Evaluation of Aspergillus flavus Growth on Weathered HDPE Plastics Contaminated with Diesel Fuel
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Objective and Experimental Design
2.2. Chemical Characterization of Diesel Fuel in Study Samples
2.2.1. Virgin Diesel
2.2.2. Weathered Diesel
2.3. Preparation of HDPE Samples
2.4. Preparation of Culture Medium
2.5. Inoculation and Incubation
2.6. Monitoring and Evaluation of Fungal Growth
3. Results
3.1. Composition of Commercial Diesel Fuel
3.2. Composition of Weathered Diesel Fuel Extracted from HDPE Fuel Container Surfaces
3.3. Fungal Growth Dynamics on Plastic Substrates
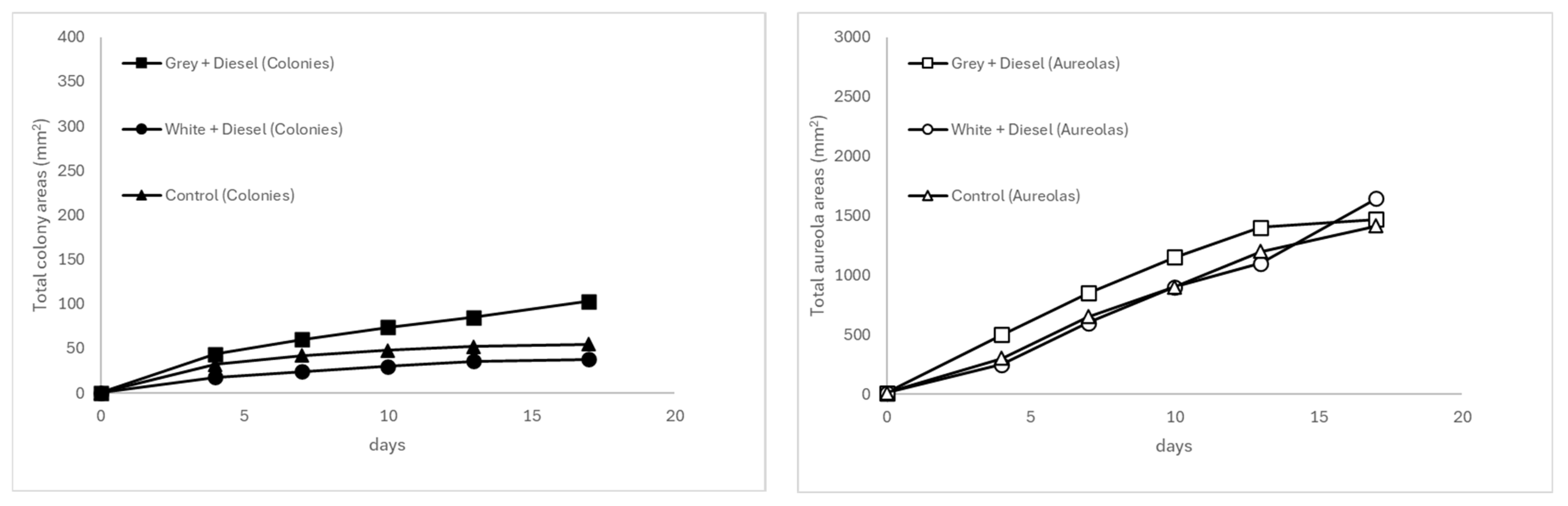
3.3.1. Comparative Fungal Behavior at 30 °C According to Plastic Substrate Type
3.3.2. Comparison of Fungal Behavior at 25 °C by Plastic Matrix Type
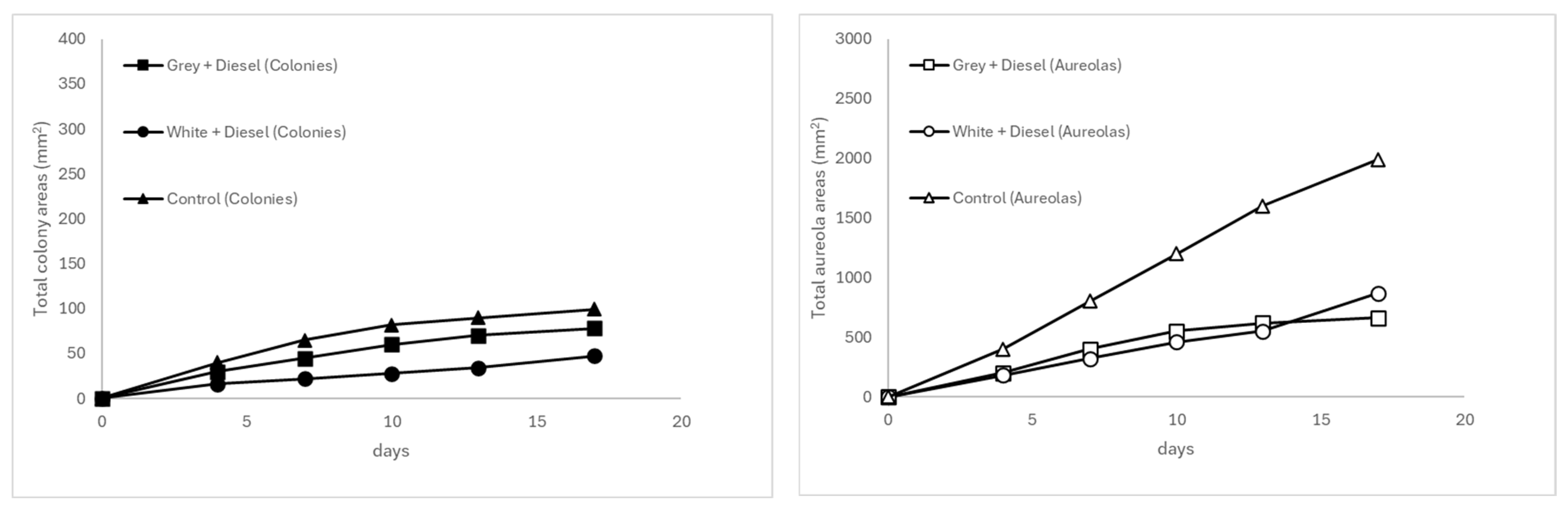
3.3.3. Comparison of Fungal Behavior at 20 °C According to Plastic Matrix Type
3.3.4. Analysis of Experimental Variability Across Treatments
3.3.5. Influence of Incubation Temperature on Fungal Colonization
3.3.6. Temporal Evolution of Mycelial Growth and Halo Formation
3.3.7. Influence of Temperature and Substrate Type on Fungal Growth: ANOVA Results
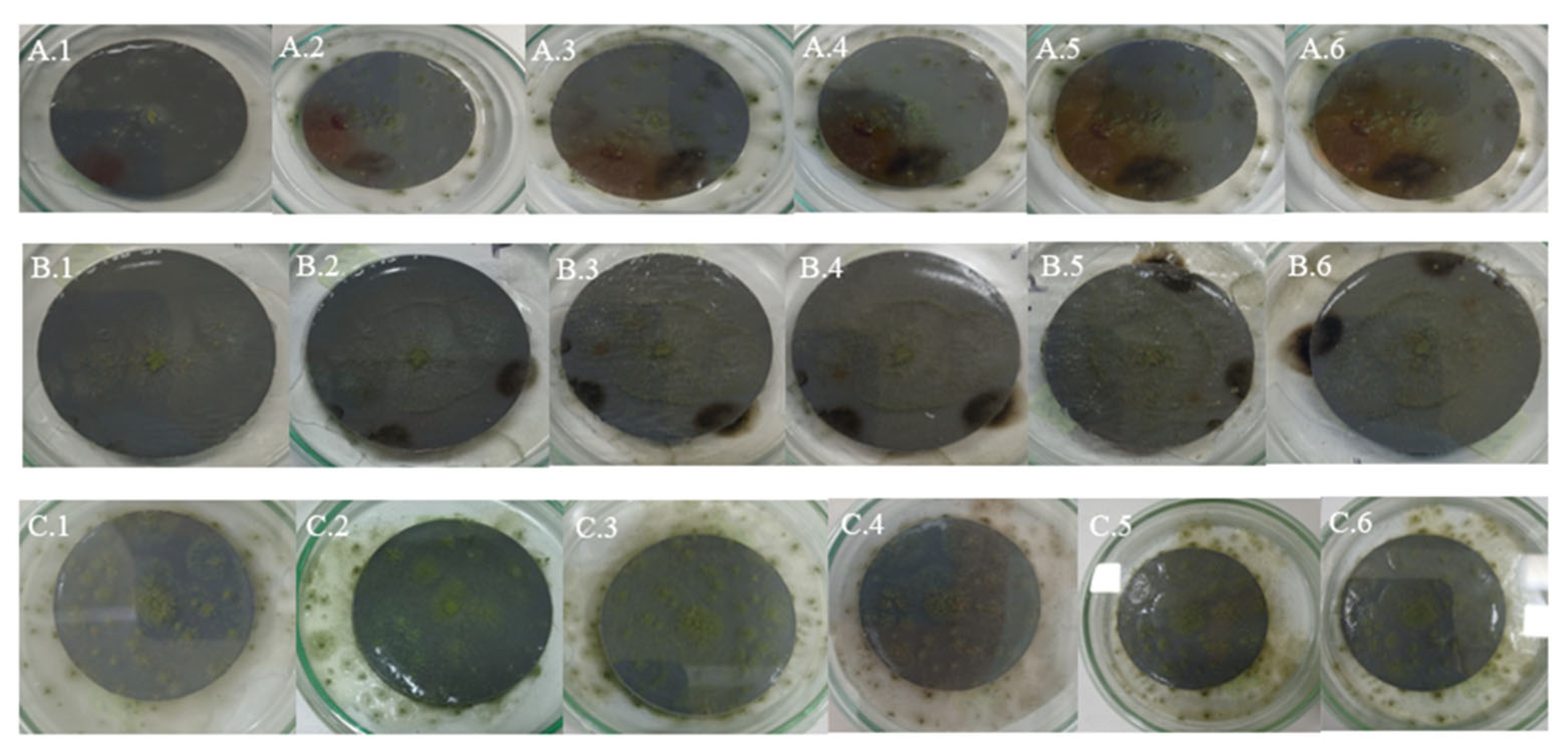
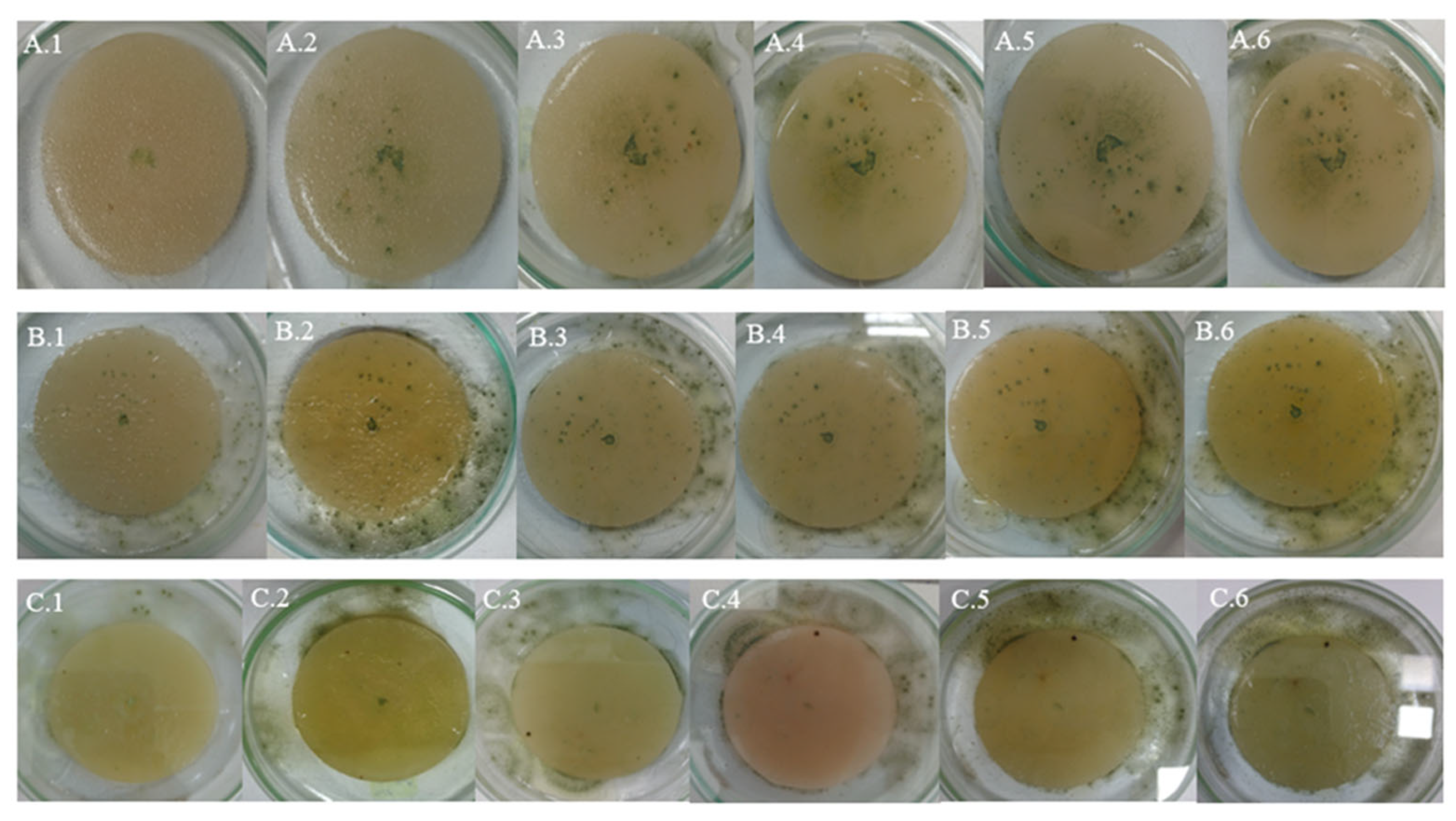
4. Qualitative Analysis of Photographic Images of Fungal Cultures
4.1. Samples of Gray Plastic Contaminated with Weathered Diesel Fuel
4.2. White Plastic Contaminated with Non-Weathered Diesel Fuel
4.3. Gray Plastic Without Contamination Used as Control
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HPDE | High-Density Polyethylene |
| PDA | Potato Dextrose Agar |
| CV | Coefficient of Variation |
| BTEX | Benzene, Toluene, Ethylbenzene, and Xylenes |
| PAHs | Polycyclic Aromatic Hydrocarbons |
| GC-MS | Gas Chromatography–Mass Spectrometry |
