]
- Giraldo-Silva, A.; Nelson, C.; Barger, N.N.; Garcia-Pichel, F. Nursing Biocrusts: Isolation, Cultivation, and Fitness Test of Indigenous Cyanobacteria. Restor. Ecol. 2019, 27, 793–803. [Google Scholar] [CrossRef]
- Flechtner, V.R.; Johansen, J.R.; Belnap, J. The Biological Soil Crusts of the San Nicolas Island: Enigmatic Algae from a Geographically Isolated Ecosystem. West. N. Am. Nat. 2008, 68, 405–436. [Google Scholar] [CrossRef]
- Dulić, T.; Meriluoto, J.; Palanački Malešević, T.; Gajić, V.; Važić, T.; Tokodi, N.; Obreht, I.; Kostić, B.; Kosijer, P.; Khormali, F.; et al. Cyanobacterial Diversity and Toxicity of Biocrusts from the Caspian Lowland Loess Deposits, North Iran. Quat. Int. 2017, 429, 74–85. [Google Scholar] [CrossRef]
- Couradeau, E.; Giraldo-Silva, A.; De Martini, F.; Garcia-Pichel, F. Spatial Segregation of the Biological Soil Crust Microbiome around Its Foundational Cyanobacterium, Microcoleus Vaginatus, and the Formation of a Nitrogen-Fixing Cyanosphere. Microbiome 2019, 7, 55. [Google Scholar] [CrossRef]
- Karimi, A.; Tahmourespour, A.; Hoodaji, M. Cyanobacterial Biocrust Alters Soil Physical Properties Reducing Soil Erosion and Aerosol Production. Braz. J. Microbiol. 2024, 55, 2453–2461. [Google Scholar] [CrossRef]
- Lan, S.; Wu, L.; Adessi, A.; Hu, C. Cyanobacterial Persistence and Influence on Microbial Community Dynamics over 15 Years in Induced Biocrusts. Environ. Microbiol. 2022, 24, 66–81. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Zhang, C.; Vadiveloo, A.; Montes, M.L.; Xia, L.; Song, S.; Fernandez, M.A.; Lan, S. Efficient Nutrient Recycling from Wastewater to Deserts: A Comparative Study on Biocrust Cyanobacteria Performance. Chem. Eng. J. 2024, 491, 151927. [Google Scholar] [CrossRef]
- Kimura, K.; Okuro, T. Cyanobacterial Biocrust on Biomineralized Soil Mitigates Freeze–Thaw Effects and Preserves Structure and Ecological Functions. Microb. Ecol. 2024, 87, 69. [Google Scholar] [CrossRef] [PubMed]
- Kheirfam, H. Increasing Soil Potential for Carbon Sequestration Using Microbes from Biological Soil Crusts. J. Arid. Environ. 2020, 172, 104022. [Google Scholar] [CrossRef]
- Alameda-Martín, A.; Chamizo, S.; Maggioli, L.; Roman, R.; Machado-de-Lima, N.; Muñoz-Rojas, M.; Cantón, Y. Optimizing Survival and Growth of Inoculated Biocrust-Forming Cyanobacteria through Native Plant-Based Habitat Amelioration. J. Environ. Manag. 2024, 370, 122960. [Google Scholar] [CrossRef]
- Águila-Carricondo, P.; Román, R.; Marín-Guirao, J.I.; Cantón, Y.; de Cara, M. Native Biocrust Cyanobacteria Strains Showing Antagonism against Three Soilborne Pathogenic Fungi. Pathogens 2024, 13, 579. [Google Scholar] [CrossRef]
- Chamizo, S.; Adessi, A.; Certini, G.; De Philippis, R. Cyanobacteria Inoculation as a Potential Tool for Stabilization of Burned Soils. Restor. Ecol. 2020, 28, S106–S114. [Google Scholar] [CrossRef]
