A Review of the Epidemiology of Lassa Fever in Nigeria
Abstract
1. Background
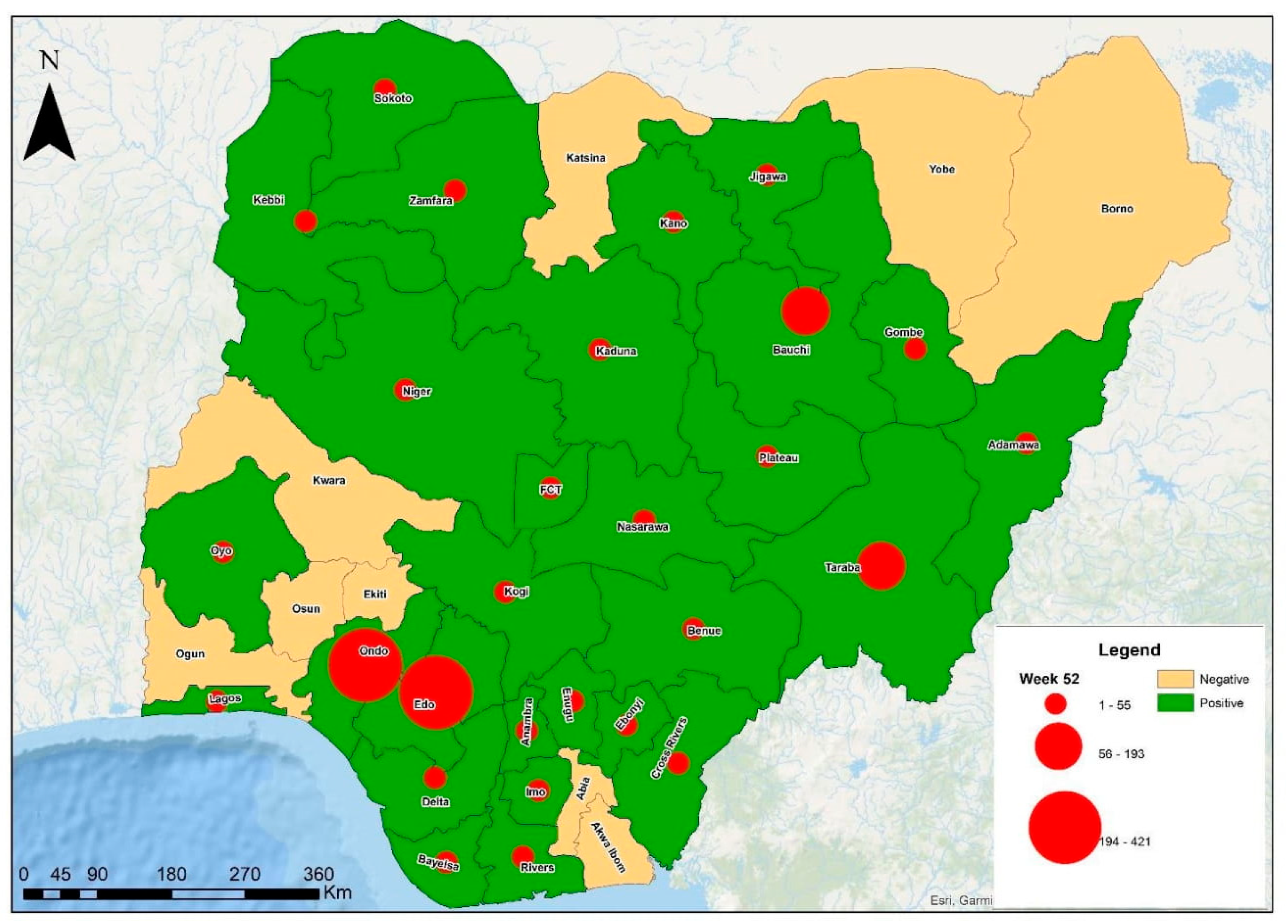
2. Geographical Distribution
3. Lassa Fever Incidence, Cases, and Trends in Nigeria
| Year | Suspected Cases | Confirmed Cases | Calculated Case Positivity (%) | No. of Death | Calculated CFR (%) | States with at Least 1 Confirmed Case |
|---|---|---|---|---|---|---|
| 2018 | 3498 | 633 | 18.1 | 171 | 27.0 | 23 states, 93 LGA |
| 2019 | 5057 | 833 | 16.5 | 174 | 20.9 | 23 states, 86 LGA |
| 2020 | 6791 | 1189 | 17.5 | 244 | 20.5 | 27 states, 131 LGA |
