Reimagining Microbially Induced Concrete Deterioration: A Novel Approach Through Coupled Confocal Laser Scanning Microscope–Avizo Three-Dimensional Modeling of Biofilms
Abstract
1. Introduction
2. Materials and Methods
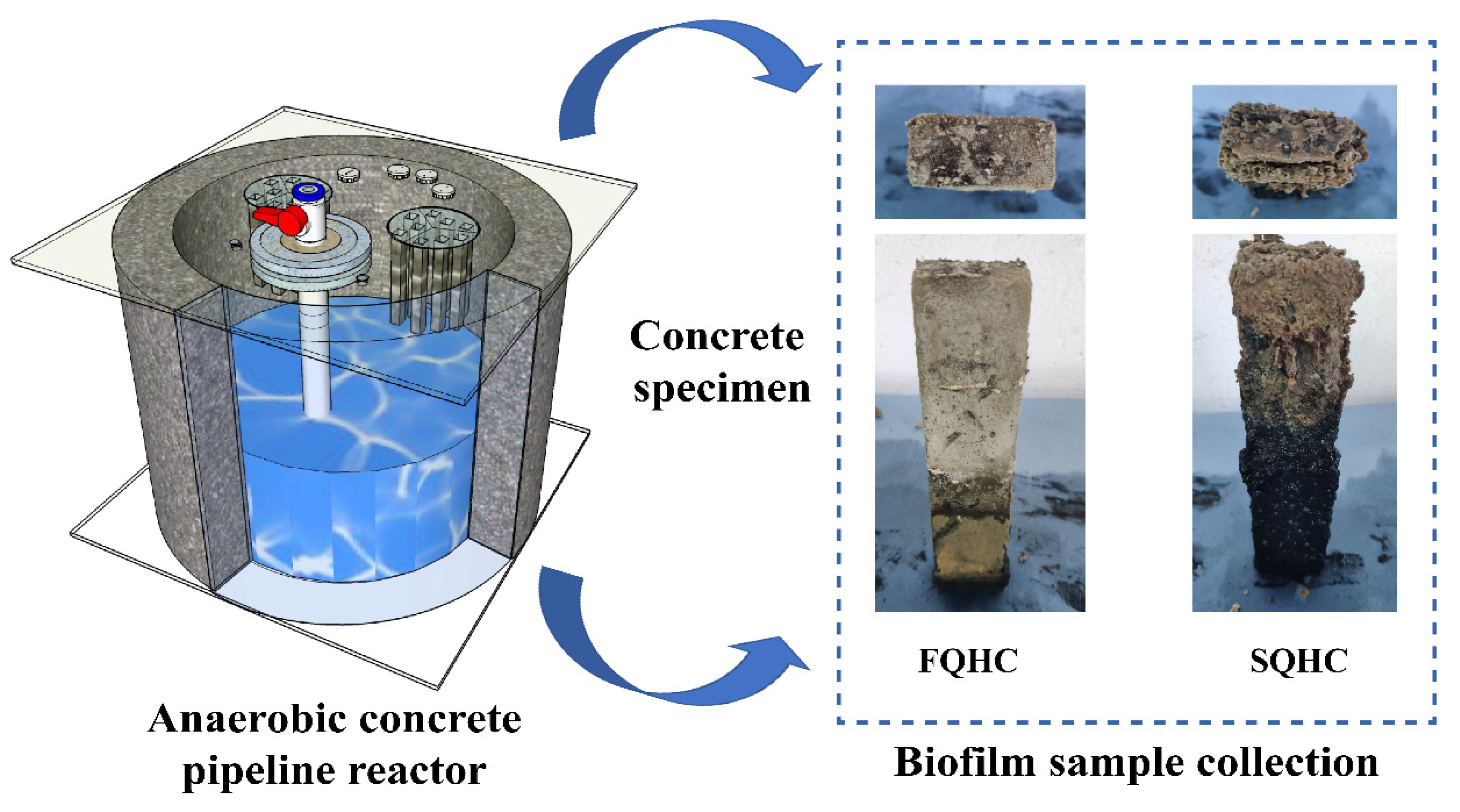
2.1. Preparation of Concrete Material Specimens
2.2. Design and Operating Conditions of Anaerobic Concrete Pipeline
2.3. Inoculation and Domestication of Activated Sludge
2.4. Monitoring of Chemical Parameters Inside the Pipeline
2.5. Temenos Collection and Measurement of EPS Component Content
2.6. Determination of Protein and Polysaccharide Content in EPS
2.7. SEM Observation
2.8. EPS Fluorescent Staining and CLSM Observation
2.9. Determination of Fluorescence Spectroscopy
2.10. Avizo Modeling
3. Results and Discussion
3.1. Analysis of Chemical Parameters of Concrete Pipeline Reactor
3.2. Microbial Community Composition and Diversity
3.3. Components and Physicochemical Properties of Temenoses
3.4. SEM Image Analysis
3.5. Three-Dimensional Reconstruction of Temenos
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Anbari, M.J.; Tabesh, M.; Roozbahani, A. Risk assessment model to prioritize sewer pipes inspection in wastewater collection networks. J. Environ. Manag. 2017, 190, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Grengg, C.; Mittermayr, F.; Ukrainczyk, N.; Koraimann, G.; Kienesberger, S.; Dietzel, M. Advances in concrete materials for sewer systems affected by microbial induced concrete corrosion: A review. Water Res. 2018, 134, 341–352. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Johnson, I.; Mueller, K.; Wilkie, S.; Hanzic, L.; Bond, P.L.; O’Moore, L.; Yuan, Z.; Jiang, G. corrosion mitigation by nitrite spray on corroded concrete in a real sewer system. Sci. Total Environ. 2022, 806, 151328. [Google Scholar] [CrossRef] [PubMed]
- Gaylarde, C.C.; Ortega-Morales, B.O. Biodeterioration and Chemical corrosion of Concrete in the Marine Environment: Too Complex for Prediction. Microorganisms 2023, 11, 2438. [Google Scholar] [CrossRef]
- Kip, N.; van Veen, J.A. The dual role of microbes in corrosion. ISME J. 2014, 9, 542–551. [Google Scholar] [CrossRef]
- Zhou, J.; Yin, S.; Fu, Q.; Wang, Q.; Huang, Q.; Wang, J. Microbial-induced concrete corrosion under high-salt conditions: Microbial community composition and environmental multivariate association analysis. Int. Biodeterior. Biodegrad. 2021, 164, 105287. [Google Scholar] [CrossRef]
- Xie, Y.; Lin, X.; Ji, T.; Liang, Y.; Pan, W. Comparison of corrosion resistance mechanism between ordinary Portland concrete and alkali-activated concrete subjected to biogenic sulfuric acid attack. Constr. Build. Mater. 2019, 228, 117071. [Google Scholar] [CrossRef]
- Windt, L.D.; Devillers, P. Modeling the degradation of Portland cement pastes by biogenic organic acids. Cem. Concr. Res. 2010, 40, 1165–1174. [Google Scholar] [CrossRef]
