Detection and Prevalence of Rabies in Bats from Oaxaca
Abstract
1. Introduction
2. Materials and Methods
2.1. Desk-Research
2.2. Fieldwork (Sample Collection)
2.3. Brain Extraction
2.4. Preparation of Imprints
2.5. Direct Immunofluorescence Technique (DIF)
2.6. DIF Microscopic Observation and Interpretation of Results
2.7. Rabies Prevalence
3. Results
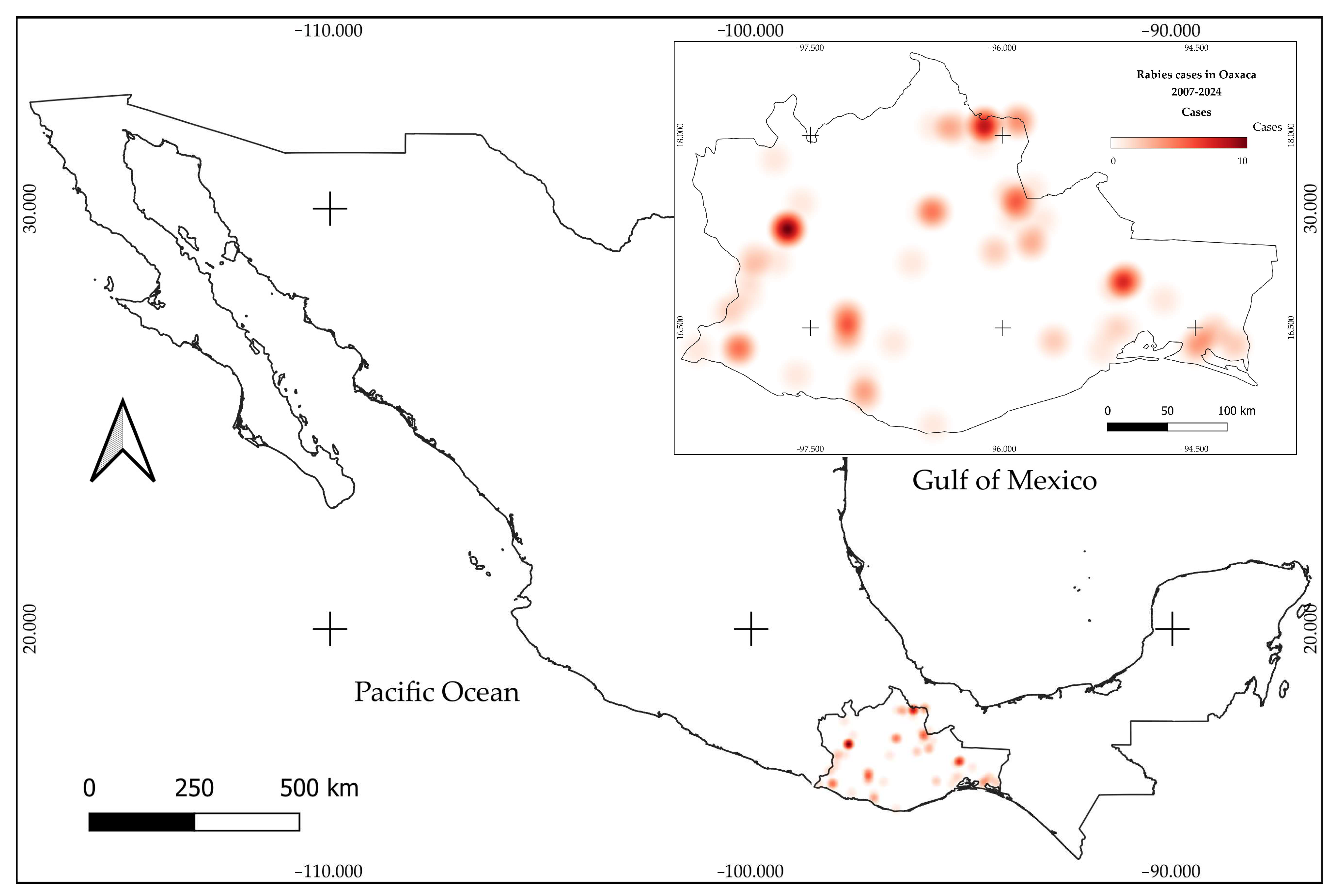
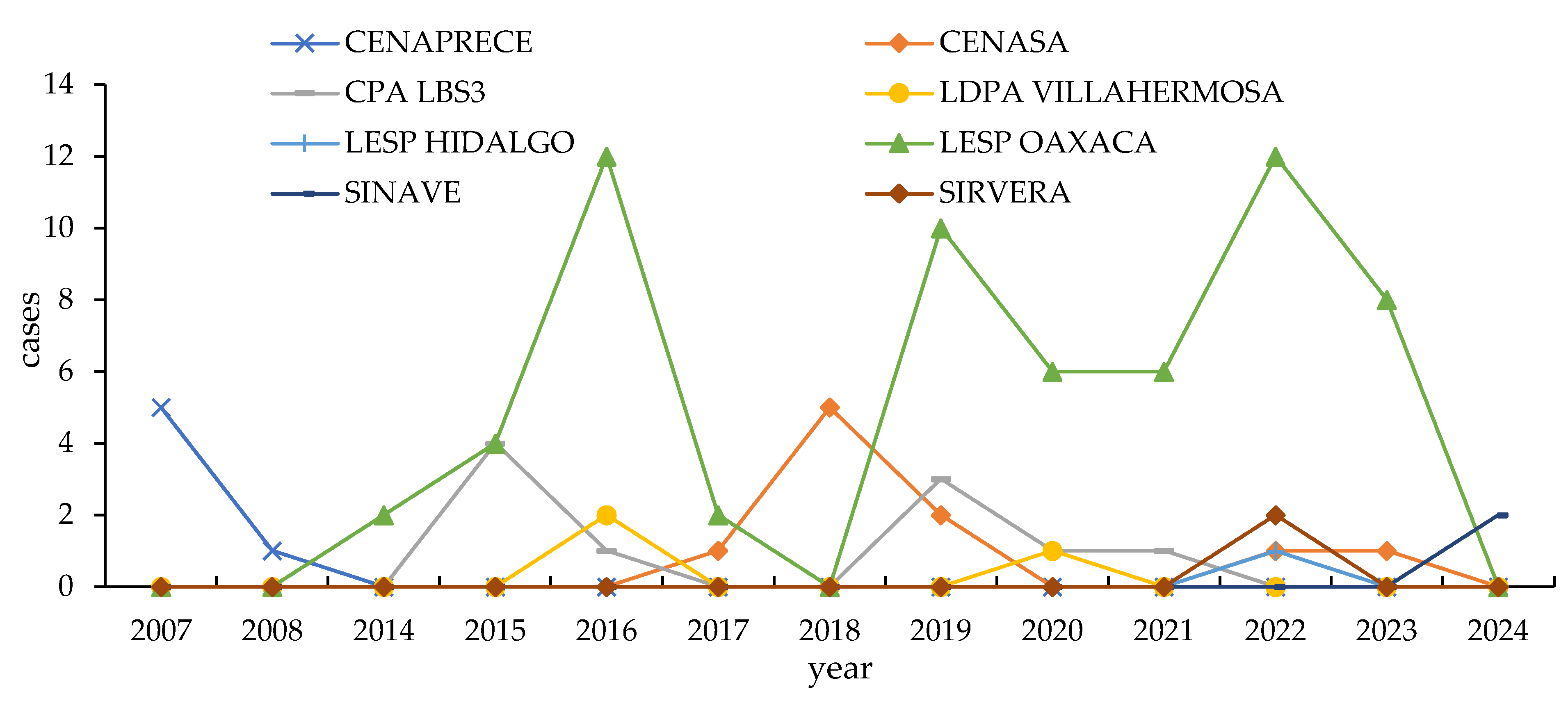
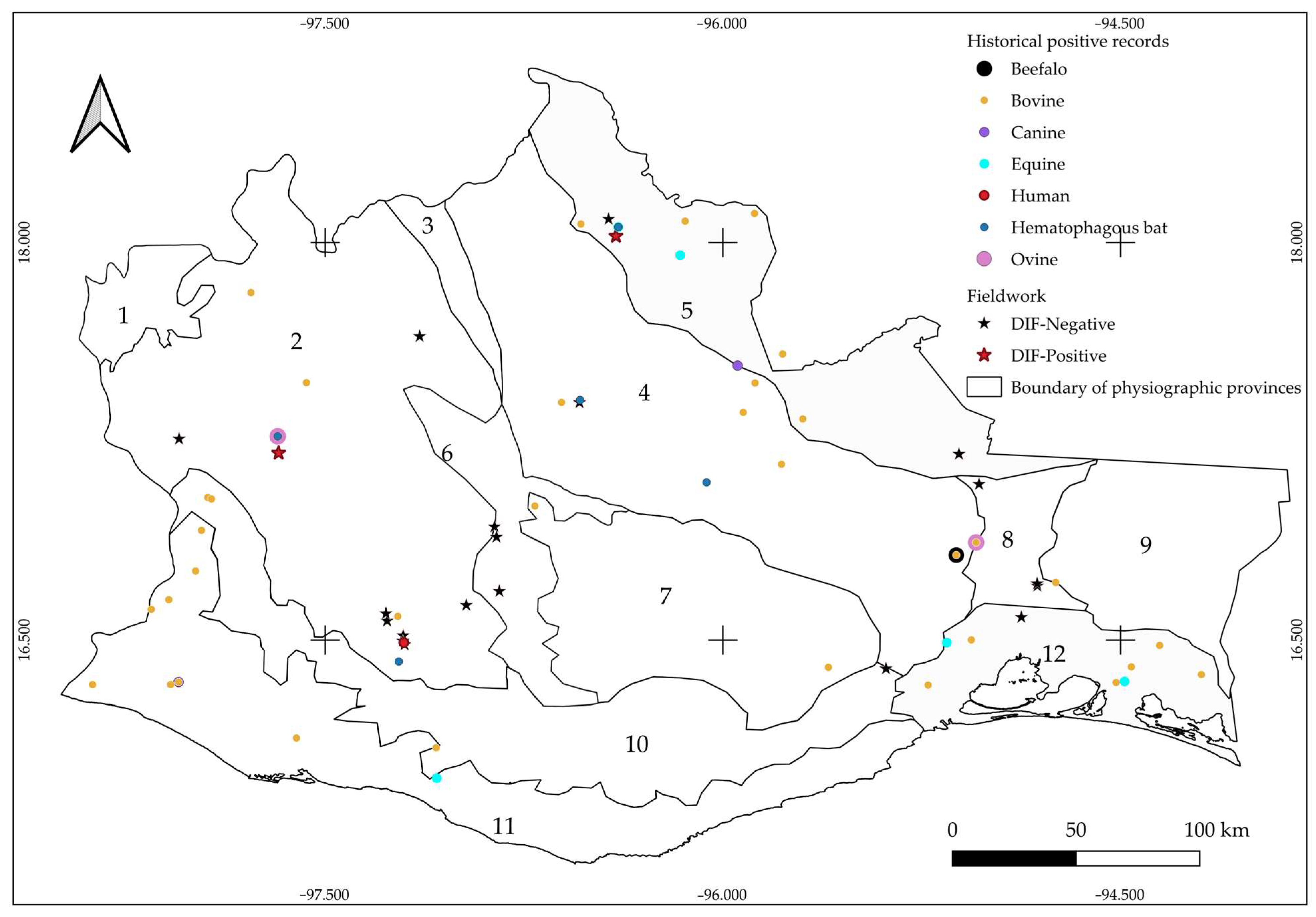
3.1. Historic Records
3.2. Active Bats Surveillance (2022–2024)
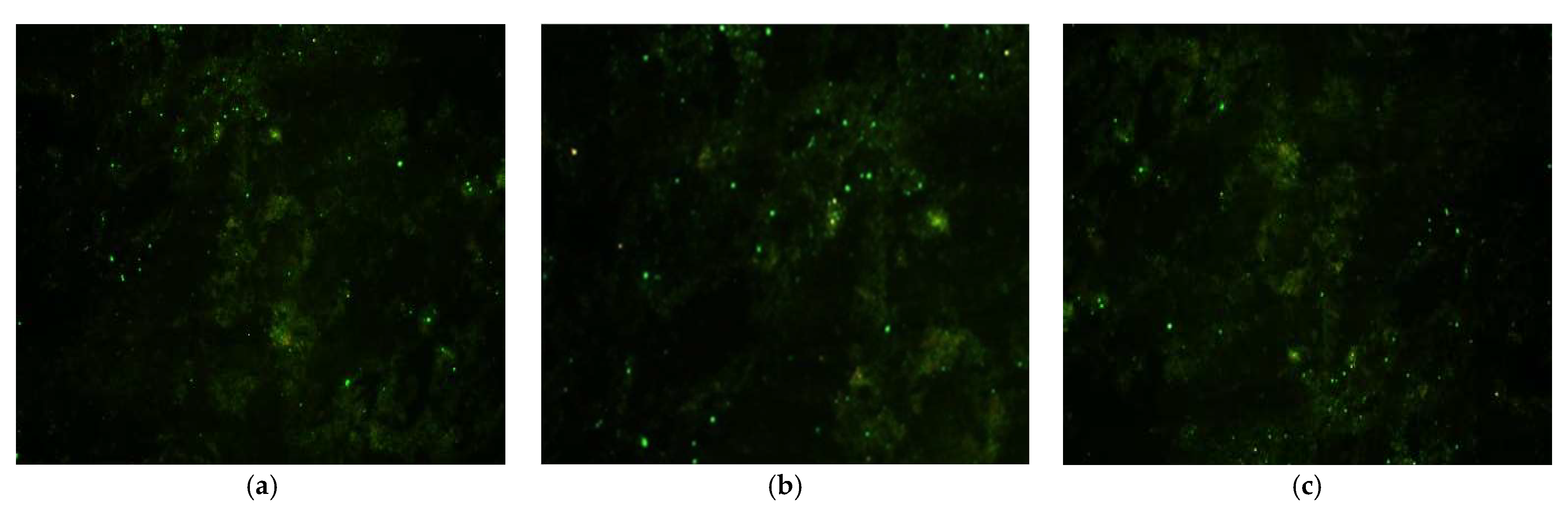
3.3. Rabies Virus Detection by Direct Immunofluorescence (DIF)
3.4. Prevalence of Rabies Virus in Bats
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| LESP | Laboratorio Estatal de Salud Pública |
| CPA LBS3s | Laboratorio de Bioseguridad Nivel 3 de la Comisión México-Estados Unidos para la Prevención de Fiebre Aftosa |
| LDPA Villahermosa | Laboratorio De Patología Animal Villahermosa |
| CENAPRECE | Centro Nacional de Programas Preventivos y Control de Enfermedades |
| SENASICA | Servicio Nacional de Sanidad, Inocuidad y Calidad Agroalimentaria |
| CENASA | Centro Nacional de Servicios de Diagnóstico en Salud Animal |
| DIF | Direct Immunofluorescence |
| WHOA | World Organization for Animal Health |
| SAGARPA | Secretaría de Agricultura, Ganadería, Desarrollo Rural, Pesca y Alimentación |
| OMS | Organización Mundial de la Salud |
| CEFPPO | Comité Estatal para el Fomento y Protección Pecuaria del Estado de Oaxaca |
| SINAVE | Sistema Nacional de Vigilancia Epidemiológica |
| SIRVERA | Sistema de Información Regional para la Vigilancia Epidemiológica de la Rabia |
Appendix A
Appendix A.1
| Year | Municipality | Physiographic Province | Species | Laboratory |
|---|---|---|---|---|
| 2007 | San Pedro Mixtepec | Sierra Madre del Sur | Canine | CENAPRECE |
| 2007 | Pinotepa Nacional | Pacific Coastal Plain | Canine | CENAPRECE |
| 2007 | Pinotepa Nacional | Pacific Coastal Plain | Canine | CENAPRECE |
| 2007 | Pinotepa Nacional | Pacific Coastal Plain | Canine | CENAPRECE |
| 2007 | San Vicente Coatlán | Valles Centrales | Human | CENAPRECE |
| 2008 | Pinotepa Nacional | Pacific Coastal Plain | Canine | CENAPRECE |
| 2014 | Santiago Jocotepec | Sierra Madre de Oaxaca | Bovine | LESP Oaxaca |
| 2014 | San Juan Cotzocon | Sierra Madre de Oaxaca | Bovine | LESP Oaxaca |
| 2015 | Santiago Jocotepec | Sierra Madre de Oaxaca | Bovine | CPA LBS3 |