- Pagli, C.; Chamizo, S.; Migliore, G.; Rugnini, L.; De Giudici, G.; Braglia, R.; Canini, A.; Cantón, Y. Isolation of Biocrust Cyanobacteria and Evaluation of Cu, Pb, and Zn Immobilisation Potential for Soil Restoration and Sustainable Agriculture. Sci. Total Environ. 2024, 946, 174020. [Google Scholar] [CrossRef]
- Muñoz-Rojas, M.; Chilton, A.; Liyanage, G.S.; Erickson, T.E.; Merritt, D.J.; Neilan, B.A.; Ooi, M.K.J. Effects of Indigenous Soil Cyanobacteria on Seed Germination and Seedling Growth of Arid Species Used in Restoration. Plant Soil 2018, 429, 91–100. [Google Scholar] [CrossRef]
- Machado-de-Lima, N.M.; Charlesworth, J.; Stewart, J.; Ooi, M.K.J.; Muñoz-Rojas, M. Seed Biopriming at Different Concentrations to Assess the Effects of Cyanobacteria on Germination and Seedling Performance of Keystone Arid Species. J. Sustain. Agric. Environ. 2023, 2, 266–275. [Google Scholar] [CrossRef]
- Yadav, P.; Singh, R.P.; Alodaini, H.A.; Hatamleh, A.A.; Santoyo, G.; Kumar, A.; Gupta, R.K. Impact of Dehydration on the Physiochemical Properties of Nostoc Calcicola BOT1 and Its Untargeted Metabolic Profiling through UHPLC-HRMS. Front. Plant Sci. 2023, 14, 1147390. [Google Scholar] [CrossRef]
- Fattahi, S.M.; Soroush, A.; Huang, N. Wind Erosion Control Using Inoculation of Aeolian Sand with Cyanobacteria. Land Degrad. Dev. 2020, 31, 2104–2116. [Google Scholar] [CrossRef]
- Hakkoum, Z.; Minaoui, F.; Douma, M.; Mouhri, K.; Loudiki, M. Diversity and Spatial Distribution of Soil Cyanobacteria Along an Altitudinal Gradient in Marrakesh Area (Morocco). Appl. Ecol. Environ. Res. 2020, 18, 5527–5545. [Google Scholar] [CrossRef]
- Moia, I.C.; Pereira, S.B.; Domizio, P.; De Philippis, R.; Adessi, A. Phormidium Ambiguum and Leptolyngbya Ohadii Exopolysaccharides under Low Water Availability. Polymers 2023, 15, 1889. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Quan, L.; Deng, Z.; Vadiveloo, A.; Cheng, Y.; Yang, L.; Zhang, Z.; Saber, A.A.; Lan, S. Performance of a Biocrust Cyanobacteria-Indigenous Bacteria (BCIB) Co-Culture System for Nutrient Capture and Transfer in Municipal Wastewater. Sci. Total Environ. 2023, 888, 164236. [Google Scholar] [CrossRef]
- Büdel, B.; Williams, W.J.; Reichenberger, H. Annual Net Primary Productivity of a Cyanobacteria-Dominated Biological Soil Crust in the Gulf Savannah, Queensland, Australia. Biogeosciences 2018, 15, 491–505. [Google Scholar] [CrossRef]
- Cano-Díaz, C.; Mateo, P.; Muñoz-Martín, M.Á.; Maestre, F.T. Diversity of Biocrust-Forming Cyanobacteria in a Semiarid Gypsiferous Site from Central Spain. J. Arid. Environ. 2018, 151, 83–89. [Google Scholar] [CrossRef]
- Giraldo-Silva, A.; Fernandes, V.M.C.; Bethany, J.; Garcia-Pichel, F. Niche Partitioning with Temperature among Heterocystous Cyanobacteria (Scytonema spp., Nostoc spp., and Tolypothrix spp.) from Biological Soil Crusts. Microorganisms 2020, 8, 396. [Google Scholar] [CrossRef]