| 2021 | 4654 | 511 | 11.0 | 102 | 20.0 | 17 states, 68 LGA |
| 2022 | 8202 | 1067 | 13.0 | 189 | 17.7 | 27 states, 112 LGA |
| 2023 | 9155 | 1270 | 13.9 | 227 | 17.9 | 28 states, 124 LGA |
| 2024 | 10,098 | 1309 | 13.0 | 214 | 16.3 | 28 states, 139 LGA |
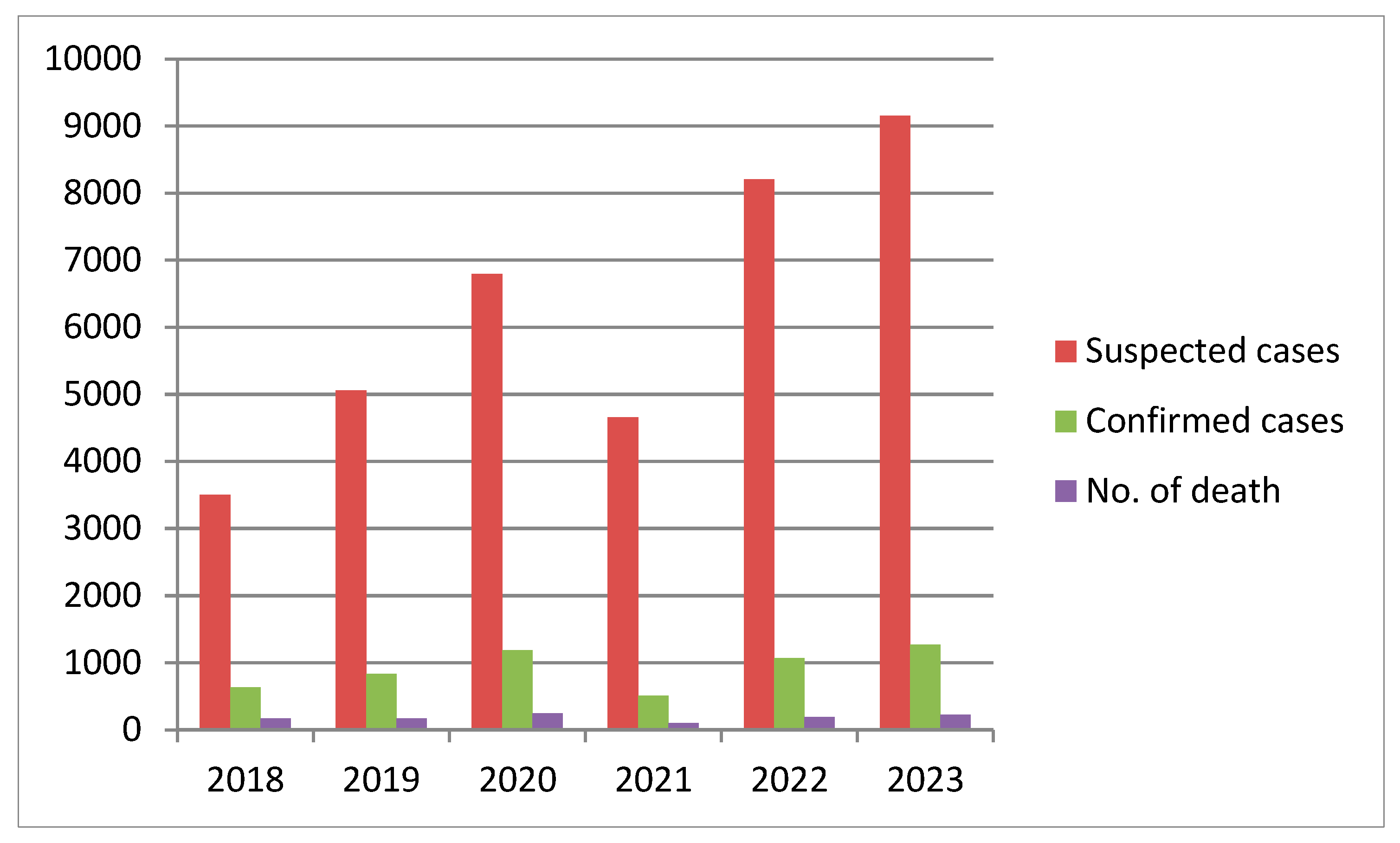

4. Seasonal Variation
5. Risk Factors
6. Surveillance of Lassa Fever in Nigeria
7. Current Control Efforts of Lassa Fever
- i.
- Case Management:
- ii.
- Infection Prevention and Control (IPC):
- iii.
- Public Health Education and Risk Communication:
- iv.
- Environmental and Rodent Control:
- v.