| SPME | Solid Phase Microextraction |
| UV | Ultraviolet |
| AFB1 | Aflatoxin B1 |
| AFB2 | Aflatoxin B2 |
| WRF | White-Rot Fungi |
| A. flavus | Aspergillus flavus |
References
- Center for Sustainable Systems. Plastic Production and Disposal Factsheet; Pub. No. CSS05-06; University of Michigan: Ann Arbor, MI, USA, 2023; Available online: https://css.umich.edu/publications/factsheets/material-resources/plastic-waste-factsheet (accessed on 13 July 2023).
- Ministry of Economy, Development and Reconstruction, Chile. Supreme Decree No. 160: Approves the Safety Regulation for the Facilities and Operations of Production and Refining, Transportation, Storage, Distribution, and Supply of Liquid Fuels. Superintendence of Electricity and Fuels (SEC). 2009. Available online: https://vlex.cl/vid/abastecimiento-combustibles-liquidos-239601602 (accessed on 13 July 2023).
- Erdmann, M.; Boehning, M.; Niebergall, U. Physical and chemical effects of biodiesel storage on high-density polyethylene: Evidence of co-oxidation. Polym. Degrad. Stab. 2019, 161, 206–215. [Google Scholar] [CrossRef]
- Bacha, J.D.; Freel, J.; Gibbs, L.; Gibbs, J.; Hemighaus, G.; Hoekman, S.K.; Horn, J.; Ingham, M.; Organ, R.; Olsen, E.; et al. Diesel Fuels Technical Review, 5th ed.; Chevron Products Company: San Ramon, CA, USA, 2007. [Google Scholar]
- Kuppusamy, S.; Maddela, N.R.; Megharaj, M.; Venkateswarlu, K. An overview of total petroleum hydrocarbons. In En Total Petroleum Hydrocarbons: Environmental Fate, Toxicity and Remediation; Springer International Publishing: Cham, Switzerland, 2020; pp. 1–27. [Google Scholar] [CrossRef]
- Almeida, C.M.R.; Mucha, A.P.; Bordalo, A.A. Microbial communities in estuarine sediments: Response to petroleum hy-drocarbons and metals. Sci. Total Environ. 2013, 454–455, 20–29. [Google Scholar]