| 2015 | Constancia del Rosario | Sierra Madre del Sur | Bovine | CPA LBS3 |
| 2015 | Heroica Ciudad de Tlaxiaco | Western Mountains and Valleys | Ovine | CPA LBS3 |
| 2015 | Matias Romero Avendaño | Isthmus Depression of Tehuantepec | Bovine | LESP Oaxaca |
| 2015 | Matias Romero Avendaño | Isthmus Depression of Tehuantepec | Bovine | LESP Oaxaca |
| 2015 | Santiago Minas | Western Mountains and Valleys | Hematophagous bat | LESP Oaxaca |
| 2015 | Santiago Minas | Western Mountains and Valleys | Hematophagous bat | LESP Oaxaca |
| 2015 | San Pedro Mixtepec | Sierra Madre del Sur | Bovine | CPA LBS3 |
| 2016 | San Pedro Tapanatepec | Tehuantepec Coastal Plain | Bovine | LESP Oaxaca |
| 2016 | San Juan Bautista Tuxtepec | Gulf Coastal Plain | Bovine | LESP Oaxaca |
| 2016 | Matias Romero Avendaño | Isthmus Depression of Tehuantepec | Bovine | LDPA Villahermosa |
| 2016 | Matias Romero Avendaño | Isthmus Depression of Tehuantepec | Bovine | LDPA Villahermosa |
| 2016 | Santa Maria Tlahuitoltepec | Sierra Madre de Oaxaca | Bovine | LESP Oaxaca |
| 2016 | Heroica Ciudad de Tlaxiaco | Western Mountains and Valleys | Ovino | LESP Oaxaca |
| 2016 | Santa Maria Tlahuitoltepec | Sierra Madre de Oaxaca | Hematophagous bat | LESP Oaxaca |
| 2016 | Heroica Ciudad de Tlaxiaco | Western Mountains and Valleys | Hematophagous bat | LESP Oaxaca |
| 2016 | San Agustin de Las Juntas | Central Mountains and Valleys | Bovine | CPA LBS3 |
| 2016 | Mesones Hidalgo | Pacific Coastal Plain | Bovine | LESP Oaxaca |
| 2016 | Pinotepa Nacional | Pacific Coastal Plain | Bovine | LESP Oaxaca |
| 2016 | Heroica Ciudad de Tlaxiaco | Western Mountains and Valleys | Bovine | LESP Oaxaca |
| 2016 | Heroica Ciudad de Tlaxiaco | Western Mountains and Valleys | Bovine | LESP Oaxaca |
| 2016 | Heroica Ciudad de Tlaxiaco | Western Mountains and Valleys | Bovine | LESP Oaxaca |
| 2016 | San Pedro Amuzgos | Pacific Coastal Plain | Bovine | LESP Oaxaca |
| 2017 | San Juan Cacahuatepec | Pacific Coastal Plain | Bovine | LESP Oaxaca |
| 2017 | Loma Bonita | Gulf Coastal Plain | Bovine | LESP Oaxaca |
| 2017 | Santiago Yolomecatl | Western Mountains and Valleys | Bovine | CENASA |
| 2018 | San Juan Evangelista Analco | Sierra Madre de Oaxaca | Bovine | CENASA |
| 2018 | San Juan Bautista Tuxtepec | Gulf Coastal Plain | Bovine | CENASA |
| 2018 | Putla Villa de Guerrero | Sierra Madre del Sur | Bovine | CENASA |
