Propagule-Type Specificity in Arbuscular Mycorrhizal Fungal Communities in Early Growth of Allium tuberosum
Abstract
1. Introduction
2. Materials and Methods
2.1. Soil Sources for AMF Inocula
2.2. Soil Preparation and Experimental Setup
2.3. Seed Sterilization, Germination, and Plant Cultivation
2.4. DNA Extraction from Root Samples
2.5. DNA Extraction from Hyphae and Spore Fractions
2.6. PCR Amplification and Sequencing
2.7. Bioinformatics and Statistical Analysis
3. Results
3.1. Overall AMF Taxonomic Diversity
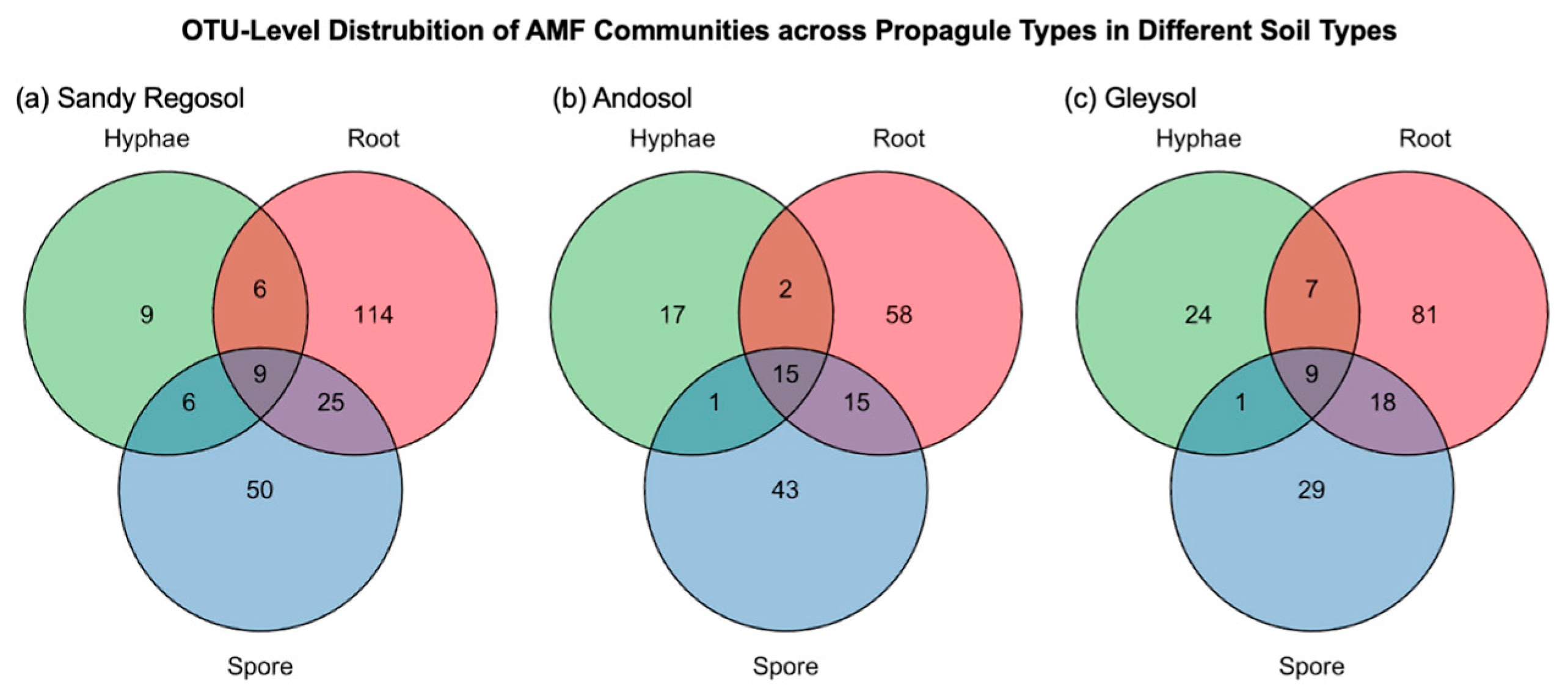
3.2. AMF Diversity Patterns by Propagule Type
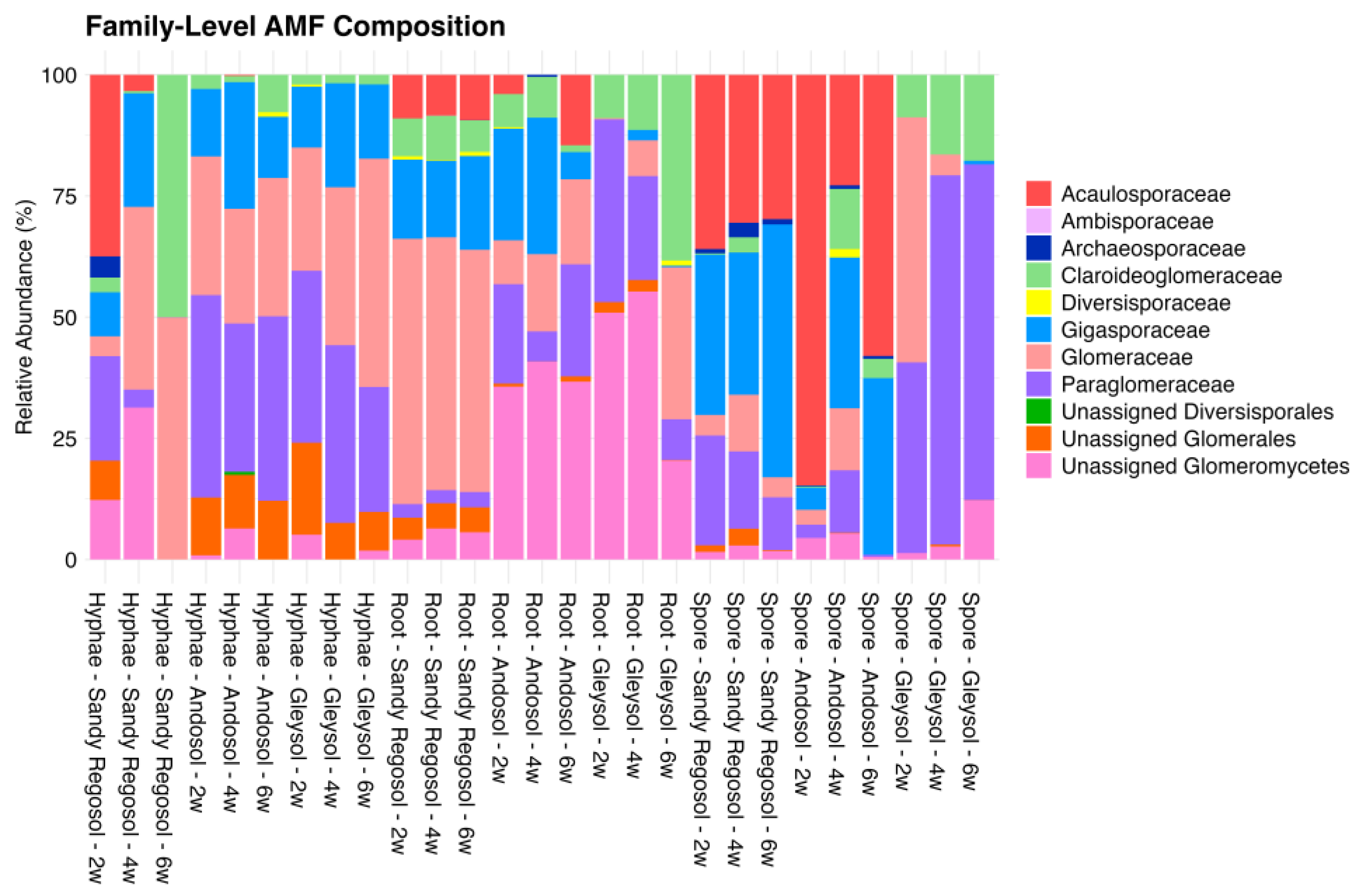
3.3. Dominant Taxa Show Distinct Propagule Preferences
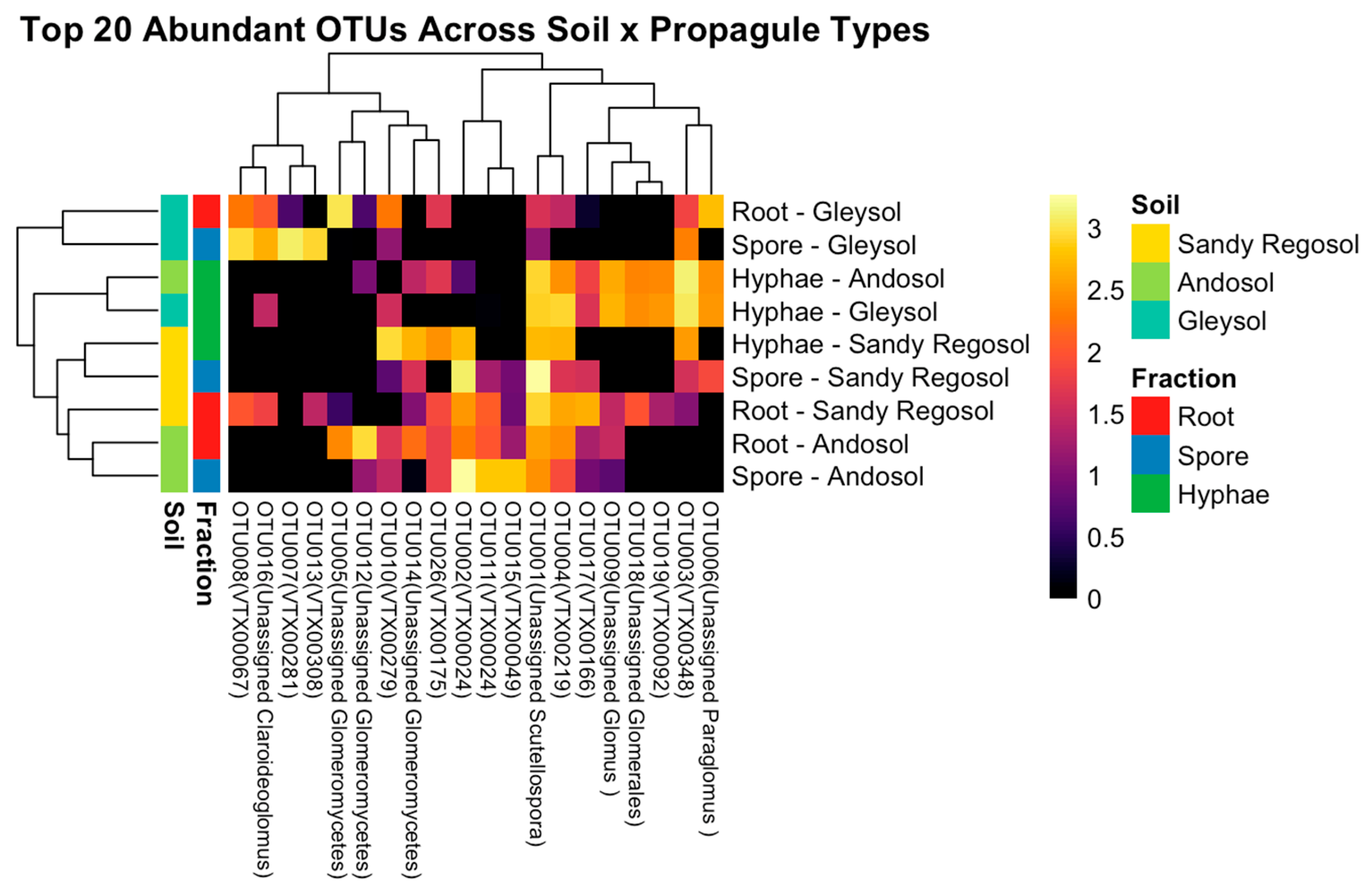
3.4. Propagule Preferences of Dominant AMF OTUs
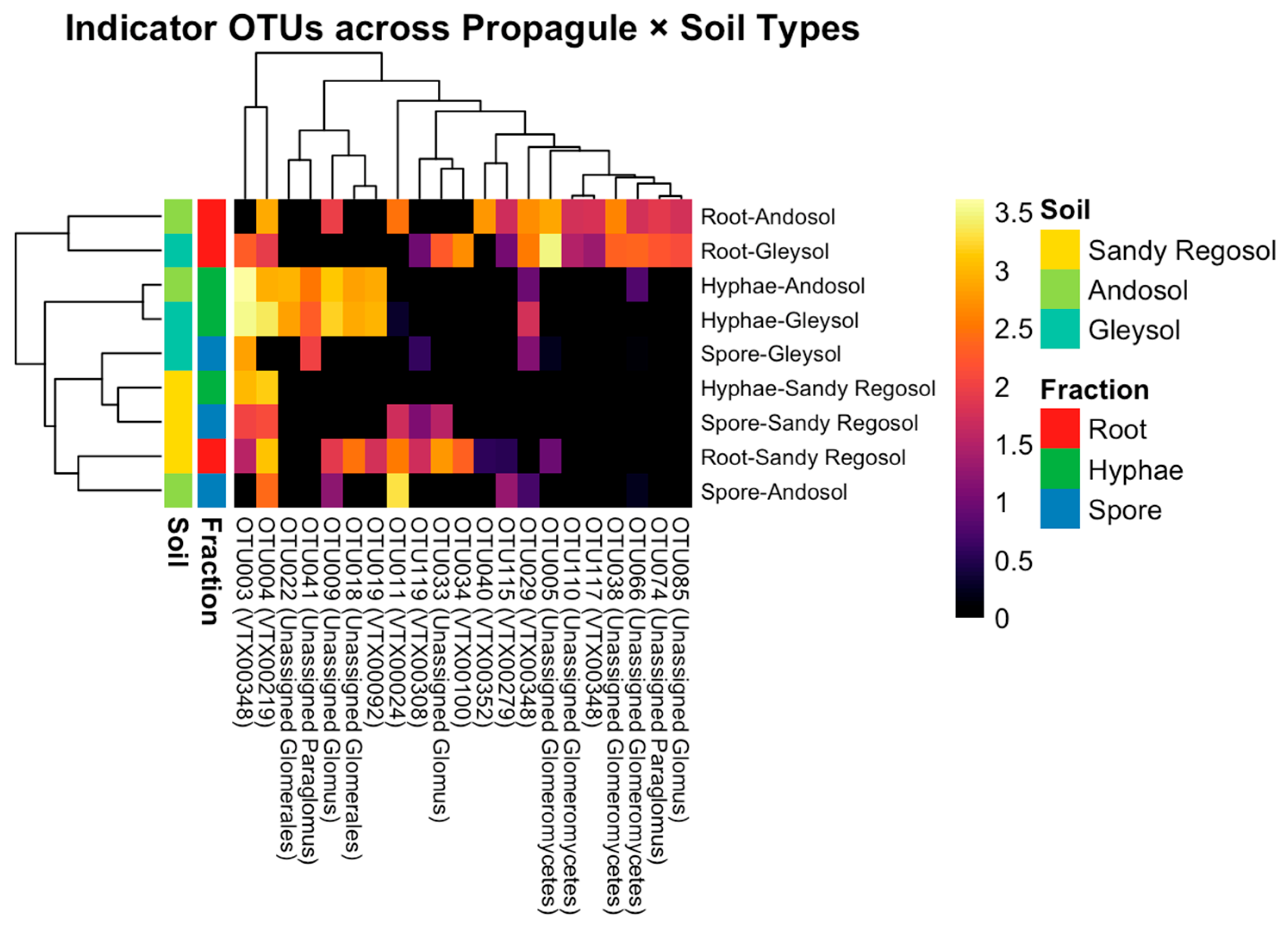
3.5. Indicator Taxa Differentiate Propagule Fractions
4. Discussion
4.1. High AMF Taxonomic Diversity and Propagule-Specific Functional Specialization Characterize Community Assembly
4.2. Inoculum Origin Effect on AMF Community Composition
4.3. Propagule Preferences Exemplify Functional Specialization and Survival Strategies Among AMF Taxa
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AMF | Arbuscular Mycorrhizal Fungi |
| OTU | Operational Taxonomic Unit |
| DNA | Deoxyribonucleic Acid |
| PCR | Polymerase Chain Reaction |
| rRNA | Ribosomal Ribonucleic Acid |
| PCoA | Principal Coordinates Analysis |
References
- Smith, S.E.; Read, D.J. Mycorrhizal Symbiosis, 3rd ed.; Academic Press: London, UK, 2008. [Google Scholar]
- Wang, W.; Zhong, Z.; Wang, Q.; Wang, H.; Fu, Y.; He, X. Glomalin Contributed More to Carbon, Nutrients in Deeper Soils, and Differently Associated with Climates and Soil Properties in Vertical Profiles. Sci. Rep. 2017, 7, 13003. [Google Scholar] [CrossRef] [PubMed]
- Delaeter, M.; Magnin-Robert, M.; Randoux, B.; Lounès-Hadj Sahraoui, A. Arbuscular Mycorrhizal Fungi as Biostimulant and Biocontrol Agents: A Review. Microorganisms 2024, 12, 1281. [Google Scholar] [CrossRef]