- Lourmpas, N.; Papanikos, P.; Efthimiadou, E.K.; Fillipidis, A.; Lekkas, D.F.; Alexopoulos, N.D. Degradation assessment of high density polyethylene (HDPE) debris after long exposure to marine conditions. Sci. Total Environ. 2024, 954, 176847. [Google Scholar] [CrossRef] [PubMed]
- Freeman, D.; Reddy, C.M.; Valentine, D.L. Photochemical weathering of petroleum in cold waters. Environ. Sci. Technol. Lett. 2023, 10, 25–30. [Google Scholar]
- Alexandrino, G.L.; Malmborg, J.; Augusto, F.; Christensen, J.H. Investigating weathering in light diesel oils using compre-hensive two-dimensional gas chromatography-High resolution mass for spectrometry and pixel-based analysis: Possibilities and limitations. J. Chromatogr. A 2019, 1591, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Guo, F.; Li, Y.; Liu, X. Biodegradation of petroleum hydrocarbons using white rot fungi: Mechanisms and environmental applications. J. Hazard. Mater. 2024, 457, 131759. [Google Scholar]
- Horel, A.; Schiewer, S. Impact of VOC removal by activated carbon on biodegradation rates of diesel, Syntroleum and biodiesel in contaminated sand. Sci. Total Environ. 2016, 563–564, 420–431. [Google Scholar] [CrossRef] [PubMed]
- Nrior, R.R.; Odokuma, L.O. Bioremediation of petroleum-contaminated environments using fungal isolates. J. Bioremediat. Biodegrad. 2017, 8, 403. [Google Scholar]
- Vaksmaa, A.; Guerrero-Cruz, S.; Ghosh, P.; Zeghal, E.; Hernando-Morales, V.; Niemann, H. Role of fungi in bioremediation of emerging pollutants. Front. Mar. Sci. 2023, 10, 1070905. [Google Scholar] [CrossRef]