| 2018 | San Juan Evangelista Analco | Sierra Madre de Oaxaca | Bovine | CENASA |
| 2018 | Santa Ana Yareni | Sierra Madre de Oaxaca | Bovine | CENASA |
| 2019 | San Francisco del Mar | Tehuantepec Coastal Plain | Bovine | LESP Oaxaca |
| 2019 | San Blas Atempa | Tehuantepec Coastal Plain | Bovine | LESP Oaxaca |
| 2019 | Santo Domingo Zanatepec | Tehuantepec Coastal Plain | Bovine | LESP Oaxaca |
| 2019 | Santo Domingo Zanatepec | Tehuantepec Coastal Plain | Bovine | LESP Oaxaca |
| 2019 | Santo Domingo Teojomulco | Western Mountains and Valleys | Bovine | CENASA |
| 2019 | Reforma de Pineda | Tehuantepec Coastal Plain | Bovine | CENASA |
| 2019 | San Juan Bautista Tuxtepec | Gulf Coastal Plain | Bovine | LESP Oaxaca |
| 2019 | Santo Domingo Armenta | Pacific Coastal Plain | Bovine | LESP Oaxaca |
| 2019 | Loma Bonita | Gulf Coastal Plain | Bovine | LESP Oaxaca |
| 2019 | San Pedro Comitancillo | Tehuantepec Coastal Plain | Equine | CPA LBS3 |
| 2019 | San Francisco Ixhuatan | Tehuantepec Coastal Plain | Equine | CPA LBS3 |
| 2019 | San Juan Bautista Tuxtepec | Gulf Coastal Plain | Bovine | CPA LBS3 |
| 2019 | Reforma de Pineda | Tehuantepec Coastal Plain | Bovine | LESP Oaxaca |
| 2019 | San Pedro Tapanatepec | Tehuantepec Coastal Plain | Bovine | LESP Oaxaca |
| 2019 | San Juan Bautista Tuxtepec | Gulf Coastal Plain | Bovine | LESP Oaxaca |
| 2020 | San Miguel Chimalapa | Sierra Madre del Sur de Oaxaca y Chiapas | Bovine | LESP Oaxaca |
| 2020 | San Juan Cotzocon | Sierra Madre de Oaxaca | Bovine | LDPA Villahermosa |
| 2020 | San Juan Bautista Tuxtepec | Gulf Coastal Plain | Bovine | LESP Oaxaca |
| 2020 | San Jose Chiltepec | Gulf Coastal Plain | Equine | CPA LBS3 |
| 2020 | Santo Domingo Teojomulco | Western Mountains and Valleys | Bovine | LESP Oaxaca |
| 2020 | San Juan Evangelista Analco | Sierra Madre de Oaxaca | Hematophagous bat | LESP Oaxaca |
| 2020 | San Juan Evangelista Analco | Sierra Madre de Oaxaca | Hematophagous bat | LESP Oaxaca |
| 2020 | Matias Romero Avendaño | Isthmus Depression of Tehuantepec | Bovine | LESP Oaxaca |
| 2021 | Santiago Yaveo | Gulf Coastal Plain | Bovine | LESP Oaxaca |
| 2021 | Asuncion Ixtaltepec | Tehuantepec Coastal Plain | Bovine | LESP Oaxaca |
| 2021 | San Juan Cotzocon | Sierra Madre de Oaxaca | Bovine | LESP Oaxaca |
| 2021 | San Lorenzo | Gulf Coastal Plain | Bovine | LESP Oaxaca |
| 2021 | San Juan Bautista Tuxtepec | Gulf Coastal Plain | Bovine | LESP Oaxaca |