- Wingender, J.; Neu, T.R.; Flemming, H.-C. What are Bacterial Extracellular Polymeric Substances? Springer: Berlin/Heidelberg, Germany, 1999; pp. 1–19. [Google Scholar] [CrossRef]
- Zhu, L.; Zhou, J.; Lv, M.; Yu, H.; Zhao, H.; Xu, X. Specific component comparison of extracellular polymeric substances (EPS) in flocs and granular sludge using EEM and SDS-PAGE. Chemosphere 2015, 121, 26–32. [Google Scholar] [CrossRef]
- Zhang, X.; Bishop, P.L. Spatial Distribution of Extracellular Polymeric Substances in Biofilms. J. Environ. Eng. 2001, 127, 850–856. [Google Scholar] [CrossRef]
- Dreszer, C.; Wexler, A.D.; Drusová, S.; Overdijk, T.; Zwijnenburg, A.; Flemming, H.-C.; Kruithof, J.C.; Vrouwenvelder, J.S. In-situ biofilm characterization in membrane systems using Optical Coherence Tomography: Formation, structure, detachment and impact of flux change. Water Res. 2014, 67, 243–254. [Google Scholar] [CrossRef] [PubMed]
- West, S.; Wagner, M.; Engelke, C.; Horn, H. Optical coherence tomography for the in situ three-dimensional visualization and quantification of feed spacer channel fouling in reverse osmosis membrane modules. J. Membr. Sci. 2016, 498, 345–352. [Google Scholar] [CrossRef]
- VandeWalle, J.L.; Goetz, G.W.; Huse, S.M.; Morrison, H.G.; Sogin, M.L.; Hoffmann, R.G.; Yan, K.; McLellan, S.L. Acinetobacter, Aeromonas and Trichococcus populations dominate the microbial community within urban sewer infrastructure. Environ. Microbiol. 2012, 14, 2538–2552. [Google Scholar] [CrossRef] [PubMed]
- Ennazii, A.-E.; Beaudoin, A.; Fatu, A.; Doumalin, P.; Bouyer, J.; Jolly, P.; Henry, Y.; Laçaj, E.; Couderc, B. Pore-scale numerical analysis of fluid flows in compressed polyurethane foams with a workflow of open-cell foams modeling. J. Fluids Struct. 2024, 125, 104065. [Google Scholar] [CrossRef]
- Chrostek, E.; Peralta, S.; Fiani, N. Morphological study of pulp cavity anatomy of canine teeth in domestic cats using micro-computed tomography. Front. Vet. Sci. 2024, 11, 1373517. [Google Scholar] [CrossRef]
- Li, Z.; Lin, L.; Liu, X.; Wan, C.; Lee, D.-J. Understanding the role of extracellular polymeric substances in the rheological properties of aerobic granular sludge. Sci. Total Environ. 2020, 705, 135948. [Google Scholar] [CrossRef]
- Sand, W.; Gehrke, T. Extracellular polymeric substances mediate bioleaching/bio corrosion via interfacial processes involving iron(III) ions and acidophilic bacteria. Res. Microbiol. 2006, 157, 49–56. [Google Scholar] [CrossRef]
- JGJ55; Standard for Specification for Mix Proportion Design of Ordinary Concrete Construction Engineering Industry Standard of the P.R. China. National Standards of the People’s Republic of China: Beijing, China, 2011. (In Chinese)
- Balemans, S.; Vlaeminck, S.E.; Torfs, E.; Hartog, L.; Zaharova, L.; Rehman, U.; Nopens, I. The Impact of Local Hydrodynamics on High-Rate Activated Sludge Flocculation in Laboratory and Full-Scale Reactors. Processes 2020, 8, 131. [Google Scholar] [CrossRef]