- Jia, Y.; van der Heijden, M.G.A.; Wagg, C.; Feng, G.; Walder, F. Symbiotic Soil Fungi Enhance Resistance and Resilience Of An Experimental Grassland to Drought and Nitrogen Deposition. J. Ecol. 2021, 109, 3171–3181. [Google Scholar] [CrossRef]
- Bedini, S.; Pellegrino, E.; Avio, L.; Pellegrini, S.; Bazzoffi, P.; Argese, E.; Giovannetti, M. Changes in Soil Aggregation and Glomalin-Related Soil Protein Content as Affected by The Arbuscular Mycorrhizal Fungal Species Glomus mosseae and Glomus intraradices. Soil Biol. Biochem. 2009, 41, 1491–1496. [Google Scholar] [CrossRef]
- Lutgen, E.R.; Rillig, M.C. Influence of Spotted Knapweed (Centaurea maculosa) Management Treatments on Arbuscular Mycorrhizae and Soil Aggregation. Weed Sci. 2004, 52, 172–177. [Google Scholar] [CrossRef]
- Singh, P.K.; Singh, M.K.; Tripathi, B.N. Glomalin: An Arbuscular Mycorrhizal Fungal Soil Protein. Protoplasma 2012, 250, 663–669. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.G.; Bi, Y.L.; Jiang, B.; Zhakypbek, Y.; Peng, S.P.; Liu, W.W.; Liu, H. Arbuscular Mycorrhizal Fungi Enhance Soil Carbon Sequestration in the Coalfields, Northwest China. Sci. Rep. 2016, 6, 34336. [Google Scholar] [CrossRef]
- McGonigle, T.P.; Miller, M.H.; Evans, D.G.; Fairchild, G.L.; Swan, J.A. A New Method Which Gives An Objective Measure of Colonization of Roots by Vesicular-Arbuscular Mycorrhizal Fungi. New Phytol. 1990, 115, 495–501. [Google Scholar] [CrossRef]
- Silva, I.R.D.; Silva, D.K.A.D.; Souza, F.A.D.; Oehl, F.; Maia, L.C. Changes in Arbuscular Mycorrhizal Fungal Communities Along A River Delta Island in Northeastern Brazil. Acta Oecol. 2017, 79, 8–17. [Google Scholar] [CrossRef]
- Iffis, B.; St-Arnaud, M.; Hijri, M. Bacteria Associated with Arbuscular Mycorrhizal Fungi Within Roots of Plants Growing in A Soil Highly Contaminated with Aliphatic and Aromatic Petroleum Hydrocarbons. FEMS Microbiol. Lett. 2014, 358, 44–54. [Google Scholar] [CrossRef]