- Ferreira, V.C.R.; de Sá Lima, L.G.; Branco, L.H.Z.; Santoro, K.R.; Corrêa, M.M.; Molica, R.J.R. Distinct Responses of Scytonema Hyalinum and Leptolyngbya sp. to Water Availability and Biocrust Formation. Braz. J. Microbiol. 2025, 56, 1263–1275. [Google Scholar] [CrossRef]
- Alameda-Martín, A.; Chamizo, S.; Rodríguez-Caballero, E.; Muñoz-Rojas, M.; Cantón, Y. The Potential of Biocrust-Forming Cyanobacteria to Enhance Seedling Growth of Native Semi-Arid Plants Through Seed Biopriming. J. Plant Growth Regul. 2024, 1–18. [Google Scholar] [CrossRef]
- De, D.; Nayak, T.; Das, G.; Dhal, P.K. Metagenomics and Bioinformatics in Microbial Ecology: Current Status and Beyond. In Applications of Metagenomics: Agriculture, Environment, and Health; Academic Press: Cambridge, MA, USA, 2024; pp. 359–385. [Google Scholar] [CrossRef]
- Moore, L.R.; Caspi, R.; Campbell, D.A.; Casey, J.R.; Crevecoeur, S.; Lea-Smith, D.J.; Long, B.; Omar, N.M.; Paley, S.M.; Schmelling, N.M.; et al. CyanoCyc Cyanobacterial Web Portal. Front. Microbiol. 2024, 15, 1340413. [Google Scholar] [CrossRef] [PubMed]
- Cantalapiedra, C.P.; Hernández-Plaza, A.; Letunic, I.; Bork, P.; Huerta-Cepas, J. EggNOG-Mapper v2: Functional Annotation, Orthology Assignments, and Domain Prediction at the Metagenomic Scale. Mol. Biol. Evol. 2021, 38, 5825–5829. [Google Scholar] [CrossRef] [PubMed]
- Bai, D.; Ma, C.; Xun, J.; Luo, H.; Yang, H.; Lyu, H.; Zhu, Z.; Gai, A.; Yousuf, S.; Peng, K.; et al. MicrobiomeStatPlots: Microbiome Statistics Plotting Gallery for Meta-Omics and Bioinformatics. iMeta 2025, 4, e70002. [Google Scholar] [CrossRef]
- Jones, M.R.; Pinto, E.; Torres, M.A.; Dörr, F.; Mazur-Marzec, H.; Szubert, K.; Tartaglione, L.; Dell’Aversano, C.; Miles, C.O.; Beach, D.G.; et al. CyanoMetDB, a Comprehensive Public Database of Secondary Metabolites from Cyanobacteria. Water Res. 2021, 196, 117017. [Google Scholar] [CrossRef] [PubMed]
- Blin, K.; Shaw, S.; Vader, L.; Szenei, J.; Reitz, Z.L.; Augustijn, H.E.; Cediel-Becerra, J.D.D.; de Crécy-Lagard, V.; Koetsier, R.A.; Williams, S.E.; et al. AntiSMASH 8.0: Extended Gene Cluster Detection Capabilities and Analyses of Chemistry, Enzymology, and Regulation. Nucleic Acids Res. 2013, 1, 13–14. [Google Scholar] [CrossRef]
- Biswas, A.; Staals, R.H.J.; Morales, S.E.; Fineran, P.C.; Brown, C.M. CRISPRDetect: A Flexible Algorithm to Define CRISPR Arrays. BMC Genom. 2016, 17, 356. [Google Scholar] [CrossRef]
- Wu, L.Y.; Wijesekara, Y.; Piedade, G.J.; Pappas, N.; Brussaard, C.P.D.; Dutilh, B.E. Benchmarking Bioinformatic Virus Identification Tools Using Real-World Metagenomic Data across Biomes. Genome Biol. 2024, 25, 97. [Google Scholar] [CrossRef]
- Guo, J.; Bolduc, B.; Zayed, A.A.; Varsani, A.; Dominguez-Huerta, G.; Delmont, T.O.; Pratama, A.A.; Gazitúa, M.C.; Vik, D.; Sullivan, M.B.; et al. VirSorter2: A Multi-Classifier, Expert-Guided Approach to Detect Diverse DNA and RNA Viruses. Microbiome 2021, 9, 37. [Google Scholar] [CrossRef]