- Wang, J.; Yin, S.; Lu, L.; Zhou, J.; Fu, Q. Characterization of microbial-induced concrete corrosion by combining morphology observation and fluorescence staining. Case Stud. Constr. Mater. 2022, 17, e1586. [Google Scholar] [CrossRef]
- Pan, X.; Liu, J.; Zhang, D. Binding of phenanthrene to extracellular polymeric substances (EPS) from aerobic activated sludge: A fluorescence study. Colloids Surf. B Biointerfaces 2010, 80, 103–106. [Google Scholar] [CrossRef]
- Chen, M.Y.; Lee, D.J.; Tay, J.H. Distribution of extracellular polymeric substances in aerobic granules. Appl. Microbiol. Biotechnol. 2007, 73, 1463–1469. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Wang, T.; Wu, K.; Kan, L. Microbiologically induced corrosion of concrete in sewer structures: A review of the mechanisms and phenomena. Constr. Build. Mater. 2020, 239, 117813. [Google Scholar] [CrossRef]
- Liu, Z.; Gan, L.; Rong, H. Durability of concrete exposed to laboratory-simulated marine microbe-induced corrosion. Constr. Build. Mater. 2023, 400, 132563. [Google Scholar] [CrossRef]
- Van den Brand, T.P.H.; Roest, K.; Brdjanovic, D.; Chen, G.H.; van Loosdrecht, M.C.M. Temperature effect on acetate and propionate consumption by sulfate-reducing bacteria in saline wastewater. Appl. Microbiol. Biotechnol. 2014, 98, 4245–4255. [Google Scholar] [CrossRef]
- Brisolara, K.F.; Qi, Y. Biosolids and Sludge Management. Water Environ. Res. 2013, 85, 1283–1297. [Google Scholar] [CrossRef]
- Gutierrez, O.; Park, D.; Sharma, K.R.; Yuan, Z. Effects of long-term pH elevation on the sulfate-reducing and methanogenic activities of anaerobic sewer biofilms. Water Res. 2009, 43, 2549–2557. [Google Scholar] [CrossRef]
- Hao, T.; Xiang, P.; Mackey, H.R.; Chi, K.; Lu, H.; Chui, H.; van Loosdrecht, M.C.M.; Chen, G.-H. A review of biological sulfate conversions in wastewater treatment. Water Res. 2014, 65, 1–21. [Google Scholar] [CrossRef]
- Diao, C.; Ye, W.; Yan, J.; Hao, T.; Huang, L.; Chen, Y.; Long, J.; Xiao, T.; Zhang, H. Application of microbial sulfate-reduction process for sulfate-laden wastewater treatment: A review. J. Water Process Eng. 2023, 52, 103537. [Google Scholar] [CrossRef]
- Kong, L.; Zhao, W.; Xuan, D.; Wang, X.; Liu, Y. Application potential of alkali-activated concrete for antimicrobial induced corrosion: A review. Constr. Build. Mater. 2022, 317, 126169. [Google Scholar] [CrossRef]
- Rong, H.; Zhang, S.; Ma, G.; Zheng, X.; Qian, C.; Zhang, L.; Zhang, Y.; Xu, R. Formation, growth and corrosion effect of sulfur oxidizing bacteria biofilm on mortar. Constr. Build. Mater. 2021, 268, 121218. [Google Scholar] [CrossRef]
- Annuk, H.; Moran, A.P. Microbial biofilm-related polysaccharides in biofouling and corrosion. In Microbial Glycobiology; Elsevier: Amsterdam, The Netherlands, 2010; pp. 781–801. [Google Scholar] [CrossRef]