- Öpik, M.; Moora, M.; Liira, J.; Kõljalg, U.; Zobel, M.; Sen, R. Divergent Arbuscular Mycorrhizal Fungal Communities Colonize Roots of Pulsatilla spp. in Boreal Scots Pine Forest and Grassland Soils. New Phytol. 2003, 160, 581–593. [Google Scholar] [CrossRef] [PubMed]
- Öpik, M.; Vanatoa, A.; Vanatoa, E.; Moora, M.; Davison, J.; Kalwij, J.M.; Reier, Ü.; Zobel, M. The online database MaarjAM reveals global and ecosystemic distribution patterns in arbuscular mycorrhizal fungi (Glomeromycota). New Phytologist 2010, 188, 223–241. [Google Scholar] [CrossRef] [PubMed]
- Varela-Cervero, S.; Vasar, M.; Davison, J.; Barea, J.M.; Öpik, M.; Azcón-Aguilar, C. The Composition of Arbuscular Mycorrhizal Fungal Communities Differs Among the Roots, Spores and Extraradical Mycelia Associated with Five Mediterranean Plant Species. Environ. Microbiol. 2015, 17, 2882–2895. [Google Scholar] [CrossRef]
- Varela-Cervero, S.; López-García, A.; Barea, J.M.; Azcón-Aguilar, C. Spring to Autumn Changes in The Arbuscular Mycorrhizal Fungal Community Composition in The Different Propagule Types Associated to A Mediterranean Shrubland. Plant Soil 2016, 408, 107–120. [Google Scholar] [CrossRef]
- Klironomos, J.N.; Hart, M.M. Colonization of Roots by Arbuscular Mycorrhizal Fungi Using Different Sources of Inoculum. Mycorrhiza 2002, 12, 181–184. [Google Scholar] [CrossRef] [PubMed]
- Santos-González, J.C.; Finlay, R.D.; Tehler, A. Seasonal Dynamics of Arbuscular Mycorrhizal Fungal Communities in Roots in A Seminatural Grassland. Appl. Environ. Microbiol. 2007, 73, 5613–5623. [Google Scholar] [CrossRef]
- Sweeney, C.J.; de Vries, F.T.; van Dongen, B.E.; Bardgett, R.D. Root Traits Explain Rhizosphere Fungal Community Composition Among Temperate Grassland Plant Species. New Phytol. 2021, 229, 1492–1507. [Google Scholar] [CrossRef]