- Kieft, K.; Zhou, Z.; Anantharaman, K. VIBRANT: Automated Recovery, Annotation and Curation of Microbial Viruses, and Evaluation of Viral Community Function from Genomic Sequences. Microbiome 2020, 8, 90. [Google Scholar] [CrossRef]
- Durairaj, J.; Waterhouse, A.M.; Mets, T.; Brodiazhenko, T.; Abdullah, M.; Studer, G.; Tauriello, G.; Akdel, M.; Andreeva, A.; Bateman, A.; et al. Uncovering New Families and Folds in the Natural Protein Universe. Nature 2023, 622, 646–653. [Google Scholar] [CrossRef]
- Liu, H. AlphaFold and Structural Mass Spectrometry Enable Interrogations on the Intrinsically Disordered Regions in Cyanobacterial Light-Harvesting Complex Phycobilisome. J. Mol. Biol. 2022, 434, 167831. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.E.; Agdanowski, M.P.; Dolinsky, J.L.; Sawaya, M.R.; Cascio, D.; Rodriguez, J.A.; Yeates, T.O. AlphaFold-Assisted Structure Determination of a Bacterial Protein of Unknown Function Using X-Ray and Electron Crystallography. Acta Crystallogr. D Struct. Biol. 2024, 80, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Koehler Leman, J.; Szczerbiak, P.; Renfrew, P.D.; Gligorijevic, V.; Berenberg, D.; Vatanen, T.; Taylor, B.C.; Chandler, C.; Janssen, S.; Pataki, A.; et al. Sequence-Structure-Function Relationships in the Microbial Protein Universe. Nat. Commun. 2023, 14, 2351. [Google Scholar] [CrossRef] [PubMed]
- Barrio-Hernandez, I.; Yeo, J.; Jänes, J.; Mirdita, M.; Gilchrist, C.L.M.; Wein, T.; Varadi, M.; Velankar, S.; Beltrao, P.; Steinegger, M. Clustering Predicted Structures at the Scale of the Known Protein Universe. Nature 2023, 622, 637–645. [Google Scholar] [CrossRef]
- Suttle, C.A. Cyanophages and Their Role in the Ecology of Cyanobacteria. In The Ecology of Cyanobacteria; Springer: Dordrecht, The Netherlands, 2000; pp. 563–589. [Google Scholar] [CrossRef]
- Sullivan, M.B.; Waterbury, J.B.; Chisholm, S.W. Cyanophages Infecting the Oceanic Cyanobacterium Prochlorococcus. Nature 2003, 424, 1047–1051. [Google Scholar] [CrossRef]
- Broman, E.; Holmfeldt, K.; Bonaglia, S.; Hall, P.O.J.; Nascimento, F.J.A. Cyanophage Diversity and Community Structure in Dead Zone Sediments. mSphere 2021, 6, e00208-21. [Google Scholar] [CrossRef]
- Kour, B.; Sharma, P.; Ramya, S.; Gawdiya, S.; Sudheer, K.; Ramakrishnan, B. Cyanobacterial Biofertilizer Inoculation Has a Distinctive Effect on the Key Genes of Carbon and Nitrogen Cycling in Paddy Rice. J. Appl. Phycol. 2024, 36, 1859–1874. [Google Scholar] [CrossRef]
- Tian, F.; Wainaina, J.M.; Howard-Varona, C.; Domínguez-Huerta, G.; Bolduc, B.; Gazitúa, M.C.; Smith, G.; Gittrich, M.R.; Zablocki, O.; Cronin, D.R.; et al. Prokaryotic-Virus-Encoded Auxiliary Metabolic Genes throughout the Global Oceans. Microbiome 2024, 12, 159. [Google Scholar] [CrossRef]
- Zablocki, O.; Adriaenssens, E.M.; Cowan, D. Diversity and Ecology of Viruses in Hyperarid Desert Soils. Appl. Environ. Microbiol. 2016, 82, 770–777. [Google Scholar] [CrossRef]