| 2021 | San Juan Bautista Tuxtepec | Gulf Coastal Plain | Bovine | LESP Oaxaca |
| 2021 | Huajuapan de Leon | Western Mountains and Valleys | Bovine | CPA LBS3 |
| 2022 | Magdalena Tequisistlan | Central Mountains and Valleys | Bovine | LESP Oaxaca |
| 2022 | Magdalena Tequisistlan | Central Mountains and Valleys | Bovine | LESP Oaxaca |
| 2022 | San Lorenzo | Western Mountains and Valleys | Human | LESP Oaxaca |
| 2022 | San Lorenzo | Western Mountains and Valleys | Human | LESP Oaxaca |
| 2022 | Santiago Choapam | Sierra Madre de Oaxaca | Bovine | LESP Oaxaca |
| 2022 | Heroica Ciudad de Tlaxiaco | Western Mountains and Valleys | Bovine | LESP Oaxaca |
| 2022 | Heroica Ciudad de Tlaxiaco | Western Mountains and Valleys | Bovine | LESP Oaxaca |
| 2022 | Heroica Ciudad de Tlaxiaco | Western Mountains and Valleys | Bovine | LESP Oaxaca |
| 2022 | Santa Maria Petapa | Sierra Madre de Oaxaca | Beefalo | LESP Oaxaca |
| 2022 | San Juan Lalana | Sierra Madre de Oaxaca | Bovine | LESP Oaxaca |
| 2022 | Matias Romero Avendaño | Isthmus Depression of Tehuantepec | Bovine | LESP Oaxaca |
| 2022 | San Pedro Mixtepec | Sierra Madre del Sur | Equine | CENASA |
| 2022 | Jalapa de Diaz | Gulf Coastal Plain | Bovine | LESP Hidalgo |
| 2022 | San Gabriel Mixtepec | Sierra Madre del Sur | Bovine | LESP Oaxaca |
| 2022 | San Lorenzo Texmelucan | Sierra Madre del Sur | Human | SIRVERA |
| 2022 | San Lorenzo Texmelucan | Sierra Madre del Sur | Human | SIRVERA |
| 2023 | San Juan Lalana | Sierra Madre de Oaxaca | Bovine | LESP Oaxaca |
| 2023 | Loma Bonita | Gulf Coastal Plain | Bovine | LESP Oaxaca |
| 2023 | San Lucas Ojitlan | Gulf Coastal Plain | Equine | CENASA |
| 2023 | Loma Bonita | Gulf Coastal Plain | Bovine | LESP Oaxaca |
| 2023 | San Juan Lalana | Sierra Madre de Oaxaca | Bovine | LESP Oaxaca |
| 2023 | San Juan Lalana | Sierra Madre de Oaxaca | Bovine | LESP Oaxaca |
| 2023 | Santa Maria Zacatepec | Pacific Coastal Plain | Bovine | LESP Oaxaca |
| 2023 | Tututepec de Melchor Ocampo | Pacific Coastal Plain | Bovine | LESP Oaxaca |
| 2023 | Santa Maria Petapa | Sierra Madre de Oaxaca | Bovine | LESP Oaxaca |
| 2024 | Santa Maria Yucuiti | Western Mountains and Valleys | Human | SINAVE |
| 2024 | Santa María Tonameca | Sierra Madre del Sur | Human | SINAVE |
Appendix A.2
| Scientific Name | Location | Specimens |
|---|---|---|
| Desmodus rotundus | San Miguel Cuevas, Santiago Juxtlahuaca, Western Mountains and Valleys | 11 |