- Scheublin, T.R.; Sanders, I.R.; Keel, C.; van der Meer, J.R. Characterisation Of Microbial Communities Colonising The Hyphal Surfaces Of Arbuscular Mycorrhizal Fungi. ISME J. 2010, 4, 752–763. [Google Scholar] [CrossRef]
- Zhang, L.; Shi, N.; Fan, J.; Wang, F.; George, T.; Feng, G. Arbuscular Mycorrhizal Fungi Stimulate Organic Phosphate Mobilization Associated with Changing Bacterial Community Structure Under Field Conditions. Environ. Microbiol. 2018, 20, 2639–2651. [Google Scholar] [CrossRef]
- Mafa-Attoye, T.; Borden, K.A.; Obregón, D.; Thevathasan, N.; Isaac, M.E.; Dunfield, K.E. Roots Alter Soil Microbial Diversity and Interkingdom Interactions in Diversified Agricultural Landscapes. Oikos 2023, 2023. [Google Scholar] [CrossRef]
- Emery, S.M.; Reid, M.L.; Bell-Dereske, L.; Gross, K.L. Soil Mycorrhizal and Nematode Diversity Vary in Response to Bioenergy Crop Identity and Fertilization. GCB Bioenergy 2017, 9, 1644–1656. [Google Scholar] [CrossRef]
- Wurst, S.; Gebhardt, K.; Rillig, M.C. Independent Effects of Arbuscular Mycorrhiza and Earthworms on Plant Diversity and Newcomer Plant Establishment. J. Veg. Sci. 2011, 22, 1021–1030. [Google Scholar] [CrossRef]
- Lekberg, Y.; Koide, R.T.; Rohr, J.R.; Aldrich-Wolfe, L.; Morton, J.B. Role of Niche Restrictions and Dispersal in The Composition of Arbuscular Mycorrhizal Fungal Communities. J. Ecol. 2006, 95, 95–105. [Google Scholar] [CrossRef]
- Xu, X.; Chen, C.; Zhang, Z.; Sun, Z.; Chen, Y.; Jiang, J.; Shen, Z. The Influence of Environmental Factors on Communities of Arbuscular Mycorrhizal Fungi Associated with Chenopodium Ambrosioides Revealed by Miseq Sequencing Investigation. Sci. Rep. 2017, 7, 45134. [Google Scholar] [CrossRef]
- Zhang, M.; Shi, Z.; Xu, X.; Wang, X. Arbuscular Mycorrhizal Fungi Associated with Roots Reveal High Diversity Levels at Different Elevations in Tropical Montane Rainforests. Diversity 2022, 14, 587. [Google Scholar] [CrossRef]