- Di Napoli, M.; Silvestri, B.; Castagliuolo, G.; Carpentieri, A.; Luciani, G.; Di Maro, A.; Sorbo, S.; Pezzella, A.; Zanfardino, A.; Varcamonti, M. High density polyethylene (HDPE) biodegradation by the fungus Cladosporium halotolerans. FEMS Microbiol. Ecol. 2023, 99, fiac148. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, S.; Maroof, L.; Iqbal, M.; Farman, S.; Lubna; Faisal, S.; Bhatt, K. Biodegradation of Low-Density Polyethylene (LDPE) Bags by Fungi Isolated from Waste Disposal Soil. Appl. Environ. Soil. Sci. 2022, 2022, 8286344. [Google Scholar] [CrossRef]
- Ong, G.H.; Liew, L.M.; Wong, K.K.; Wong, R.R.; Barasarathi, J.; Loh, K.E.; Tanee, T. Screening of Native Fungi For Biodeg-radation of High-Density Polyethylene (HDPE) Plastic in Mangroves Ecosystem. Malays. Appl. Biol. 2024, 53, 97–103. [Google Scholar] [CrossRef]
- Erdmann, M.; Kleinbub, S.; Wachtendorf, V.; Schutter, J.D.; Niebergall, U.; Boehning, M.; Koerdt, A. Photo-oxidation of PE-HD affecting polymer/fuel interaction and bacterial attachment. npj Mater. Degrad. 2020, 4, 18. [Google Scholar] [CrossRef]
- Dantas, C.P.; Pinchemel, J.P.D.; de Jesus, G.M.; Pimentel, M.B.; Oliveira, O.M.C.; Queiroz, A.F.S.; Lima, D.F. Bioprospection of ligninolytic enzymes from marine origin filamentous fungi. An. Acad. Bras. Ciências 2021, 93 (Suppl. 3), e20210296. [Google Scholar] [CrossRef] [PubMed]
- Fariña, J.I.; Raya Tonetti, G.; Perotti, N.I. A mathematical model applied to the fungal colony growth of Sclerotium rolfsii. Biotechnol. Tech. 1997, 11, 217–219. [Google Scholar] [CrossRef]
- Tsang, M.Y.; Chen, L.H.; Huang, F.C. UV-C sterilization on polypropylene and polyethylene surfaces: Efficacy evaluated in solid-phase microbial assays. J. Appl. Microbiol. 2022, 133, 2041–2050. [Google Scholar] [CrossRef]
- Nrior, R.R.; Uzochukwu, S.C.; Uzochukwu, C.I. Biodegradation of petroleum oils by fungi isolated from oil palm fruit and mechanic village. J. Environ. Sci. Technol. 2017, 10, 85–94. [Google Scholar]