- Research and Development:
8. Response Strategies
- I.
- Policy and Governance:
- II.
- Outbreak Preparedness and Emergency Response
- III.
- Surveillance and Contact Tracing:
- IV.
- Capacity Building:
9. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lukashevich, I.S.; Stelmakh, T.A.; Golubev, V.P.; Stchesljenok, E.P.; Lemeshko, N.N. Ribonucleic acids of Machupo and Lassa viruses. Arch. Virol. 1984, 79, 189–203. [Google Scholar] [CrossRef] [PubMed]
- Klitting, R.; Mehta, S.B.; Oguzie, J.U.; Oluniyi, P.E.; Pauthner, M.G.; Siddle, K.J.; Andersen, K.G.; Happi, C.T.; Sabeti, P.C. Lassa virus genetics. In Lassa Fever: Epidemiology, Immunology, Diagnostics, and Therapeutics; Current Topics in Microbiology and Immunology; Springer: Cham, Switzerland, 2020; pp. 23–65. [Google Scholar] [CrossRef]
- Frame, J.D.; Baldwin, J.J.; Gocke, D.J.; Troup, J.M. Lassa fever, a new virus disease of man from West Africa. I. Clinical description and pathological findings. Am. J. Trop. Med. Hyg. 1970, 19, 670–676. [Google Scholar] [CrossRef] [PubMed]
- Akpede, G.O.; Asogun, D.A.; Okogbenin, S.A.; Okokhere, P.O. Lassa fever outbreaks in Nigeria. Expert Rev. Anti. Infect. Ther. 2018, 16, 663–666. [Google Scholar] [CrossRef] [PubMed]
- Agbonlahor, D.E.; Akpede, G.O.; Happi, C.T.; Tomori, O. 52 Years of Lassa Fever outbreaks in Nigeria, 1969–2020: An epidemiologic analysis of the temporal and spatial trends. Am. J. Trop. Med. Hyg. 2021, 105, 974–985. [Google Scholar] [CrossRef]
- Meulen, J.T.; Lukashevich, I.; Sidibe, K.; Inapogui, A.; Marx, M.; Dorlemann, A.; Yansane, M.L.; Koulemou, K.; Chang-Claude, J.; Schmitz, H. Hunting of Peridomestic Rodents and Consumption of Their Meat as Possible Risk Factors for Rodent-to-Human Transmission of Lassa Virus in the Republic of Guinea. Am. J. Trop. Med. Hyg. 1996, 55, 661–666. [Google Scholar] [CrossRef]
- Morsy, T.A.; El-Bahnasawy, M.M.; Saleh, H.A.A.; Megahed, L.A.-M. Lassa Fever or Lassa Hemorrhagic Fever Risk to Humans from Rodent-Borne Zoonoses. J. Egypt. Soc. Parasitol. 2015, 45, 61–70. [Google Scholar] [CrossRef]
- Fichet-Calvet, E.; Koulémou, K.; Koivogui, L.; Soropogui, B.; Lecompte, E.; Daffis, S.; Kouadio, A.; Kouassi, S.; Denys, C. Spatial Distribution of Commensal Rodents in Regions with High and Low Lassa Fever Prevalence in Guinea. Belg. J. Zool. 2005, 135 (Supplement), 63–67. [Google Scholar]
- Thomas, P.J.; Dadzie, D.; Amedzro, I.; Kenu, E.; Hilary, J. Investigation of Lassa Fever Outbreak in Grand Bassa County, Liberia, 2021. J. Interv. Epidemiol. Public Health 2024, 7, 50. [Google Scholar]
- McCormick, J.B.; Webb, P.A.; Krebs, J.W.; Johnson, K.M.; Smith, E.S. A Prospective Study of the Epidemiology and Ecology of Lassa Fever. J. Infect. Dis. 1987, 155, 437–444. [Google Scholar] [CrossRef]
- Richmond, J.K.; Baglole, D.J. Lassa Fever: Epidemiology, Clinical Features, and Social Consequences. BMJ 2003, 327, 1271–1275. [Google Scholar] [CrossRef]
- Ogbu, O.; Ajuluchukwu, E.; Uneke, C.J. Lassa Fever in West African Sub-Region: An Overview. J. Vector Borne Dis. 2007, 44, 1–11. [Google Scholar]
- Akpede, G.O.; Lawal, W. Part nine: Chapter 61. Viral Haemorrhagic fevers (VHF) with particular focus on Lassa and Ebola. In Paediatrics and Child Health in a Tropical Region, 3rd ed.; Azubuike, J.C., Nkanginieme, K.E.O., Eds.; Educational Printing and Publishing: Lagos, Nigeria, 2016; pp. 625–656. [Google Scholar]