- Balestrini, R.; Magurno, F.; Walker, C.; Lumini, E.; Bianciotto, V. Cohorts of Arbuscular Mycorrhizal Fungi (AMF) in Vitis Vinifera, A Typical Mediterranean Fruit Crop. Environ. Microbiol. Rep. 2010, 2, 594–604. [Google Scholar] [CrossRef] [PubMed]
- Alguacil, M.D.M.; Torres, P.; Montesinos-Navarro, A.; Roldán, A. Soil Characteristics Driving Arbuscular Mycorrhizal Fungal Communities in Semiarid Mediterranean Soils. Appl. Environ. Microbiol. 2016, 82, 3348–3356. [Google Scholar] [CrossRef] [PubMed]
- Greenwood, D.J.; Gerwitz, A.; Stone, D.A.; Barnes, A. Root Development of Vegetable Crops. Plant Soil 1982, 68, 75–96. [Google Scholar] [CrossRef]
- Eo, J.K.; Eom, A.H. Differential growth response of various crop species to arbuscular mycorrhizal inoculation. Mycobiology 2009, 37, 72–76. [Google Scholar] [CrossRef]
- Sato, K.; Suyama, Y.; Saito, M.; Sugawara, K. A New Primer for Discrimination of Arbuscular Mycorrhizal Fungi with Polymerase Chain Reaction-Denature Gradient Gel Electrophoresis. Grassl. Sci. 2005, 51, 179–181. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, Interactive, Scalable and Extensible Microbiome Data Science Using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.; Holmes, S.P. DADA2: High-resolution Sample Inference from Illumina Amplicon Data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [PubMed]
- De Cáceres, M.; Legendre, P. Associations Between Species and Groups of Sites: Indices and Statistical Inference. Ecology 2009, 90, 3566–3574. [Google Scholar] [CrossRef] [PubMed]
- Öpik, M.; Metsis, M.; Daniell, T.J.; Zobel, M.; Moora, M. Large-scale Parallel 454 Sequencing Reveals Host Ecological Group Specificity of Arbuscular Mycorrhizal Fungi in A Boreonemoral Forest. New Phytol. 2009, 184, 424–437. [Google Scholar] [CrossRef] [PubMed]
- Bonfante, P.; Genre, A. Mechanisms Underlying Beneficial Plant–Fungus Interactions in Mycorrhizal Symbiosis. Nat. Commun. 2010, 1, 48. [Google Scholar] [CrossRef]