| Anoura geoffroyi | 1 | |
| Leptonycteris yerbabuenae | 1 | |
| Eptesicus fuscus | 3 | |
| Myotis sp. | 2 | |
| Dermanura azteca | 1 | |
| Artibeus jamaicensis | La Ventosa, Juchitán, Tehuantepec Coastal Plain | 2 |
| Desmodus rotundus | San Sebastián de las Grutas, Villa Sola de Vega, Western Mountains and Valleys | 2 |
| Dermanura azteca | 5 | |
| Artibeus jamaicensis | 2 | |
| Anoura geoffroyi | 1 | |
| Desmodus rotundus | Santo Domingo Teojomulco, Sola de Vega, Western Mountains and | 26 |
| Glossophaga soricina | Valleys | 2 |
| Anoura geoffroyi | 1 | |
| Sturnira hondurensis | 1 | |
| Desmodus rotundus | Ayoquezco de Aldama, Central Valleys | 15 |
| Desmodus rotundus | Heroica Ciudad de Tlaxiaco, Western Mountains and Valleys | 13 (1) |
| Desmodus rotundus | Santiago Apoala Nochixtlán, Western Mountains and Valleys | 8 |
| Dermanura azteca | 15 | |
| Anoura geoffroyi | 2 | |
| Desmodus rotundus | San Juan Evangelista Analco, Sierra Madre de Oaxaca | 15 |
| Desmodus rotundus | La Cañada, Santa Inés del Monte Zaachila, Central Valleys | 2 |
| Desmodus rotundus | La Soledad, Santa Inés del Monte Zaachila, Central Valleys | 2 |
| Desmodus rotundus | Santa María Mixtequilla, Sierra Madre del Sur | 8 |
| Macrotus waterhousii | 3 | |
| Balantiopteryx plicata | 12 | |
| Desmodus rotundus | Rancho 1 uvero, Palomares Matías Romero Juchitán, Isthmus Depression of Tehuantepec | 3 |
| Desmodus rotundus | Rancho 2, Palomares, Matías Romero Juchitán, Gulf Coastal Plain | 7 |
| Sturnira parvidens | 4 | |
| Sturnira hondurensis | 2 | |
| Dermanura phaeotis | 1 | |
| Dermanura watsoni | 1 | |
| Desmodus rotundus | San Miguel Chimalapas, Tehuantepec Coastal Plain | 17 |
| Glossophaga soricina | San Lucas Ojitlán, Tuxtepec, Gulf Coastal Plain | 1 |
| Sturnira hondurensis | 1 | |
| Desmodus rotundus | 4 (2) | |
| Desmodus rotundus | Palo de lima, San Lorenzo Texmelucan Juquila, Western Mountains and | 4 |
| Sturnira parvidens | Valleys | 1 |
| Desmodus rotundus | El Carrizal, San Lorenzo Texmelucan Juquila, Western Mountains and Valleys | 5 |
References
- Ramírez-Pulido, J.; González-Ruiz, N.; Gardner, A.L.; Arroyo-Cabrales, J. List of recent land mammals of México. Spec. Publ. Mus. Tex. Tech Univ. 2014, 63, 1–69. Available online: https://repository.si.edu/server/api/core/bitstreams/a85c9675-d1fa-44cb-95e5-b1e04993b02b/content (accessed on 8 January 2025).