- Sogoba, N.; Feldmann, H.; Safronetz, D. Lassa Fever in West Africa: Evidence for an Expanded Region of Endemicity. Zoonoses Public Health 2012, 59, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Dwalu, E.; Jetoh, R.W.; Shobayo, B.I.; Pewu, I.; Taweh, F.; Wilson-Sesay, H.W.; Akpan, G.E.; Shannon, F.; Joseph, B.O.; Umeokonkwo, C.D.; et al. Trend of Lassa Fever Cases and Factors Associated with Mortality in Liberia, 2016–2021: A Secondary Data Analysis. Pan Afr. Med. J. 2024, 47, 22. [Google Scholar] [CrossRef] [PubMed]
- Fichet-Calvet, E.; Rogers, D.J. Risk Maps of Lassa Fever in West Africa. PLoS Negl. Trop. Dis. 2009, 3, e388. [Google Scholar] [CrossRef]
- Ajala, B.O.; Adejumo, A.O.; Akinyemi, J.A.; Ekum, M.I.; Barrah, V.; Barrah-Ajala, J.U.; Nwanya, J.C. Optimizing Lassa Fever Outbreak Control: A Comparative Study on the Efficacy of Contact Tracing Integration in Epidemiological Models. Res. Sq. 2024. [Google Scholar] [CrossRef]
- Adesola, R.O.; Idris, I.; Bakre, A.A.; Arthur, J.F.; D’Souza, J.N. Challenges Associated with Re-Emergence of Lassa Fever in Nigeria: An Exploratory Study of Epidemiology, Phylogenomics, and Recommendations Toward Its Eradication. Health Sci. Rep. 2024, 7, e70225. [Google Scholar] [CrossRef]
- Moore, S.M.; Rapheal, E.; Guerrero, S.M.; Dean, N.E.; Stoddard, S.T. Estimation of Lassa Fever Incidence Rates in West Africa: Development of a Modeling Framework to Inform Vaccine Trial Design. medRxiv 2024. [Google Scholar] [CrossRef]
- Monath, T.P.; Newhouse, V.F.; Kemp, G.E.; Setzer, H.W.; Cacciapuoti, A. Lassa Virus Isolation from Mastomys Natalensis Rodents During an Epidemic in Sierra Leone. Science 1974, 185, 263–265. [Google Scholar] [CrossRef]
- Okwor, T.J.; Ndu, A.C.; Okeke, T.A.; Aguwa, E.N.; Arinze-Onyia, S.U.; Chinawa, A.; Kassy, W.C.; Ochie, C.N. A review of Lassa fever outbreaks in Nigeria from 1969 to 2017: Epidemiologic profile, determinants and public health response. Niger. J. Med. 2018, 27, 219–233. [Google Scholar] [CrossRef]
- Dalhat, M.M.; Olayinka, A.; Meremikwu, M.M.; Dan-Nwafor, C.; Iniobong, A.; Ntoimo, L.F.; Onoh, I.; Mba, S.; Ohonsi, C.; Arinze, C.; et al. Epidemiological Trends of Lassa Fever in Nigeria, 2018–2021. PLoS ONE 2022, 17, e0279467. [Google Scholar] [CrossRef]
- Sylvester Chibueze, I.; Kurotimipa Frank, O.; Matthew Chidozie, O. Lassa Fever in Nigeria: Social and Ecological Risk Factors Exacerbating Transmission and Sustainable Management Strategies. Int. J. Trop. Dis. 2022, 5, 065. [Google Scholar] [CrossRef]
- Safronetz, D.; Sogoba, N.; Diawara, S.I.; Bane, S.; Rosenke, K.; Maiga, O.; Boisen, M.; Garry, R.F.; Branco, L.M.; Lindsay, L.R.; et al. Annual Incidence of Lassa Virus Infection in Southern Mali. Am. Soc. Trop. Med. Hyg. 2017, 96, 944–946. [Google Scholar] [CrossRef] [PubMed]
- WHO (World Health Organization). Lassa Fever Key Facts. 2025. Available online: https://www.who.int/news-room/fact-sheets/detail/Lassa-fever (accessed on 28 May 2025).
- WHO (World Health Organization). Lassa Fever—Fact Sheet. 2017. Available online: https://www.who.int (accessed on 20 December 2024).
- NCDC (Nigeria Centre for Disease Control). Lassa Fever—Monthly Situation Report. 2023. Available online: https://ncdc.gov.ng/ (accessed on 20 December 2024).