- Bruns, T.D.; Corradi, N.; Redecker, D.; Taylor, J.W.; Öpik, M. Glomeromycotina: What Is A Species and Why Should We Care? New Phytol. 2018, 220, 963–967. [Google Scholar] [CrossRef]
- Hart, M.M.; Reader, R.J. Taxonomic Basis for Variation in The Colonization Strategy of Arbuscular Mycorrhizal Fungi. New Phytol. 2002, 155, 361–370. [Google Scholar] [CrossRef]
- Akiyama, K.; Matsuzaki, K.; Hayashi, H. Plant Sesquiterpenes Induce Hyphal Branching in Arbuscular Mycorrhizal Fungi. Nature 2005, 435, 824–827. [Google Scholar] [CrossRef]
- Besserer, A.; Puech-Pagès, V.; Kiefer, P.; Gomez-Roldan, V.; Jauneau, A.; Roy, S.; Portais, J.C.; Roux, C.; Bécard, G.; Séjalon-Delmas, N. Strigolactones Stimulate Arbuscular Mycorrhizal Fungi by Activating Mitochondria. PLoS Biol. 2006, 4, e226. [Google Scholar] [CrossRef]
- Bergmann, J.; Weigelt, A.; van Der Plas, F.; Laughlin, D.C.; Kuyper, T.W.; Guerrero-Ramirez, N.; Valverde-Barrantes, O.J.; Bruelheide, H.; Freschet, G.T.; Iversen, C.M.; et al. The Fungal Collaboration Gradient Dominates The Root Economics Space in Plants. Sci. Adv. 2020, 6, eaba3756. [Google Scholar] [CrossRef]
- Camenzind, T.; Aguilar-Trigueros, C.A.; Heuck, M.K.; Maerowitz-McMahan, S.; Rillig, M.C.; Cornwell, W.K.; Powell, J.R. Progressing Beyond Colonization Strategies to Understand Arbuscular Mycorrhizal Fungal Life History. New Phytol. 2024, 243, 2217–2230. [Google Scholar] [CrossRef] [PubMed]
- Maherali, H.; Klironomos, J.N. Influence of Phylogeny on Fungal Community Assembly and Ecosystem Functioning. Science 2007, 316, 1746–1748. [Google Scholar] [CrossRef] [PubMed]
- Sýkorová, Z.; Ineichen, K.; Wiemken, A.; Redecker, D. The Cultivation Bias: Different Communities of Arbuscular Mycorrhizal Fungi Detected in Roots from The Field, from Bait Plants Transplanted To The Field, and from A Greenhouse Trap Experiment. Mycorrhiza 2007, 18, 1–14. [Google Scholar] [CrossRef]