- Mickleburgh, S.P.; Hutson, A.M.; Racey, P.A. A review of the global conservation status of bats. Oryx 2002, 36, 18–34. [Google Scholar] [CrossRef]
- Briones-Salas, M.; Cortés-Marcial, M.; Lavariega, M.C. Diversidad y distribución geográfica de los mamíferos terrestres del estado de Oaxaca, México. Rev. Mex. Biodivers. 2015, 86, 685–710. [Google Scholar] [CrossRef]
- Zárate, M.; Serrato, A.; López-Willchis, R. Importancia ecológica de los murciélagos. Contactos 2012, 85, 19–27. [Google Scholar]
- Castillo, D.L.L. Diagnóstico de la Problemática Social y Económica Asociada al Murciélago Vampiro (Desmodus rotundus) en Ranchos Ganaderos de la Zona Centro y Oriente del Estado de Yucatán. Master’s Thesis, INECOL, Xalapa Veracruz, Mexico, 2012. Available online: https://inecol.repositorioinstitucional.mx/jspui/bitstream/1005/46/1/6718_2012-10348.pdf (accessed on 23 May 2024).
- Medellín, R.A.; Equihua, M.; Amin, M.A. Bat diversity and abundance as indicators of disturbance in neotropical rainforest. Conserv. Biol. 2000, 14, 1666–1675. [Google Scholar] [CrossRef] [PubMed]
- Galindo, G.J.; Sosa, V. Frugivorous bats in isolated trees and riparian vegetation associated with human-made pastures in a fragmented tropical landscape. Southwest. Nat. 2003, 48, 579–589. [Google Scholar] [CrossRef]
- Calisher, C.H.; Childs, J.E.; Field, H.E.; Holmes, K.V.; Schountz, T. Bats: Important reservoir hosts of emerging viruses. Clin. Microbiol. Rev. 2006, 19, 531–545. [Google Scholar] [CrossRef] [PubMed]
- Irving, A.T.; Ahn, M.; Goh, G.; Anderson, D.E.; Wang, L.F. Lessons from the host defenses of bats, a unique viral reservoir. Nature 2021, 589, 363–370. [Google Scholar] [CrossRef] [PubMed]
- OMS. Rabia. Organización Mundial de la Salud. 2025. Available online: https://www.paho.org/es/temas/rabia (accessed on 13 January 2025).
- Gutiérrez, C.V.; Chávez, F.I.A.; Fernández, C.J.R.; Rodríguez, M.J.D.; Navarro, A.O.; Gómez, M.J.I. Programa de Acción Específico de Prevención y Control de Enfermedades Zoonóticas y Emergentes 2020–2024. Secretaría de Salud. 1-81, 2021. Available online: https://www.gob.mx/cms/uploads/attachment/file/738300/PAE_Zoonosis.pdf (accessed on 25 February 2025).
- Servicio Nacional de Sanidad, Inocuidad y Calidad Agroalimentaria (SENASICA). Panorama Nacional de Rabia Paralitica Bovina. 2020. Available online: https://dj.senasica.gob.mx/AtlasSanitario/storymaps/rpb.html (accessed on 25 February 2025).