- Caceres-Zambrano, J.Z.; Rodríguez-Córdova, L.A.; Sáez-Navarrete, C.A.; Rives, Y.C. Biodegradation capabilities of fila-mentous fungi in high-concentration heavy crude oil environments. Arch. Microbiol. 2024, 206, 123. [Google Scholar] [CrossRef] [PubMed]
- Norlia, M.; Jinap, S.; Nor-Khaizura, M.A.R.; Radu, S.; John, J.M.; Rahman, M.A.H.; Peter, M.L.; Sharif, Z. Modelling the effect of temperature and water activity on the growth rate of Aspergillus flavus and aflatoxin production in peanut meal extract agar. Int. J. Food Microbiol. 2020, 335, 108836. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Jun, S.; Han, K.; Hong, S.; Yu, J. Diversity, Application, and Synthetic Biology of Industrially Important Aspergillus Fungi. Adv. Appl. Microbiol. 2017, 100, 161–202. [Google Scholar] [CrossRef] [PubMed]


| Commercial Diesel | Weathered Diesel | |||
|---|---|---|---|---|
| Component | % w/w | Error (%) | % w/w | Error (%) |
| n-Alkanes C10–C14 | 8.5 | 0.38 | 0.5 | 0.1 |
| n-Alkanes C15–C20 | 20.0 | 0.42 | 5.0 | 0.3 |
| n-Alkanes C21–C25 | 25.0 | 0.39 | 8.0 | 0.4 |
| Aromatic hydrocarbons (BTEX) | 15.0 | 0.16 | 0.2 | 0.05 |
| Naphthenes | 12.0 | 0.31 | 3.0 | 0.2 |
| Polycyclic aromatic hydrocarbons (PAHs) | 5.0 | 0.32 | 1.5 | 0.1 |
| Pristane and phytane | 1.5 | 0.19 | 0.7 | 0.05 |
| Unidentified fraction (residuals) | 13.0 | 0.24 | 81.1 | 0.5 |
| Weathered Samples | Unweathered Samples | Control Samples | ||||
|---|---|---|---|---|---|---|
| Sample (30 °C) | Mean ± SD (mm2) | Mean ± SD (mm2) | Mean ± SD (mm2) | Mean ± SD (mm2) | Mean ± SD (mm2) | Mean ± SD (mm2) |
| 1 | 168.01 ± 22.3 | 1647.17 ± 463.91 | 11.72 ± 4.12 | n.a. | 101.07 ± 56.03 | n.a. |
| 2 | 154.62 ± 63.01 | 1632.86 ± 470.5 | 18.54 ± 9.16 | 257.85 ± 311.29 | 108.89 ± 61.16 | 1788.58 ± 178.13 |
| 3 | 275.51 ± 30.16 | 2363.83 ± 488.25 | 18.31 ± 9.55 | 273.85 ± 296.04 | 101.12 ± 52.90 | 1951.67 ± 426.44 |