- Oehl, F.; Laczko, E.; Bogenrieder, A.; Stahr, K.; Bösch, R.; van der Heijden, M.; Sieverding, E. Soil Type and Land Use Intensity Determine The Composition of Arbuscular Mycorrhizal Fungal Communities. Soil Biol. Biochem. 2010, 42, 724–738. [Google Scholar] [CrossRef]
- IUSS Working Group WRB. World Reference Base for Soil Resources 2014, Update 2015. In World Soil Resources Reports No. 106; Food and Agriculture Organization of the United Nations: Rome, Italy, 2015. [Google Scholar]
- Prado-Tarango, D.E.; Mata-González, R.; Hovland, M. Response of Sagebrush Steppe Grass Species to AMF Inoculum Sources and Biochar. Microorganisms 2023, 11, 1113. [Google Scholar] [CrossRef]
- Hazard, C.; Gosling, P.; Gast, C.; Mitchell, D.; Doohan, F.; Bending, G. The Role of Local Environment and Geographical Distance in Determining Community Composition of Arbuscular Mycorrhizal Fungi at The Landscape Scale. ISME J. 2013, 7, 498–508. [Google Scholar] [CrossRef]
- Barceló, M.; van Bodegom, P.M.; Tedersoo, L.; den Haan, N.; Veen, G.F.; Ostonen, I.; Trimbos, K.; Soudzilovskaia, N.A. The Abundance of Arbuscular Mycorrhiza in Soils Is Linked to The Total Length of Roots Colonized at Ecosystem Level. PLoS ONE 2020, 15, e0237256. [Google Scholar] [CrossRef]
- Lanfranco, L.; Bonfante, P.; Genre, A. The Mutualistic Interaction between Plants and Arbuscular Mycorrhizal Fungi. Microbiol. Spectr. 2016, 4, 10-1128. [Google Scholar] [CrossRef]
- Bonneau, L.; Recorbet, G.; van Tuinen, D.; Wipf, D.; Courty, P.E. Analysis of Common Mycorrhizal Networks in Microcosms. In Methods in Rhizosphere Biology Research; Reinhardt, D., Sharma, A., Eds.; Springer: Singapore, 2019; pp. 171–181. [Google Scholar]
- Tipton, A.G.; Nelsen, D.; Koziol, L.; Duell, E.B.; House, G.; Wilson, G.W.; Schultz, P.A.; Bever, J.D. Arbuscular Mycorrhizal Fungi Taxa Show Variable Patterns of Micro-Scale Dispersal in Prairie Restorations. Front. Microbiol. 2022, 13, 827293. [Google Scholar] [CrossRef]