- Meng, Y.; Yin, C.; Zhou, Z.; Meng, F. Increased salinity triggers significant changes in the functional proteins of ANAMMOX bacteria within a biofilm community. Chemosphere 2018, 207, 655–664. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Yang, L.; Hu, Z.; Chen, Y.; Mei, N.; Yao, H. Critical role of extracellular DNA in the establishment and maintenance of anammox biofilms. Sci. Total Environ. 2023, 869, 161897. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Luo, J.; Wang, Y.; Dong, J.; Tian, Y. Biofouling mitigation by D-tyrosine in membrane bioreactor: Short-term performance. J. Environ. Chem. Eng. 2023, 11, 109554. [Google Scholar] [CrossRef]
- Kolodkin-Gal, I.; Romero, D.; Cao, S.; Clardy, J.; Kolter, R.; Losick, R. d-Amino Acids Trigger Biofilm Disassembly. Science 2010, 328, 627–629. [Google Scholar] [CrossRef]
- Su, X.; Cheng, X.; Wang, Y.; Luo, J. Effect of different D-amino acids on biofilm formation of mixed microorganisms. Water Sci. Technol. 2021, 85, 116–124. [Google Scholar] [CrossRef]
- Li, H.; Wang, Y.; Zhao, X.; Yan, Z.; Song, C.; Wang, S. Chirality of tyrosine controls biofilm formation via the regulation of bacterial adhesion. Biochem. Eng. J. 2023, 192, 108844. [Google Scholar] [CrossRef]
- Li, J.; Ye, W.; Wei, D.; Ngo, H.H.; Guo, W.; Qiao, Y.; Xu, W.; Du, B.; Wei, Q. System performance and microbial community succession in a partial nitrification biofilm reactor in response to salinity stress. Bioresour. Technol. 2018, 270, 512–518. [Google Scholar] [CrossRef]
- Kaplan, J.B.; Ragunath, C.; Ramasubbu, N.; Fine, D.H. Detachment of Actinobacillus actinomycetemcomitans Biofilm Cells by an Endogenous β-Hexosaminidase Activity. J. Bacteriol. 2003, 185, 4693–4698. [Google Scholar] [CrossRef]
- Ahimou, F.; Semmens, M.J.; Haugstad, G.; Novak, P.J. Effect of Protein, Polysaccharide, and Oxygen Concentration Profiles on Biofilm Cohesiveness. Appl. Environ. Microbiol. 2007, 73, 2905–2910. [Google Scholar] [CrossRef]
- Hartmann, R.; Singh, P.K.; Pearce, P.; Mok, R.; Song, B.; Díaz-Pascual, F.; Dunkel, J.; Drescher, K. Emergence of three-dimensional order and structure in growing biofilms. Nat. Phys. 2018, 15, 251–256. [Google Scholar] [CrossRef]
- Boyd, A.; Chakrabarty, A.M. Role of alginate lyase in cell detachment of Pseudomonas aeruginosa. Appl. Environ. Microbiol. 1994, 60, 2355–2359. [Google Scholar] [CrossRef] [PubMed]
- Flemming, H.C.; Wingender, J.; Moritz, R.; Mayer, C. The forces that keep biofilms together. Dechema Monogr. 1996, 311–316. [Google Scholar]
- Jones, J.M.; Grinberg, I.; Eldar, A.; Grossman, A.D. A mobile genetic element increases bacterial host fitness by manipulating development. Elife 2021, 10, e65924. [Google Scholar] [CrossRef] [PubMed]
- Ji, B.; Zhang, H.; Zhou, L.; Yang, J.; Zhang, K.; Yuan, X.; Ma, J.; Qian, Y. Effect of the rapid increase of salinity on anoxic-oxic biofilm reactor for treatment of high-salt and high-ammonia–nitrogen wastewater. Bioresour. Technol. 2021, 337, 125363. [Google Scholar] [CrossRef]