| 4 | 269.97 ± 14.44 | 2347.39 ± 580.89 | 18.31 ± 9.55 | 273.85 ± 296.04 | 103.15 ± 41.69 | 2068.26 ± 320.96 |
| 5 | 355.8 ± 125.13 | 2823.45 ± 365.65 | 18.23 ± 15.3 | 786.62 ± 597.94 | 103.15 ± 41.69 | 2068.26 ± 320.96 |
| 6 | 355.8 ± 125.13 | 2823.45 ± 365.65 | 20.1 ± 14.16 | 1036.23 ± 454.27 | 103.15 ± 41.69 | 2290.89 ± 698.34 |
| Colony area | Halo area | Colony area | Halo area | Colony area | Halo area | |
| Weathered Samples | Unweathered Samples | Control Samples | ||||
|---|---|---|---|---|---|---|
| Sample (25 °C) | Mean ± SD (mm2) | Mean ± SD (mm2) | Mean ± SD (mm2) | Mean ± SD (mm2) | Mean ± SD (mm2) | Mean ± SD (mm2) |
| 1 | 43.88 ± 6.87 | 754.65 ± 188.26 | 25.63 ± 5.04 | n.a. | 42.3 ± 24.32 | n.a. |
| 2 | 64.01 ± 15.76 | 1640.63 ± 1044.26 | 23.79 ± 7.49 | 781.98 ± 214.36 | 42.3 ± 24.32 | 587.02 ± 313.9 |
| 3 | 47.88 ± 8.18 | 1344.78 ± 723.93 | 35.55 ± 19.23 | 598.39 ± 352.26 | 54.21 ± 32.73 | 1538.96 ± 949.21 |
| 4 | 47.88 ± 8.18 | 1344.78 ± 723.93 | 34.49 ± 17.11 | 1039.12 ± 728.00 | 53.86 ± 31.24 | 1628.69 ± 899.63 |
| 5 | 72.19 ± 27.19 | 1437.97 ± 260.99 | 38.17 ± 17.47 | 814.66 ± 737.94 | 55.14 ± 13.24 | 1268.75 ± 126.77 |
| 6 | 74.19 ± 25.08 | 1437.97 ± 260.99 | 45.24 ± 26.37 | 870.22 ± 984.13 | 49.15 ± 23.26 | 1229.19 ± 174.54 |
| Colony area | Halo area | Colony area | Halo area | Colony area | Halo area | |
| Weathered Samples | Unweathered Samples | Control Samples | ||||
|---|---|---|---|---|---|---|
| Sample (20 °C) | Mean ± SD (mm2) | Mean ± SD (mm2) | Mean ± SD (mm2) | Mean ± SD (mm2) | Mean ± SD (mm2) | Mean ± SD (mm2) |
| 1 | 29.31 ± 7.82 | 314.65 ± 173.9 | 14.78 ± 10.97 | n.a. | 24.24 ± 10.51 | n.a. |
| 2 | 119.42 ± 56.37 | 735.88 ± 428.1 | 33.92 ± 20.04 | 157.17 ± 133.89 | 69.55 ± 26.68 | 1024.85 ± 86.90 |
| 3 | 90.86 ± 52.75 | 625.89 ± 343.95 | 53.27 ± 30.78 | n.a. | 69.55 ± 26.68 | 1024.85 ± 86.90 |