- Ehichioya, D.U.; Dellicour, S.; Pahlmann, M.; Rieger, T.; Oestereich, L.; Becker-Ziaja, B.; Cadar, D.; Ighodalo, Y.; Olokor, T.; Omomoh, E.; et al. Phylogeography of Lassa Virus in Nigeria. J. Virol. 2019, 93, e00929-19. [Google Scholar] [CrossRef] [PubMed]
- Ibukun, F.I. Inter-Lineage Variation of Lassa Virus Glycoprotein Epitopes: A Challenge to Lassa Virus Vaccine Development. Viruses 2020, 12, 386. [Google Scholar] [CrossRef]
- Garry, R.F. Lassa Fever—The Road Ahead. Nat. Rev. Microbiol. 2023, 21, 87–96. [Google Scholar] [CrossRef]
- Al-Mustapha, A.I.; Adesiyan, I.M.; Orum, T.G.; Ogundijo, O.A.; Lawal, A.N.; Nzedibe, O.E.; Onyeka, L.O.; Muhammad, K.U.; Odetayo, L.; Oyewo, M.; et al. Lassa fever in Nigeria: Epidemiology and risk perception. Sci. Rep. 2024, 14, 27669. [Google Scholar] [CrossRef]
- Nigeria Centre for Disease Control and Prevention (NCDC). Lassa Fever Situation Report. Epi Week: 52 2024. Available online: https://www.ncdc.gov.ng/themes/common/files/sitreps/a45a720bd6ddd1e75a5a3e7e6195e412.pdf (accessed on 28 April 2025).
- Nigeria Centre for Disease Control and Prevention (NCDC). Lassa Fever Situation Report. Epi Week: 52 2018. Available online: https://ncdc.gov.ng/themes/common/files/sitreps/733d856bae4b2afa5d29c0465e6c335e.pdf (accessed on 11 December 2024).
- Nigeria Centre for Disease Control and Prevention (NCDC). Lassa Fever Situation Report. Epi Week: 52 2019. Available online: https://ncdc.gov.ng/themes/common/files/sitreps/8c02d1bf6e3e02aa2adfe144dda40db2.pdf (accessed on 11 December 2024).
- Nigeria Centre for Disease Control and Prevention (NCDC). Lassa Fever Situation Report. Epi Week: 53 2020. Available online: https://ncdc.gov.ng/themes/common/files/sitreps/ba1e917c531710377786f683ef4aaec4.pdf (accessed on 11 December 2024).
- Nigeria Centre for Disease Control and Prevention (NCDC). Lassa Fever Situation Report. Epi Week: 52 2021. Available online: https://ncdc.gov.ng/themes/common/files/sitreps/65ad32fe92ed2be30726b44e99204790.pdf (accessed on 11 December 2024).
- Nigeria Centre for Disease Control and Prevention (NCDC). Lassa Fever Situation Report. Epi Week: 52 2022. Available online: https://ncdc.gov.ng/themes/common/files/sitreps/2fd923c3f16ae99e0ddbfa1ced8a0ae9.pdf (accessed on 11 December 2024).
- Nigeria Centre for Disease Control and Prevention (NCDC). Lassa Fever Situation Report. Epi Week: 52 2023. Available online: https://ncdc.gov.ng/themes/common/files/sitreps/60b4a539bd9b9852ac1a5059ec0f3433.pdf (accessed on 11 December 2024).
- Worldometer. Nigeria Population. 2024. Available online: https://www.worldometers.info/world-population/nigeria-population/ (accessed on 11 December 2024).