- Belotto, A.; Leanes, L.F.; Schneider, M.C.; Tamayo, H.; Correa, E. Overview of rabies in the Americas. Virus Res. 2005, 111, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Pan American Health Organization (PAHO). Core Indicators Dataset Download, Region of the Americas. 2025. Available online: https://opendata.paho.org/en/core-indicators/download-dataset (accessed on 26 May 2025).
- Secretaría de Salud (SSA). Boletin Epidemiológico, Vigilancia Epidemiológica Semana 50, Sistema Nacional de Vigilancia Epidemiógica (SINAVE). 2024. Available online: https://www.gob.mx/cms/uploads/attachment/file/963614/BolEpid_SE50_5024.pdf#page=1.00 (accessed on 25 May 2025).
- Servicio Nacional de Sanidad, Inocuidad y Calidad Agroalimentaria (SENASICA). Panorama Nacional de Rabia Paralítica Bovina (PANR). 2021. Available online: https://dj.senasica.gob.mx/Contenido/files/2021/abril/PANRabiaparal%C3%ADticabovina16-12-20_5578cfed-e4da-4982-858d-0433072fdc9e.pdf (accessed on 27 May 2025).
- World Health Organization (WHO). Global Burden of Dog-Transmitted Human Rabies. 2025. Available online: https://www.who.int/teams/control-of-neglected-tropical-diseases/rabies/epidemiology-and-burden#:~:text=Rabies%20is%20estimated%20to%20cause,attributable%20to%20children%20under%2015 (accessed on 27 May 2025).
- Müller, T.; Freuling, C.; Stoffel, C.; Torres, G. Control and Elimination of Rabies in Europe: Challenges and Strategies for a Rabies-Free Europe. OIE Regional Comissión, 2016. Available online: https://www.woah.org/app/uploads/2021/03/2016-eur1-muller-a.pdf (accessed on 26 May 2025). [CrossRef]
- World Organization for Animal Health (WOAH). OIE’s Terrestrial Manual; Chapter 3.1.18 for the Diagnosis of Rabies in Bats; World Organization for Animal Health: Paris, France, 2023; pp. 1–42. [Google Scholar]
- Ribeiro, J.; Staudacher, C.; Martins, C.M.; Ullmann, L.S.; Ferreira, F.; Araujo, J.P., Jr.; Biondo, A.W. Bat rabies surveillance and risk factors in Brazil. BMC Vet. Res. 2018, 14, 173. [Google Scholar] [CrossRef] [PubMed]
- Salgado-Cardoso, Á.M.; Olave-Leyva, J.I.; Morales, I.; Aguilar-Setién, A.; López-Martínez, I.; Aréchiga-Ceballos, N. Cats: The New Challenge for Rabies Control in Yucatán, Mexico. Pathogens 2024, 13, 907. [Google Scholar] [CrossRef] [PubMed]
- Castillo, J.L.R.; Mora-Valle, A.D.L.; Medina-Basulto, G.E.; Monge-Navarro, F.J.; Hurtado, R.; Valencia, G.L. Report of Rabies in Feedlot Cattle Introduced to Baja California from the State of Guerrero, Mexico. Turk. J. Vet. Anim. Sci. 2015, 39, 241–244. [Google Scholar] [CrossRef]
- García, M.A.; Ordoñez, M.J.; Briones-Salas, M.A. Biodiversidad de Oaxaca; Universidad Nacional Autónoma de México, Fondo Oaxaqueño para la Conservación de la Naturaleza, World Wildlife Fund: Mexico City, Mexico, 2004. [Google Scholar]
- Torres, C.R. Tipos de vegetación. In Biodiversidad de Oaxaca; García-Mendoza, A.J., Ordoñez, M.J., Briones-Salas, M.A., Eds.; Universidad Nacional Autónoma de México, Fondo Oaxaqueño para la Conservación de la Naturaleza, World Wildlife Fund: Ciudad de México, México, 2004; pp. 105–117. [Google Scholar]
- Trejo, I. Clima. In Biodiversidad de Oaxaca, 1st ed.; García-Mendoza, A.J., Ordoñez, M.J., Briones-Salas, M.A., Eds.; Universidad Nacional Autónoma de México, Fondo Oaxaqueño para la Conservación de la Naturaleza, World Wildlife Fund: Ciudad de México, México, 2004; pp. 67–85. [Google Scholar]
- Medellín, R.A.; Arita, H.T.; Sánchez, O. Identificación de los Murciélagos de México, Clave de Campo, 2nd ed.; Consejo Nacional de Ciencia y Tecnología, Instituto de Ecología UNAM: Mexico City, Mexico, 2007; pp. 1–81. [Google Scholar]
- The Humane Society of the United States. Manual de Referencia Sobre la Eutanasia, 2nd ed.; The Humane Society of the United States: Washington, DC, USA, 2013; pp. 81–82. [Google Scholar]
- Romero-Almaraz, L.; Sánchez, H.; García, E.; Owen, R.D. Manual de Técnicas de Captura, Preparación, Preservación y Estudio de Mamíferos Pequeños; Universidad Nacional Autónoma de México, Facultad de Ciencias, Instituto de Biología: Mexico City, Mexico, 2007; pp. 1–204. [Google Scholar]
- NOM ZOO-056-ZOO-1995; Especificaciones Técnicas para las Pruebas Diagnósticas que Realicen los Laboratorios de Pruebas Aprobados en Materia Zoosanitaria. Servicio Nacional de Sanidad, Inocuidad y Calidad Agroalimentaria (SENASICA): Mexico City, Mexico, 1999. Available online: https://www.gob.mx/senasica/documentos/nom-056-zoo-1995 (accessed on 15 February 2025).