- Zhao, Y.; Lian, Y.C.; Zhao, Y.Q.; Xu, W.W.; Zhao, Y.X.; Zhang, Z.S. Biocrust Succession Significantly Influences Soil Virus Composition and Alpha Diversity in a Sandy Desert. Appl. Soil Ecol. 2024, 195, 105255. [Google Scholar] [CrossRef]
- Williamson, K.E.; Fuhrmann, J.J.; Wommack, K.E.; Radosevich, M. Viruses in Soil Ecosystems: An Unknown Quantity Within an Unexplored Territory. Annu. Rev. Virol. 2017, 4, 201–219. [Google Scholar] [CrossRef] [PubMed]
- Chevallereau, A.; Pons, B.J.; van Houte, S.; Westra, E.R. Interactions between Bacterial and Phage Communities in Natural Environments. Nat. Rev. Microbiol. 2022, 20, 49–62. [Google Scholar] [CrossRef] [PubMed]
- Thompson, L.R.; Zeng, Q.; Kelly, L.; Huang, K.H.; Singer, A.U.; Stubbe, J.A.; Chisholm, S.W. Phage Auxiliary Metabolic Genes and the Redirection of Cyanobacterial Host Carbon Metabolism. Proc. Natl. Acad. Sci. USA 2011, 108, E757–E764. [Google Scholar] [CrossRef]
- Zhu, D.; Liu, S.Y.; Sun, M.M.; Yi, X.Y.; Duan, G.L.; Ye, M.; Gillings, M.R.; Zhu, Y.G. Adaptive Expression of Phage Auxiliary Metabolic Genes in Paddy Soils and Their Contribution toward Global Carbon Sequestration. Proc. Natl. Acad. Sc.i USA 2024, 121, e2419798121. [Google Scholar] [CrossRef]
- Fuchsman, C.A.; Palevsky, H.I.; Widner, B.; Duffy, M.; Carlson, M.C.G.; Neibauer, J.A.; Mulholland, M.R.; Keil, R.G.; Devol, A.H.; Rocap, G. Cyanobacteria and Cyanophage Contributions to Carbon and Nitrogen Cycling in an Oligotrophic Oxygen-Deficient Zone. ISME J. 2019, 13, 2714–2726. [Google Scholar] [CrossRef]
- Hirose, Y.; Ohtsubo, Y.; Misawa, N.; Yonekawa, C.; Nagao, N.; Shimura, Y.; Fujisawa, T.; Kanesaki, Y.; Katoh, H.; Katayama, M.; et al. Genome Sequencing of the NIES Cyanobacteria Collection with a Focus on the Heterocyst-Forming Clade. DNA Res. 2021, 28, dsab024. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Jeraldo, P.; Herbert, W.; McDonough, S.; Eckloff, B.; Schulze-Makuch, D.; de Vera, J.P.; Cockell, C.; Leya, T.; Baqué, M.; et al. Whole Genome Sequencing of Cyanobacterium Nostoc sp. CCCryo 231-06 Using Microfluidic Single Cell Technology. iScience 2022, 25, 104291. [Google Scholar] [CrossRef]
- Baunach, M.; Guljamow, A.; Miguel-Gordo, M.; Dittmann, E. Harnessing the Potential: Advances in Cyanobacterial Natural Product Research and Biotechnology. Nat. Prod. Rep. 2023, 41, 347–369. [Google Scholar] [CrossRef]
- Rajeev, L.; Da Rocha, U.N.; Klitgord, N.; Luning, E.G.; Fortney, J.; Axen, S.D.; Shih, P.M.; Bouskill, N.J.; Bowen, B.P.; Kerfeld, C.A.; et al. Dynamic Cyanobacterial Response to Hydration and Dehydration in a Desert Biological Soil Crust. ISME J. 2013, 7, 2178–2191. [Google Scholar] [CrossRef]
- Alneberg, J.; Karlsson, C.M.G.; Divne, A.M.; Bergin, C.; Homa, F.; Lindh, M.V.; Hugerth, L.W.; Ettema, T.J.G.; Bertilsson, S.; Andersson, A.F.; et al. Genomes from Uncultivated Prokaryotes: A Comparison of Metagenome-Assembled and Single-Amplified Genomes. Microbiome 2018, 6, 173. [Google Scholar] [CrossRef]