- Saavedra, A.; Martínez-Casillas, D.C.; Collet-Lacoste, J.R.; Cortón, E. Nondestructive, reagent-free, low-volume fluidic set-up to study biofilms by using a transparent electrode, allowing simultaneous electrochemical and optical measurements. J. Appl. Microbiol. 2023, 134, lxad140. [Google Scholar] [CrossRef]
- Araújo, G.R.d.S.; Viana, N.B.; Gómez, F.; Pontes, B.; Frases, S. The mechanical properties of microbial surfaces and biofilms. Cell Surf. 2019, 5, 100028. [Google Scholar] [CrossRef]
- Shen, Y.; Zhang, H.; Xu, X.; Lin, X. Biofilm formation and lipid accumulation of attached culture of Botryococcus braunii. Bioprocess Biosyst. Eng. 2014, 38, 481–488. [Google Scholar] [CrossRef]
- Dubois-Brissonnet, F. Characterization of Bacterial Membrane Fatty Acid Profiles for Biofilm Cells. In Foodborne Bacterial Pathogens: Methods and Protocols; Springer: New York, NY, USA, 2018; pp. 165–170. [Google Scholar] [CrossRef]
- Dubois-Brissonnet, F.; Trotier, E.; Briandet, R. The Biofilm Lifestyle Involves an Increase in Bacterial Membrane Saturated Fatty Acids. Front. Microbiol. 2016, 7, 01673. [Google Scholar] [CrossRef]





| Name of Fluorescein | Excitation Wavelength (nm) | Emission Wavelength (nm) |
|---|---|---|
| Calcofluor White | 385, 395, 405 | 437, 440, 445 |
| FITC | 490, 494 | 520, 525 |
| Nile Red | 515, 555, 559 | 590, 640 |
| Rhodamine | 550 | 573 |
| Propidium Iodide (PI) | (305), 536, 538 | 617 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, M.; Yu, G.; Xu, Z.; Hu, J.; Ji, Z.; Yang, Z.; Sun, Y.; Zhen, Y.; Zhou, J. Reimagining Microbially Induced Concrete Deterioration: A Novel Approach Through Coupled Confocal Laser Scanning Microscope–Avizo Three-Dimensional Modeling of Biofilms. Microorganisms 2025, 13, 1452. https://doi.org/10.3390/microorganisms13071452
Ma M, Yu G, Xu Z, Hu J, Ji Z, Yang Z, Sun Y, Zhen Y, Zhou J. Reimagining Microbially Induced Concrete Deterioration: A Novel Approach Through Coupled Confocal Laser Scanning Microscope–Avizo Three-Dimensional Modeling of Biofilms. Microorganisms. 2025; 13(7):1452. https://doi.org/10.3390/microorganisms13071452
Chicago/Turabian StyleMa, Mingyue, Guangda Yu, Zhen Xu, Jun Hu, Ziyuan Ji, Zihan Yang, Yumeng Sun, Yeqian Zhen, and Jingya Zhou. 2025. "Reimagining Microbially Induced Concrete Deterioration: A Novel Approach Through Coupled Confocal Laser Scanning Microscope–Avizo Three-Dimensional Modeling of Biofilms" Microorganisms 13, no. 7: 1452. https://doi.org/10.3390/microorganisms13071452
APA StyleMa, M., Yu, G., Xu, Z., Hu, J., Ji, Z., Yang, Z., Sun, Y., Zhen, Y., & Zhou, J. (2025). Reimagining Microbially Induced Concrete Deterioration: A Novel Approach Through Coupled Confocal Laser Scanning Microscope–Avizo Three-Dimensional Modeling of Biofilms. Microorganisms, 13(7), 1452. https://doi.org/10.3390/microorganisms13071452