| 4 | 78.39 ± 23.36 | 660.4 ± 152.58 | 54.29 ± 29.05 | n.a. | 128.11 ± 100.07 | 1568.5 ± 570.31 |
| 5 | 78.39 ± 23.36 | 660.4 ± 152.58 | 46.59 ± 24.28 | 498.91 ± 174.39 | 95.48 ± 34.63 | 1896.73 ± 248.84 |
| 6 | 78.39 ± 23.36 | 660.4 ± 152.58 | 47.68 ± 22.43 | 526.83 ± 134.9 | 99.47 ± 33.99 | 1987.47 ± 174.92 |
| Colony area | Halo area | Colony area | Halo area | Colony area | Halo area | |
| Temperature (°C) | Matrix Type | CV (%) |
|---|---|---|
| 20 | Gray + Diesel | 29.8 |
| 20 | White + Diesel | 50.2 |
| 20 | Control (Gray) | 34.2 |
| 25 | Gray + Diesel | 33.8 |
| 25 | White + Diesel | 55.1 |
| 25 | Control (Gray) | 24.0 |
| 30 | Gray + Diesel | 35.2 |
| 30 | White + Diesel | 58.3 |
| 30 | Control (Gray) | 40.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valenzuela, J.; Sáez-Navarrete, C.; Baraza, X.; Martínez, F.; Márquez, B. Evaluation of Aspergillus flavus Growth on Weathered HDPE Plastics Contaminated with Diesel Fuel. Microorganisms 2025, 13, 1418. https://doi.org/10.3390/microorganisms13061418
Valenzuela J, Sáez-Navarrete C, Baraza X, Martínez F, Márquez B. Evaluation of Aspergillus flavus Growth on Weathered HDPE Plastics Contaminated with Diesel Fuel. Microorganisms. 2025; 13(6):1418. https://doi.org/10.3390/microorganisms13061418
Chicago/Turabian StyleValenzuela, Juan, César Sáez-Navarrete, Xavier Baraza, Fernando Martínez, and Bastián Márquez. 2025. "Evaluation of Aspergillus flavus Growth on Weathered HDPE Plastics Contaminated with Diesel Fuel" Microorganisms 13, no. 6: 1418. https://doi.org/10.3390/microorganisms13061418
APA StyleValenzuela, J., Sáez-Navarrete, C., Baraza, X., Martínez, F., & Márquez, B. (2025). Evaluation of Aspergillus flavus Growth on Weathered HDPE Plastics Contaminated with Diesel Fuel. Microorganisms, 13(6), 1418. https://doi.org/10.3390/microorganisms13061418