- Vollmers, J.; Wiegand, S.; Kaster, A.K. Comparing and Evaluating Metagenome Assembly Tools from a Microbiologist’s Perspective—Not Only Size Matters! PLoS ONE 2017, 12, e0169662. [Google Scholar] [CrossRef] [PubMed]
- Van Goethem, M.W.; Osborn, A.R.; Bowen, B.P.; Andeer, P.F.; Swenson, T.L.; Clum, A.; Riley, R.; He, G.; Koriabine, M.; Sandor, L.; et al. Long-Read Metagenomics of Soil Communities Reveals Phylum-Specific Secondary Metabolite Dynamics. Commun. Biol. 2021, 4, 1302. [Google Scholar] [CrossRef] [PubMed]
| Genera | Application | Environmental Stress Tolerance | Soil Type | References |
|---|---|---|---|---|
| Chroakolemma | Restoration of biocrusts | UV radiation; drought; arid conditions | Phaeozem calcareous and Phaeozem (mollisol) | [107,108] |
| Microcoleus | Restoration of biocrusts and soils, and use as PGPR | Drought; high salinity; UV radiation; nutrient-poor soils. | Arid/semi-arid soils | [82,109,110,111,112,113,114,115,116,117,118,119] |
| Nostoc | Restoration of soils and biocrusts, and use as bioremediation and PGPR | Extreme temperatures; UV radiation; drought. | Arid/semi-arid soils; agricultural soils | [16,120,121,122,123,124,125,126,127,128] |
| Phormidium | Use as PGPR | High salinity; temperature fluctuations. | Arid soils; saline soils. | [129,130] |
| Scytonema | Restoration of soils and burned soils | UV radiation; drought; high temperatures; nutrient-poor soils. | Arid/semi-arid soils; burned soils | [16,50,110,114,117,118,121,122,125,126,131,132,133,134,135] |
| Trichocoleus | Restoration of soils | Drought; nutrient-poor soils. | Restoration soils (gypsiferous) | [50,126,133,136] |
| Leptolyngbya | Restoration of biocrusts | Drought; High salinity | Arid/semirad soils; Planosol | [81,135] |
| Tolypothrix | PGPR | Drought | Arid/semi-arid soils | [136] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garcia, M.; Bruna, P.; Duran, P.; Abanto, M. Cyanobacteria and Soil Restoration: Bridging Molecular Insights with Practical Solutions. Microorganisms 2025, 13, 1468. https://doi.org/10.3390/microorganisms13071468
Garcia M, Bruna P, Duran P, Abanto M. Cyanobacteria and Soil Restoration: Bridging Molecular Insights with Practical Solutions. Microorganisms. 2025; 13(7):1468. https://doi.org/10.3390/microorganisms13071468
Chicago/Turabian StyleGarcia, Matias, Pablo Bruna, Paola Duran, and Michel Abanto. 2025. "Cyanobacteria and Soil Restoration: Bridging Molecular Insights with Practical Solutions" Microorganisms 13, no. 7: 1468. https://doi.org/10.3390/microorganisms13071468
APA StyleGarcia, M., Bruna, P., Duran, P., & Abanto, M. (2025). Cyanobacteria and Soil Restoration: Bridging Molecular Insights with Practical Solutions. Microorganisms, 13(7), 1468. https://doi.org/10.3390/microorganisms13071468