- Asogun, D.A.; Adomeh, D.I.; Ehimuan, J.; Odia, I.; Hass, M.; Gabriel, M.; Ölschläger, S.; Becker-Ziaja, B.; Folarin, O.; Phelan, E.; et al. Molecular Diagnostics for Lassa Fever at Irrua Specialist Teaching Hospital, Nigeria: Lessons Learnt from Two Years of Laboratory Operation. PLoS Negl. Trop. Dis. 2012, 6, e1839. [Google Scholar] [CrossRef]
- Lecompte, E.; Fichet-Calvet, E.; Daffis, S.; Koulemou, K.; Sylla, O.; Kourouma, F.; Dore, A.; Soropogui, B.; Aniskin, V.; Allali, B.; et al. Mastomys natalensis and Lassa Fever, West Africa. Emerg. Infect. Dis. 2006, 12, 1971–1974. [Google Scholar] [CrossRef]
- Baillet, N.; Reynard, S.; Perthame, E.; Hortion, J.; Journeaux, A.; Mateo, M.; Carnec, X.; Schaeffer, J.; Picard, C.; Barrot, L.; et al. Systemic Viral Spreading and Defective Host Responses Are Associated with Fatal Lassa Fever in Macaques. Commun. Biol. 2021, 4, 27. [Google Scholar] [CrossRef]
- Bonwitt, J.; Sáez, A.M.; Lamin, J.; Ansumana, R.; Dawson, M.; Buanie, J.; Lamin, J.; Sondufu, D.; Borchert, M.; Sahr, F.; et al. At Home with Mastomys and Rattus: Human-Rodent Interactions and Potential for Primary Transmission of Lassa Virus in Domestic Spaces. Am. J. Trop. Med. Hyg. 2017, 96, 935–943. [Google Scholar] [CrossRef]
- Meulen, J.; Lenz, O.; Koivogui, L.; Magassouba, N.; Kaushik, S.K.; Lewis, R.; Aldis, W. Short Communication: Lassa Fever in Sierra Leone: UN Peacekeepers Are at Risk. Trop. Med. Int. Health 2001, 6, 83–84. [Google Scholar] [CrossRef] [PubMed]
- Cadmus, S.; Taiwo, O.J.; Akinseye, V.; Cadmus, E.; Famokun, G.; Fagbemi, S.; Ansumana, R.; Omoluabi, A.; Ayinmode, A.; Oluwayelu, D.; et al. Ecological Correlates and Predictors of Lassa Fever Incidence in Ondo State, Nigeria 2017–2021: An Emerging Urban Trend. Sci. Rep. 2023, 13, 20855. [Google Scholar] [CrossRef] [PubMed]
- Ilesanmi, O.; Oton, E.; Afolabi, A. Community Engagement in the Prevention and Control of Lassa Fever in Africa: A Systematic Review. Infect. Epidemiol. Microbiol. 2021, 7, 187–196. [Google Scholar] [CrossRef]
- Oboratare, O.; Chukwuyem, A.; Emmanuel, O.; Obekpa, A.S. Housing Factors and Transmission of Lassa Fever in a Rural Area of South-South Nigeria. Gen. Health Med. Sci. 2014, 1, 15–20. [Google Scholar]
- Ana, G.R.E.E.; Ulor, A.E.; Faneye, A.; Olawade, D.B. Environmental Conditions and Predictors of Lassa Fever Transmission in a Low Socioeconomic Community in Nigeria. Int. J. Trop. Dis. Health 2021, 42, 25–39. [Google Scholar] [CrossRef]
- Aigbiremolen, A.O.; Lawal-Luka, R.K.; Abejegah, C.; Aigberemwon, J.A.; Abah, E.O.; Abah, S.O. Environmental risk factors in the transmission of Lassa fever in college students hostels in Ekpoma, a semi urban town in south-south Nigeria. Annals 2017, 3, 36–42. [Google Scholar]
- Sama, D.J.; Haider, N.; Guitian, J.; Osman, A.Y.; Ntoumi, F.; Zumla, A.; Kock, R.; Ansumana, R. Identifying risk factors for Lassa fever infection in Sierra Leone, 2019–2021. medRxiv 2024. [Google Scholar] [CrossRef]
- Bonner, P.C.; Schmidt, W.-P.; Belmain, S.R.; Oshin, B.; Baglole, D.; Borchert, M. Poor Housing Quality Increases Risk of Rodent Infestation and Lassa Fever in Refugee Camps of Sierra Leone. Am. J. Trop. Med. Hyg. 2007, 77, 169–175. [Google Scholar] [CrossRef]
- Esposito, M.M.; Turku, S.; Lehrfield, L.; Shoman, A. The Impact of Human Activities on Zoonotic Infection Transmissions. Animals 2023, 13, 1646. [Google Scholar] [CrossRef]
- Abdullahi, I.N.; Anka, A.U.; Ghamba, P.E.; Onukegbe, N.B.; Amadu, D.O.; Salami, M.O. Need for Preventive and Control Measures for Lassa Fever through the One Health Strategic Approach. Proc. Singap. Healthc. 2020, 29, 190–194. [Google Scholar] [CrossRef]
- Adetola, O.O.; Adebisi, M.A. Impacts of Deforestation on the Spread of Mastomys natalensis in Nigeria. World Sci. News 2019, 130, 286–296. [Google Scholar]