- Pepe, A.; Giovannetti, M.; Sbrana, C. Lifespan and Functionality of Mycorrhizal Fungal Mycelium Are Uncoupled from Host Plant Lifespan. Sci. Rep. 2018, 8, 28354. [Google Scholar] [CrossRef]
- Behm, J.E.; Kiers, E.T. A Phenotypic Plasticity Framework for Assessing Intraspecific Variation in Arbuscular Mycorrhizal Fungal Traits. J. Ecol. 2014, 102, 315–327. [Google Scholar] [CrossRef]
- Öpik, M.; Moora, M.; Liira, J.; Zobel, M. Composition of Root-Colonizing Arbuscular Mycorrhizal Fungal Communities in Different Ecosystems Around The Globe. J. Ecol. 2006, 94, 778–790. [Google Scholar] [CrossRef]
- Sangwan, S.; Prasanna, R. Mycorrhizae Helper Bacteria: Unlocking Their Potential as Bioenhancers of Plant–Arbuscular Mycorrhizal Fungal Associations. Microb. Ecol. 2022, 84, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Duan, S.; Jin, Z.; Zhang, L.; Declerck, S. Mechanisms of Cooperation in the Plants–Arbuscular Mycorrhizal Fungi–Bacteria Continuum. ISME J. 2025, 19, wraf023. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arslan, I.; Takahashi, K.; Harada, N.; Suzuki, K. Propagule-Type Specificity in Arbuscular Mycorrhizal Fungal Communities in Early Growth of Allium tuberosum. Microorganisms 2025, 13, 1430. https://doi.org/10.3390/microorganisms13061430
Arslan I, Takahashi K, Harada N, Suzuki K. Propagule-Type Specificity in Arbuscular Mycorrhizal Fungal Communities in Early Growth of Allium tuberosum. Microorganisms. 2025; 13(6):1430. https://doi.org/10.3390/microorganisms13061430
Chicago/Turabian StyleArslan, Irem, Kohei Takahashi, Naoki Harada, and Kazuki Suzuki. 2025. "Propagule-Type Specificity in Arbuscular Mycorrhizal Fungal Communities in Early Growth of Allium tuberosum" Microorganisms 13, no. 6: 1430. https://doi.org/10.3390/microorganisms13061430
APA StyleArslan, I., Takahashi, K., Harada, N., & Suzuki, K. (2025). Propagule-Type Specificity in Arbuscular Mycorrhizal Fungal Communities in Early Growth of Allium tuberosum. Microorganisms, 13(6), 1430. https://doi.org/10.3390/microorganisms13061430