- Sánchez, P.L. Detección de Leishmania mexicana en Murciélagos de la Región de Los Tuxtlas, Veracruz, México. Bachelor’s Thesis, Universidad Nacional Autónoma de México, Mexico City, Mexico, 2019. Available online: https://repositorio.unam.mx/contenidos/3452534 (accessed on 25 May 2024).
- Salas-Rojas, M.; Sánchez-Hernández, C.; Romero-Almaraz, M.L.; Schnell, G.D.; Schmid, R.K.; Aguilar-Setién, A. Prevalence of rabies and LPM paramyxovirus antibody in non-hematophagous bats captured in the Central Pacific coast of Mexico. Trans. R. Soc. Trop. Med. Hyg. 2004, 98, 358–365. [Google Scholar] [CrossRef] [PubMed]
- Romero-Barrera, C.E.; Osorio-Rodriguez, A.N.; Juárez-Agis, A. Distribución, abundancia, control y registros de casos de murciélagos vampiro, Desmodus rotundus (E. Geoffroy), infectados de rabia en ambientes pecuarios de Guerrero, México. Acta Agrícola Pecuaria 2021, 7, E0071005. [Google Scholar] [CrossRef]
- Jiménez, S.N.G. Monitoreo del Virus de la Rabia en Animales de Vida Silvestre de la República Mexicana. Bachelor’s Thesis, Instituto Politécnico Nacional, Escuela Nacional de Ciencias Biológicas, Mexico City, Mexico, 2009. [Google Scholar]
- Pedro, W.A.; Biagi, M.B.; Carvalho, C.; Perri, S.H.V.; Queiroz, L.H. Estacionalidad de la rabia en murciélagos (Chiroptera, Mammalia) del noroeste del estado de Sao Paulo, Brasil. Rev. Educ. Contin. Med. Vet. Zootec. 2012, 10, 84. Available online: https://www.revistamvez-crmvsp.com.br/index.php/recmvz/article/view/2563 (accessed on 23 January 2025).
- Ortega-Sánchez, R.; Bárcenas-Reyes, I.; Luna-Cozar, J.; Rojas-Anaya, E.; Cuador-Gil, J.Q.; Cantó-Alarcón, G.J.; Veyna-Salazar, N.; González-Ruiz, S.; Milián-Suazo, F. Spatial-Temporal Risk Factors in the Occurrence of Rabies in Mexico. Geospat. Health 2024, 19, e1062. [Google Scholar] [CrossRef] [PubMed]
- Mexico Business News. Ministry of Health Confirms Rabies Infection in Minors. 2023. Available online: https://mexicobusiness.news/health/news/ministry-health-confirms-rabies-infection-minors (accessed on 27 May 2025).
- Mendoza-Sáenz, V.H.; Saldaña-Vázquez, R.A.; Navarrete-Gutiérrez, D.; Kraker-Castañeda, C.; Ávila-Flores, R.; Jiménez-Ferrer, G. Reducing conflict between the common vampire bat Desmodus rotundus and cattle ranching in Neotropical landscapes. Mamm. Rev. 2023, 53, 72–83. [Google Scholar] [CrossRef]
- NOM-067-ZOO-2007 (1995); Campaña Nacional para la Prevención y Control de la Rabia en Bovinos y Especies Ganaderas. Secretaría de Agricultura, Ganadería, Desarrollo Rural, Pesca y Alimentación (SAGARPA): Mexico City, Mexico, 2011. Available online: https://www.gob.mx/senasica/documentos/nom-067-zoo-2007 (accessed on 18 February 2025).
| Family | Species | N° Specimens |
|---|---|---|
| Phyllostomidae | Desmodus rotundus | 129 (3) * |
| Dermanura azteca | 21 | |
| Anoura geoffroyi | 5 | |
| Sturnira parvidens | 5 | |
| Artibeus jamaicensis | 4 | |
| Sturnira hondurensis | 4 | |
| Glossophaga soricina | 3 | |
| Macrotus waterhousii | 3 | |
| Leptonycteris yerbabuenae | 1 | |
| Dermanura phaeotis | 1 | |
| Dermanura watsoni | 1 | |
| Emballonuridae | Balantiopteryx plicata | 12 |
| Vespertilionidae | Eptesicus fuscus | 3 |
| Myotis sp. | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Medina Matías, M.I.; García-Luis, M.; Blanco Esquivel, O.E.; Nicolás Reyes, I.; Domínguez Martínez, M.Á.; Fuentes-Mascorro, G. Detection and Prevalence of Rabies in Bats from Oaxaca. Microorganisms 2025, 13, 1417. https://doi.org/10.3390/microorganisms13061417
Medina Matías MI, García-Luis M, Blanco Esquivel OE, Nicolás Reyes I, Domínguez Martínez MÁ, Fuentes-Mascorro G. Detection and Prevalence of Rabies in Bats from Oaxaca. Microorganisms. 2025; 13(6):1417. https://doi.org/10.3390/microorganisms13061417
Chicago/Turabian StyleMedina Matías, María Isabel, Margarita García-Luis, Oscar Ezequiel Blanco Esquivel, Israel Nicolás Reyes, Miguel Ángel Domínguez Martínez, and Gisela Fuentes-Mascorro. 2025. "Detection and Prevalence of Rabies in Bats from Oaxaca" Microorganisms 13, no. 6: 1417. https://doi.org/10.3390/microorganisms13061417
APA StyleMedina Matías, M. I., García-Luis, M., Blanco Esquivel, O. E., Nicolás Reyes, I., Domínguez Martínez, M. Á., & Fuentes-Mascorro, G. (2025). Detection and Prevalence of Rabies in Bats from Oaxaca. Microorganisms, 13(6), 1417. https://doi.org/10.3390/microorganisms13061417