- McCormick, J.B. Lassa fever. In Emergence and Control of Rodent-Borne Viral Diseases (Hantaviral and Arenal Diseases); Saluzzo, J.-F., Dodet, B., Eds.; Elsevier: Amsterdam, The Netherlands, 1999; pp. 177–795. [Google Scholar]
- Tobin, E.; Asogun, D.; Akpede, N.; Adomeh, D.; Odia, I.; Gunther, S. Lassa Fever in Nigeria: Insights into Seroprevalence and Risk Factors in Rural Edo State: A Pilot Study. J. Med. Trop. 2015, 17, 51. [Google Scholar] [CrossRef]
- Brown, H.; Kelly, A.H.; Marí Sáez, A.; Fichet-Calvet, E.; Ansumana, R.; Bonwitt, J.; Magassouba, N.; Sahr, F.; Borchert, M. Extending the “Social”: Anthropological Contributions to the Study of Viral Haemorrhagic Fevers. PLoS Negl. Trop. Dis. 2015, 9, e0003651. [Google Scholar] [CrossRef][Green Version]
- Mariën, J.; Kourouma, F.; Magassouba, N.; Leirs, H.; Fichet-Calvet, E. Movement Patterns of Small Rodents in Lassa Fever-Endemic Villages in Guinea. Ecohealth 2018, 15, 348–359. [Google Scholar] [CrossRef]
- Redding, D.W.; Moses, L.M.; Cunningham, A.A.; Wood, J.; Jones, K.E. Environmental-mechanistic Modelling of the Impact of Global Change on Human Zoonotic Disease Emergence: A Case Study of Lassa Fever. Methods Ecol. Evol. 2016, 7, 646–655. [Google Scholar] [CrossRef]
- Kernéis, S.; Koivogui, L.; Magassouba, N.; Koulemou, K.; Lewis, R.; Aplogan, A.; Grais, R.F.; Guerin, P.J.; Fichet-Calvet, E. Prevalence and Risk Factors of Lassa Seropositivity in Inhabitants of the Forest Region of Guinea: A Cross-Sectional Study. PLoS Negl. Trop. Dis. 2009, 3, e548. [Google Scholar] [CrossRef] [PubMed]
- WHO. Lassa Fever Factsheet. 2024. Available online: https://www.afro.who.int/health-topics/Lassa-fever (accessed on 3 April 2025).
- Carey, D.E.; Kemp, G.E.; White, H.A.; Pinneo, L.; Addy, R.F.; Fom, A.L.M.D.; Stroh, G.; Casals, J.; Henderson, B.E. Lassa Fever Epidemiological Aspects of the 1970 Epidemic, Jos, Nigeria. Trans. R. Soc. Trop. Med. Hyg. 1972, 66, 402–408. [Google Scholar] [CrossRef]
- Fisher-Hoch, S.P.; Tomori, O.; Nasidi, A.; Perez-Oronoz, G.I.; Fakile, Y.; Hutwagner, L.; McCormick, J.B. Review of Cases of Nosocomial Lassa Fever in Nigeria: The High Price of Poor Medical Practice. BMJ 1995, 311, 857–859. [Google Scholar] [CrossRef]
- Frame, J.D.; Yalley-Ogunro, J.E.; Hanson, A.P. Endemic Lassa Fever in Liberia. V. Distribution of Lassa Virus Activity in Liberia: Hospital Staff Surveys. Trans. R. Soc. Trop. Med. Hyg. 1984, 78, 761–763. [Google Scholar] [CrossRef]
- Monath, T.P.; Mertens, P.E.; Patton, R.; Moser, C.R.; Baum, J.J.; Pinneo, L.; Gary, G.W.; Kissling, R.E. A Hospital Epidemic of Lassa Fever in Zorzor, Liberia, March–April 1972. Am. J. Trop. Med. Hyg. 1973, 22, 773–779. [Google Scholar] [CrossRef]
- Gobir, A.A.; Ejembi, C.L.; Alhaji, A.A.; Garba, M.B.; Igboanusi, C.J.-C.; Usman, B.; Umar, Z.Z.; Joshua, I.A. Knowledge of Lassa Fever Disease and Its Risk Factors Among Rural People in a Nigerian Community. Proceedings 2020, 45, 9. [Google Scholar] [CrossRef]
- Usuwa, I.S.; Akpa, C.O.; Umeokonkwo, C.D.; Umoke, M.; Oguanuo, C.S.; Olorukooba, A.A.; Bamgboye, E.; Balogun, M.S. Knowledge and Risk Perception towards Lassa Fever Infection among Residents of Affected Communities in Ebonyi State, Nigeria: Implications for Risk Communication. BMC Public Health 2020, 20, 217. [Google Scholar] [CrossRef] [PubMed]
- Awosanya, E.J. Post-Epidemic Awareness and Knowledge of Lassa Fever among Residents in Affected Community in Ibadan, Oyo State, Nigeria. Vet. World 2018, 11, 1059–1063. [Google Scholar] [CrossRef] [PubMed]
- WHO Regional Office for Africa. Technical Guidelines for Integrated Disease Surveillance and Response in the WHO African Region Booklet One: Introduction, 3rd ed.; WHO Regional Office for Africa: Brazaville, Congo, 2019; Available online: http://apps.who.int/bookorders (accessed on 9 April 2025).
- Njidda, A.M.; Oyebanji, O.; Obasanya, J.; Ojo, O.; Adedeji, A.; Mba, N.; Oladejo, J.; Ihekweazu, C. The Nigeria Centre for Disease Control. BMJ Glob. Health 2018, 3, e000712. [Google Scholar] [CrossRef] [PubMed]
- Nigeria Centre for Disease Control and Prevention. Lassa Fever Emergency Operations Centre to Strengthen Coordination of Response Efforts. 2024. Available online: https://ncdc.gov.ng/news/509/ncdc-activates-lassa-fever-emergency-operations-centre-to-strengthen-coordination-of-response-efforts (accessed on 20 May 2025).
- Nigeria Centre for Disease Control and Prevention. Public Advisory on Lassa Fever. 2025. Available online: https://ncdc.gov.ng/news/527/public-advisory-on-lassa-fever (accessed on 20 May 2025).
- Nigeria Centre for Disease Control and Prevention. Departments. Available online: https://ncdc.gov.ng/departments (accessed on 22 May 2025).
- Nigeria Centre for Disease Control and Prevention. NCDC Activates Lassa Fever Emergency Operations Centre to Strengthen the Response to Rising Cases of Lassa Fever in Nigeria. 2023. Available online: https://ncdc.gov.ng/news/438/ncdc-activates-lassa-fever-emergency-operations-centre-to-strengthen-the-response-to-rising-cases-of-lassa-fever-in-nigeria (accessed on 22 May 2025).
- Niesters, H.G.; Rossen, J.W.; Van Der Avoort, H.; Baas, D.; Benschop, K.; Claas, E.C.; Kroneman, A.; Van Maarseveen, N.; Pas, S.; Van Pelt, W.; et al. Laboratory-Based Surveillance in the Molecular Era: The TYPENED Model, a Joint Data-Sharing Platform for Clinical and Public Health Laboratories. Eurosurveillance 2013, 18, 24. [Google Scholar] [CrossRef]
- Nigeria Centre for Disease Control and Prevention. NCDC intensifies activities for Lassa Fever Surveillance and Response Following Outbreaks of Cases in Nigeria. 2021. Available online: https://ncdc.gov.ng/news/349/ncdc-intensifies-activities-for-lassa-fever-surveillance-and-response-following-outbreaks-of-cases-in-nigeria (accessed on 23 May 2025).
- Ilori, E. Lassa Surveillance & Ongoing Research in Nigeria; Lassa Fever Technical Working Group, NCDC: Abuja, Nigeria, 2018; (Unpublished Data). [Google Scholar]
- WHO. One Health. 2023. Available online: https://www.who.int/news-room/fact-sheets/detail/one-health (accessed on 27 May 2025).
- Arruda, L.B.; Haider, N.; Olayemi, A.; Simons, D.; Ehichioya, D.; Yinka-Ogunleye, A.; Ansumana, R.; Thomason, M.J.; Asogun, D.; Ihekweazu, C.; et al. The Niche of One Health Approaches in Lassa Fever Surveillance and Control. Ann. Clin. Microbiol. Antimicrob. 2021, 20, 29. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Asogun, D.; Arogundade, B.; Unuabonah, F.; Olugbenro, O.; Asogun, J.; Aluede, F.; Ehichioya, D. A Review of the Epidemiology of Lassa Fever in Nigeria. Microorganisms 2025, 13, 1419. https://doi.org/10.3390/microorganisms13061419
Asogun D, Arogundade B, Unuabonah F, Olugbenro O, Asogun J, Aluede F, Ehichioya D. A Review of the Epidemiology of Lassa Fever in Nigeria. Microorganisms. 2025; 13(6):1419. https://doi.org/10.3390/microorganisms13061419
Chicago/Turabian StyleAsogun, Danny, Bosede Arogundade, Faith Unuabonah, Olorunkemi Olugbenro, Joyce Asogun, Fatelyn Aluede, and Deborah Ehichioya. 2025. "A Review of the Epidemiology of Lassa Fever in Nigeria" Microorganisms 13, no. 6: 1419. https://doi.org/10.3390/microorganisms13061419
APA StyleAsogun, D., Arogundade, B., Unuabonah, F., Olugbenro, O., Asogun, J., Aluede, F., & Ehichioya, D. (2025). A Review of the Epidemiology of Lassa Fever in Nigeria. Microorganisms, 13(6), 1419. https://doi.org/10.3390/microorganisms13061419






